Although the first study on the efficacy of methotrexate in the treatment of psoriasis was reported in 1958, scientific evidence for this indication has been scant until quite recently. We now have new data on the pharmacokinetics and mechanism of action of methotrexate and new subcutaneous formulations that have improved the bioavailability, efficacy, and ease of administration of the drug. The results of recent clinical trials comparing methotrexate with several biologic agents have shown it to be the first-line therapy among the classic systemic treatments for psoriasis. Moreover, the incremental cost-effectiveness ratio for subcutaneous methotrexate has been shown to be superior to that of ciclosporin, adalimumab, and infliximab.
Pese a que el primer estudio sobre la eficacia de metotrexato en el tratamiento de la psoriasis se remonta a 1958, hasta no hace mucho ha sido escasa la evidencia científica disponible sobre su uso en esta indicación.
Disponemos de nuevos datos acerca de la farmacocinética y el mecanismo de acción del metotrexato, así como de nuevas presentaciones por vía subcutánea que mejoran la biodisponibilidad, la eficacia y la conveniencia de la administración de este fármaco. La reciente publicación de ensayos clínicos comparativos con diversos biológicos permite considerar al metotrexato como el estándar terapéutico dentro de los tratamientos sistémicos clásicos de la psoriasis, con un cociente coste-eficacia incremental favorable al metotrexato subcutáneo si se compara con ciclosporina, adalimumab o infliximab.
Methotrexate (MTX, 4-amino-4-deoxy-N10-methyl pteroylglutamic acid) is an analog of aminopterin (4-amino-pteroylglutamic acid), a folic acid antagonist that was introduced in 1948 to treat acute leukemia in children but later replaced by MTX as this had a more favorable toxicity profile. The first study of the efficacy of MTX in the treatment of psoriasis was published in 1958,1 and the first guidelines on its use in dermatology appeared in 1972.2 Until quite recently, however, there was relatively little evidence to support the use of MTX in the treatment of psoriasis.
Among the advances that justify this review are new efficacy data that have led MTX to be considered the first-line therapy among the classic systemic treatments for psoriasis and new subcutaneous formulations that offer improved bioavailability and convenience of administration.
PharmacokineticsMTX can be administered orally, subcutaneously, intramuscularly, or intravenously. In the case of psoriasis, the standard dose (7.5-25mg/wk) is nearly always administered orally or subcutaneously. The bioavailability of low-dose oral MTX is high (70%),3 although it varies from patient to patient. At doses of more than 15mg/wk bioavailability drops to 30%, as the uptake of MTX by the gastrointestinal tract is mediated by a saturable transporter, reduced folate carrier 1 (RFC1).3 The preferred route of administration in such cases is thus generally parenteral, which is also associated with better gastrointestinal tolerability,4 although the use of split doses of oral MTX can improve bioavailability.5 The absorption of oral MTX, which primarily occurs in the proximal jejunum, is not affected by food intake, but is decreased in cases of malabsorption or inflammatory bowel disease. The bioavailability of parenteral MTX is similar regardless of the administration route used.
Once absorbed, 10% of MTX is converted in the liver to its partly inactive metabolite, 7-hydroxymethotrexate (7-OH-MTX), thereby reducing plasma MTX concentrations. The ability to convert MTX to 7-OH-MTX via aldehyde oxidase varies greatly—relative interindividual differences ranging from 1 to 14 have been described—and exhibits a bimodal distribution in the population. Rapid metabolizers have a poorer therapeutic response to MTX; additionally, folic acid (unlike folinic acid) blocks the production of 7-OH-MTX and could therefore contribute to higher levels of MTX in these patients.6 7-OH-MTX competes with MTX for cellular uptake via RFCs, which also internalize reduced folates and folic acid, albeit with low affinity. Quantitative or qualitative alterations in RFCs, similarly to high levels of exogenous folates competing for the receptor, can result in transport-related resistance to the therapeutic action of MTX. A recent study described a single nucleotide polymorphism in the RCF1 gene that predicts therapeutic response to MTX in Japanese patients with rheumatoid arthritis,7 illustrating the role that pharmacogenetics may come to play in improving treatment outcomes.
