We present a series of 12 patients with telaprevir-induced skin toxicity. Some patients presented eczematous lesion, while others presented nonscaling macular lesions that became more purpuric in the lower limbs. Seven of the 12 patients had skin lesions affecting more than 50% of the body surface area, but none had systemic manifestations. Oral corticosteroids, prescribed in 7 patients, produced symptomatic improvement, and the response to the antiviral treatment in these patients was good. The 3 biopsies performed showed a superficial perivascular dermatitis with foci of red cell extravasation. Monitoring is central to the management of skin reactions secondary to the protease inhibitors, as severe drug eruptions have been reported. Treatment is usually symptomatic. We describe 7 cases in which oral corticosteroids—whose use continues to be controversial —were administered as a last resort for the control of pruritus.
Presentamos una serie de 12 pacientes con toxicidad cutánea relacionada con el tratamiento con telaprevir. Algunos pacientes presentaban lesiones eccematosas, otros lesiones maculares sin descamación, que se hacían más purpúricas en los miembros inferiores. La mayor parte (7/12) presentaban una afectación superior al 50% de la superficie corporal, pero ninguno manifestaciones sistémicas. Se han pautado corticoides orales en 7/12 pacientes, con mejoría de su sintomatología y también con buena respuesta al tratamiento antiviral. Se han realizado 3 biopsias que muestran una dermatitis perivascular superficial con escasos focos de extravasación hemática.
En el manejo de las reacciones cutáneas a los inhibidores de la proteasa es fundamental la monitorización, ya que se han descrito erupciones medicamentosas graves. El tratamiento es en general sintomático. El uso de corticoides orales es controvertido. Describimos 7 pacientes en los que se han administrado como último recurso para el control sintomático del prurito.
Hepatitis C virus (HCV) infection is the primary cause of cirrhosis of the liver and of hepatocellular carcinoma in Spain1 and it carries a high morbidity and mortality. The protease inhibitors (PI), such as telaprevir and boceprevir, added to double therapy with interferon (IFN) and ribavirin have revolutionized the treatment of HCV genotype 1 infection.2 However, numerous adverse effects, particularly dermatologic, gastrointestinal, and hematologic effects, have been described. These effects are common and lead to a need for strict patient selection, despite which treatment still has to be discontinued on numerous occasions.3,4
We present a series of 12 patients seen in the Dermatology Department of Hospital General Universitario de Valencia for skin rash related to treatment with PIs not in a clinical trial setting. Mostly, these patients had developed extensive rashes that were refractory to the treatment prescribed by the internists or hepatologists, including emollient creams, topical corticosteroids, and antihistamines. Management with oral corticosteroids has, in some patients, enabled the course of treatment with telaprevir to be completed without affecting the viral response.
Case DescriptionsBetween April 2012 and March 2013, 101 patients have been treated with PIs in Hospital General Universitario de Valencia, 97 with telaprevir and 4 with boceprevir; 59 patients were treated in the hepatology unit and 42 in the infectious diseases unit. Of these 42 patients, 19 presented coinfection with human immunodeficiency virus (HIV) and 1 with both HIV and hepatitis B virus (HBV). During this period, 12 patients were seen in the dermatology department for skin manifestations refractory to the prescribed treatment (Table 1), all of them on treatment with telaprevir. All were in a good general state of health, with no signs of systemic involvement, and the main reason for consultation was pruritus. The age range of the patients was 46 to 69 years (mean age, 57.4 years) and 9 of them were men. Six (50%) patients had not received previous treatment for their HCV infection. No patient with HBV or HIV coinfection was referred to our department.