MTX, like 7-OH-MTX, is metabolized intracellularly to produce monoglutamates and polyglutamates, which are the main inhibitors of a range of enzymes; the intracellular levels of these metabolites correlate with the therapeutic efficacy of MTX (which is actually a prodrug).5 Both the bioavailability of MTX and its intracellular conjugation are partly determined by the administration route. In one study of patients with rheumatoid arthritis, switching from oral to subcutaneous administration resulted in a 37% increase in the concentration of very long-chain polyglutamates and a 31% reduction in disease activity.8 Polyglutamates are released slowly from the interior of the cell by the action of active efflux carriers, which may contribute to prolonging the elimination of the drug and also reflect pharmacogenetic differences.9 While no studies have demonstrated that genetic variability in the enzymes involved in MTX metabolism is of clinical relevance in psoriasis,10 variations in the genes involved in polyglutamate efflux transporters have been associated with differences in treatment efficacy and the risk of toxicity.11
Between 35% and 50% of circulating MTX binds to albumin (compared with 91%-95% of 7-OH-MTX) and peak concentrations are reached in the kidneys, liver, gallbladder, spleen, skin, and red blood cells (RBCs). RBC MTX levels may indicate possible hematologic toxicity and hepatic accumulation of the drug. MTX tends to accumulate in the extravascular compartment, and therefore extra caution should be exercised in patients with pleural effusion, ascites, or massive edema due to the risk of toxicity from reabsorption of extravascular fluid.
Between 65% and 80% of MTX is eliminated unaltered by the kidneys (mainly in the first 12hours after administration) and 20% to 35% undergoes biliary secretion and is metabolized or transferred to other compartments. Glomerular filtration is the main pathway of renal elimination; tubular secretion and reabsorption are also involved but to a lesser extent. Biliary secretion is an important factor in patents with renal insufficiency, who have reduced drug clearance and an increased risk of toxicity. Hemodialysis and peritoneal dialysis result in limited and transient reductions in serum MTX levels due to the drug's low-to-medium protein binding and high tissue distribution.
The terminal half-life of MTX in serum is approximately 7 to 10 hours, but it can be as long as 26 hours in some patients. RBC MTX concentrations remain stable for 9 days, while serum levels become undetectable after 52hours.12 Although individual MTX clearance rates can be established with just 2 tests (at 30 and 120minutes after administration), they have not been found to be useful for defining an optimal treatment regimen in rheumatoid arthritis.13 While many drugs interact with MTX, the use of various nonsteroidal anti-inflammatory drugs (NSAIDs) was not found to significantly alter MTX serum levels, as compared with levels in patients not treated with NSAIDs.14 Nevertheless, it is very important to remember that dose reductions are necessary in older patients (>65 years) and patients with renal insufficiency.15
Mechanisms of ActionVarious mechanisms of action have been proposed for MTX. The most relevant in the case of inflammatory diseases is the promotion of adenosine release with subsequent suppression of inflammation.3
MTX inhibits the proliferation of neoplastic cells, blocking the de novo synthesis of purines and pyrimidines by irreversibly inhibiting dihydrofolate reductase, which is responsible for the production of tetrahydrofolate. Although high doses of folic or folinic acid can reverse this effect, folic and folinic acid supplementation in inflammatory diseases is not used for this purpose but rather to prevent MTX toxicity without significantly reducing treatment efficacy.16
The most important mechanism of action of MTX in inflammatory diseases is probably related to the recently described anti-inflammatory activity of adenosine through specific receptors.17 In both animal models of inflammation and patients with rheumatoid arthritis, MTX induces the extracellular release of adenosine, which acts as an anti-inflammatory agent through specific type A2 receptors18 that also participate in the pathogenesis of hepatic fibrosis.19
MTX, especially in its polyglutamate form—which accumulates in the interior of cells—inhibits AICAR (5-aminoimidazole-4-carboxamide ribonucleotide) transformylase.20 This results in the intracellular accumulation of AICAR, which competitively inhibits adenosine monophosphate desaminase. The increased intracellular levels of AICAR would then induce the release of adenine nucleotides and their conversion to adenosine by ecto-5’-nucleotidase (CD73).21,22 Adenosine has a very short half-life (of just seconds) in peripheral blood and body fluids, and it is therefore extremely difficult to measure its levels. Most of the studies published have based their measurements on the inhibition of the biological effects of adenosine using drugs that inhibit the metabolism or receptors of MTX23,24 or on animal models such as the CD73-deficient mouse21 and other mouse strains.25
Caffeine, a nonselective adenosine receptor antagonist, blocks the anti-inflammatory effects of MTX in vitro and in animal models of arthritis26; it is therefore possible that coffee and other caffeine drinks interfere with the therapeutic (and perhaps toxic) activity of MTX. This hypothesis is supported by the findings of several studies27 but was not corroborated in a retrospective study of patients with rheumatoid artritis.28 Moreover, no relationship was found between MTX at a maintenance dose and coffee consumption in patients with psoriasis or psoriatic arthritis.29
Although the antiproliferative action of MTX explains stomatitis, anemia, leukopenia, and alopecia, the release of adenosine could help to explain other adverse effects of this drug. Adenosine binding to A1 and A2B receptors induces hepatic steatosis,30 while binding to A2A receptors plays a role in the development of fibrosis31 and hepatic cirrhosis.29 Some patients have reported marked asthenia on the day they take MTX. This may be related to the release of adenosine into the central nervous system32 and could be improved with the administration of aminophylline.33
Another possible mechanism of action of MTX may involve its inhibitory effect on the proliferation of antigen-stimulated T cells, which has been reported both in vitro and in patients receiving MTX,34 or the induction of apoptosis in activated T cells,33 which may also be mediated by adenosine receptors.35
Finally, the action of MTX in psoriasis could be mediated by the suppression of T-cell activation (through folate-dependent mechanisms) and the altered expression of adhesion molecules; adenosine would also have a role in this mechanism.36
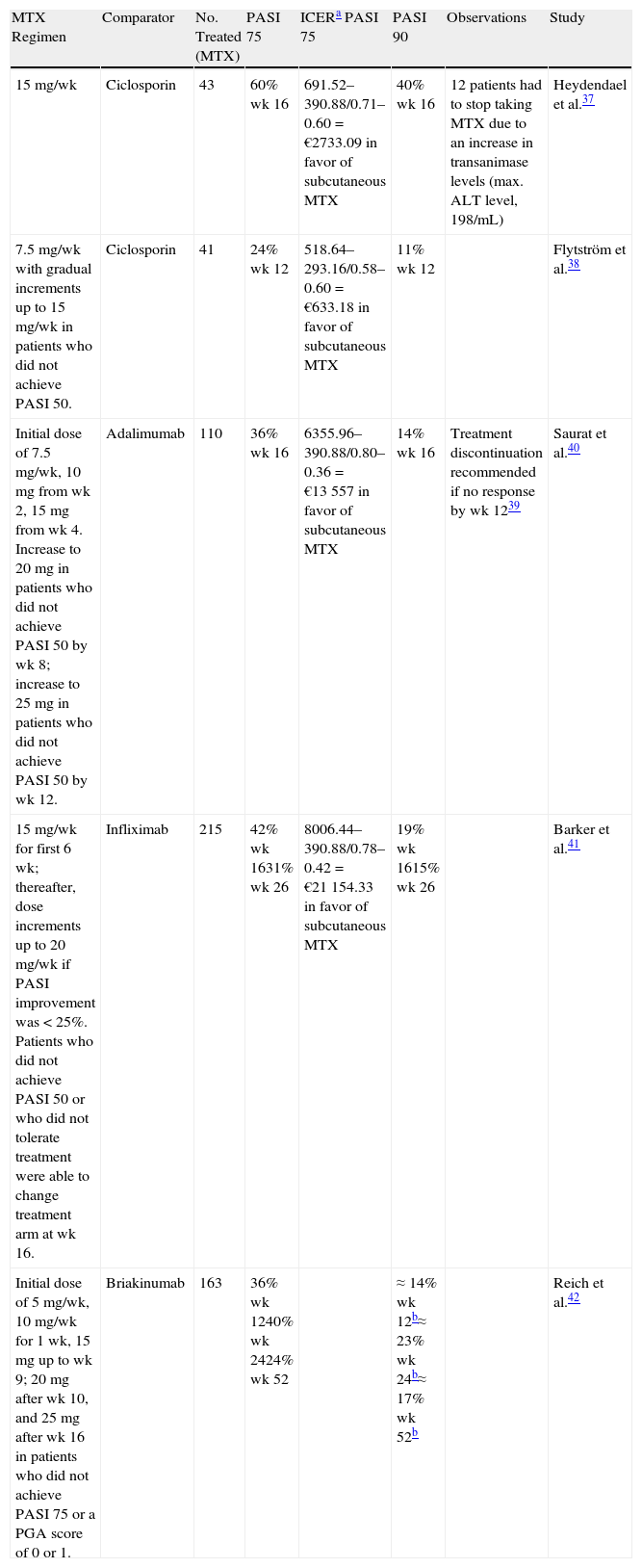
Efficacy in PsoriasisA number of clinical trials in recent years have explored the efficacy of MTX in psoriasis and compared it with other options.37–42Table 1 summarizes the findings.
Summary of Comparative Clinical Trials of Methotrexate (MTX) in Psoriasis.
| MTX Regimen | Comparator | No. Treated (MTX) | PASI 75 | ICERa PASI 75 | PASI 90 | Observations | Study |
| 15mg/wk | Ciclosporin | 43 | 60% wk 16 | 691.52–390.88/0.71–0.60=€2733.09 in favor of subcutaneous MTX | 40% wk 16 | 12 patients had to stop taking MTX due to an increase in transanimase levels (max. ALT level, 198/mL) | Heydendael et al.37 |
| 7.5mg/wk with gradual increments up to 15mg/wk in patients who did not achieve PASI 50. | Ciclosporin | 41 | 24% wk 12 | 518.64–293.16/0.58–0.60=€633.18 in favor of subcutaneous MTX | 11% wk 12 | Flytström et al.38 | |
| Initial dose of 7.5mg/wk, 10mg from wk 2, 15mg from wk 4. Increase to 20mg in patients who did not achieve PASI 50 by wk 8; increase to 25mg in patients who did not achieve PASI 50 by wk 12. | Adalimumab | 110 | 36% wk 16 | 6355.96–390.88/0.80–0.36=€13 557 in favor of subcutaneous MTX | 14% wk 16 | Treatment discontinuation recommended if no response by wk 1239 | Saurat et al.40 |
| 15mg/wk for first 6 wk; thereafter, dose increments up to 20mg/wk if PASI improvement was <25%. Patients who did not achieve PASI 50 or who did not tolerate treatment were able to change treatment arm at wk 16. | Infliximab | 215 | 42% wk 1631% wk 26 | 8006.44–390.88/0.78–0.42=€21 154.33 in favor of subcutaneous MTX | 19% wk 1615% wk 26 | Barker et al.41 | |
| Initial dose of 5mg/wk, 10mg/wk for 1 wk, 15mg up to wk 9; 20mg after wk 10, and 25mg after wk 16 in patients who did not achieve PASI 75 or a PGA score of 0 or 1. | Briakinumab | 163 | 36% wk 1240% wk 2424% wk 52 | ≈14% wk 12b≈23% wk 24b≈17% wk 52b | Reich et al.42 |
Abbreviations: ALT, alanine aminotransferase; PASI, Psoriasis Area and Severity Index; PASI 50, ≥50% improvement in PASI score with respect to baseline; PASI 75, ≥75% improvement in PASI score with respect to baseline; PASI 90, ≥90% improvement in PASI score with respect to baseline; PGA, physician's global assessment.
ICER, incremental cost-effectiveness ratio (cost of alternative−cost of subcutaneous MTX)/(efficacy alternative−efficacy subcutaneous MTX). Recommended retail prices as of October 2012. Metoject: €19.36€/wk (7.5mg); €24.43/wk (15mg) (based on maximum dose). Sandimmun Neoral 100mg/mL suspension: €128.62/50mL; €43.22/wk based on starting dose of 3mg/kg/d for a patient weighing 80kg. Remicade: 80kg, 4 vials of 100mg (€615.88) × 3.25 doses (16 wk)=€8006.44. Humira: €1127.57/2 × 9.5 doses (16 wk)=€5355.96.
Various regimens can produce a 75% improvement in the Psoriasis Area and Severity Index (PASI 75) in up to 40% of patients by week 24, although in most cases PASI response at week 12 is a good indicator of whether treatment should be continued or not.38
Experience with combination regimens of MTX and diverse biologics to treat moderate to severe psoriasis has shown improved patient responses and treatment adherence. The possible mechanisms underlying these effects include reduced clearance of the biologic agent and reduced production of antibodies against the agent.43
The results of a clinical trial comparing etanercept monotherapy and etanercept plus MTX in 239 patients per arm were recently published.44 Patients in the monotherapy arm received 50mg twice weekly for 12 weeks followed by 50mg weekly for 12 weeks, while those in the combination therapy group received 7.5mg weekly for the first 2 weeks, 10mg weekly for the next 2 weeks, and up to 15mg weekly up to week 24. Combination therapy with etanercept plus MTX was superior to etanercept alone, with PASI 75 responses observed in 70% of patients at week 12 (vs 54% in the monotherapy group) and in 77% of patients (vs 60%) at week 24. The corresponding rates of PASI 90 responses were 34% vs 23% and 54% vs 34%, respectively.
Patterns of Use and Administration RoutesPatterns of MTX use by both rheumatologists and dermatologists have changed somewhat in recent years. One recent review concluded that the bioavailability of oral MTX decreases at doses above 17.5mg,45 indicating that parenteral administration is preferable. In a clinical trial comparing the efficacy and safety of oral and subcutaneous MTX in patients with rheumatoid arthritis, 78% of patients treated with subcutaneous MTX and 70% of those treated with oral MTX achieved the treatment goal of a 20% improvement in the American College of Rheumatology criteria (ACR20) by week 48.46 There were also differences in the frequency of adverse effects between the groups (66% vs 62%) but these were not statistically significant. Thirty percent of the patients who did not respond to oral MTX 15mg achieved an ACR20 response when they were switched to subcutaneous MTX. The study is the first to provide formal proof that subcutaneous MTX has superior clinical efficacy to oral MTX,47 and one would expect these results to be extrapolated to the treatment of psoriasis, despite the current lack of scientific evidence in this setting. The main advantage of the subcutaneous route is the superior bioavailability of MTX. It has been demonstrated that the increased effectiveness seen in rheumatoid arthritis patients following a switch from oral to subcutaneous MTX without a change in dose is accompanied by an increase in RBC MTX polyglutamate concentrations, although it may take 6 months for the new steady-state concentrations to be reached.48
Data on the superior clinical efficacy of subcutaneous MTX in the treatment of rheumatoid arthritis were used as the starting point for a pharmacoeconomic analysis conducted in Spain that concluded that the additional cost associated with the use of the subcutaneous route would be offset by improved effectivness.49Table 1 summarizes the results of an incremental cost-effectiveness analysis of subcutaneous MTX and several alternatives based on data from comparative clinical trials. The results show that subcutaneous MTX has a higher incremental cost-effectiveness ratio to ciclosporin, adalimumab, and infliximab in the treatment of moderate to severe psoriasis. (We have omitted the sensitivity analysis and confidence intervals due to space constraints.)
A study of MTX use among dermatologists and rheumatologists in Canada reported that most dermatologists (97%) and rheumatologists (80%) start treating psoriasis with oral MTX and that fewer dermatologists subsequently switch to parenteral MTX (49% vs 96% of rheumatologists); of the dermatologists that do switch 63% choose the subcutaneous route (vs 98% of the rheumatologists).50
Considering that the usefulness of test doses of MTX in psoriasis is still being questioned (treatment is commonly initiated with a dose of 10 to 15mg in selected patients),51 the advantages of subcutaneous MTX in rheumatoid arthritis (greater efficacy45 and fewer gastrointestinal problems52) could probably be extrapolated to psoriasis, and it would appear reasonable to assume that subcutaneous MTX will be increasingly used in dermatology. Greater familiarity with subcutaneous injections among dermatology patients will undoubtedly contribute to this increased use, as will the introduction of higher-concentration formulations (50mg/mL) in prefilled syringes, as these offer greater tolerability and convenience53 and are preferred to the 10-mg/mL formulations by the majority of patients treated with 20mg per week.54 Single-dose formulations also have environmental and safety benefits as they eliminate the need to handle and discard unused portions of vials or syringes.55
Ethical DisclosuresProtection of humans and animalsThe authors declare that no tests were carried out in humans or animals for the purpose of this study.
Confidentiality of dataThe authors declare that they have followed their hospital's protocol on the publication of data concerning patients and that all patients included in the study have received sufficient information and have given their written informed consent to participate in the study.
Right to privacy and informed consentThe authors declare that no private patient data appear in this article.
Conflicts of InterestThe author has received speakers and/or consultancy and/or clinical trial investigator fees from Gebro Pharma and Pfizer; he has also received fees from Marge Medica Books for coordinating and co-authoring Methotraxate and psoriasis. Barcelona: Marge Medica Books; 2012, sponsored by Gebro Pharma.
Please cite this article as: Puig L. Metotrexato: novedades terapéuticas. Actas Dermosifiliogr. 2014;105:583–589.