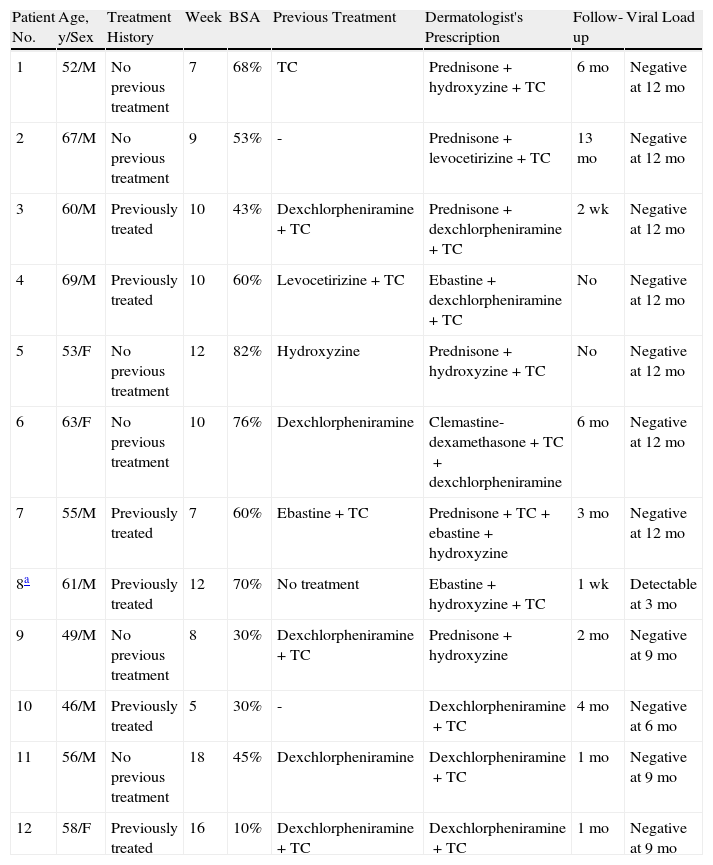
Characteristics of Patients Seen in Dermatology for Telaprevir-Induced Skin Rashes (Treatment Prior to the Visit, Treatment Prescribed by the Dermatologist, Follow-up Period, and Viral Response).
| Patient No. | Age, y/Sex | Treatment History | Week | BSA | Previous Treatment | Dermatologist's Prescription | Follow-up | Viral Load |
| 1 | 52/M | No previous treatment | 7 | 68% | TC | Prednisone+hydroxyzine+TC | 6 mo | Negative at 12 mo |
| 2 | 67/M | No previous treatment | 9 | 53% | - | Prednisone+levocetirizine+TC | 13 mo | Negative at 12 mo |
| 3 | 60/M | Previously treated | 10 | 43% | Dexchlorpheniramine+TC | Prednisone+dexchlorpheniramine+TC | 2 wk | Negative at 12 mo |
| 4 | 69/M | Previously treated | 10 | 60% | Levocetirizine+TC | Ebastine+dexchlorpheniramine+TC | No | Negative at 12 mo |
| 5 | 53/F | No previous treatment | 12 | 82% | Hydroxyzine | Prednisone+hydroxyzine+TC | No | Negative at 12 mo |
| 6 | 63/F | No previous treatment | 10 | 76% | Dexchlorpheniramine | Clemastine-dexamethasone+TC+dexchlorpheniramine | 6 mo | Negative at 12 mo |
| 7 | 55/M | Previously treated | 7 | 60% | Ebastine+TC | Prednisone+TC+ebastine+hydroxyzine | 3 mo | Negative at 12 mo |
| 8a | 61/M | Previously treated | 12 | 70% | No treatment | Ebastine+hydroxyzine+TC | 1 wk | Detectable at 3 mo |
| 9 | 49/M | No previous treatment | 8 | 30% | Dexchlorpheniramine+TC | Prednisone+hydroxyzine | 2 mo | Negative at 9 mo |
| 10 | 46/M | Previously treated | 5 | 30% | - | Dexchlorpheniramine+TC | 4 mo | Negative at 6 mo |
| 11 | 56/M | No previous treatment | 18 | 45% | Dexchlorpheniramine | Dexchlorpheniramine+TC | 1 mo | Negative at 9 mo |
| 12 | 58/F | Previously treated | 16 | 10% | Dexchlorpheniramine+TC | Dexchlorpheniramine+TC | 1 mo | Negative at 9 mo |
Abbreviations: BSA, body surface area; F, female; HBV, hepatitis B virus; HIV, human immunodeficiency virus; M male; TC, topical corticosteroids.
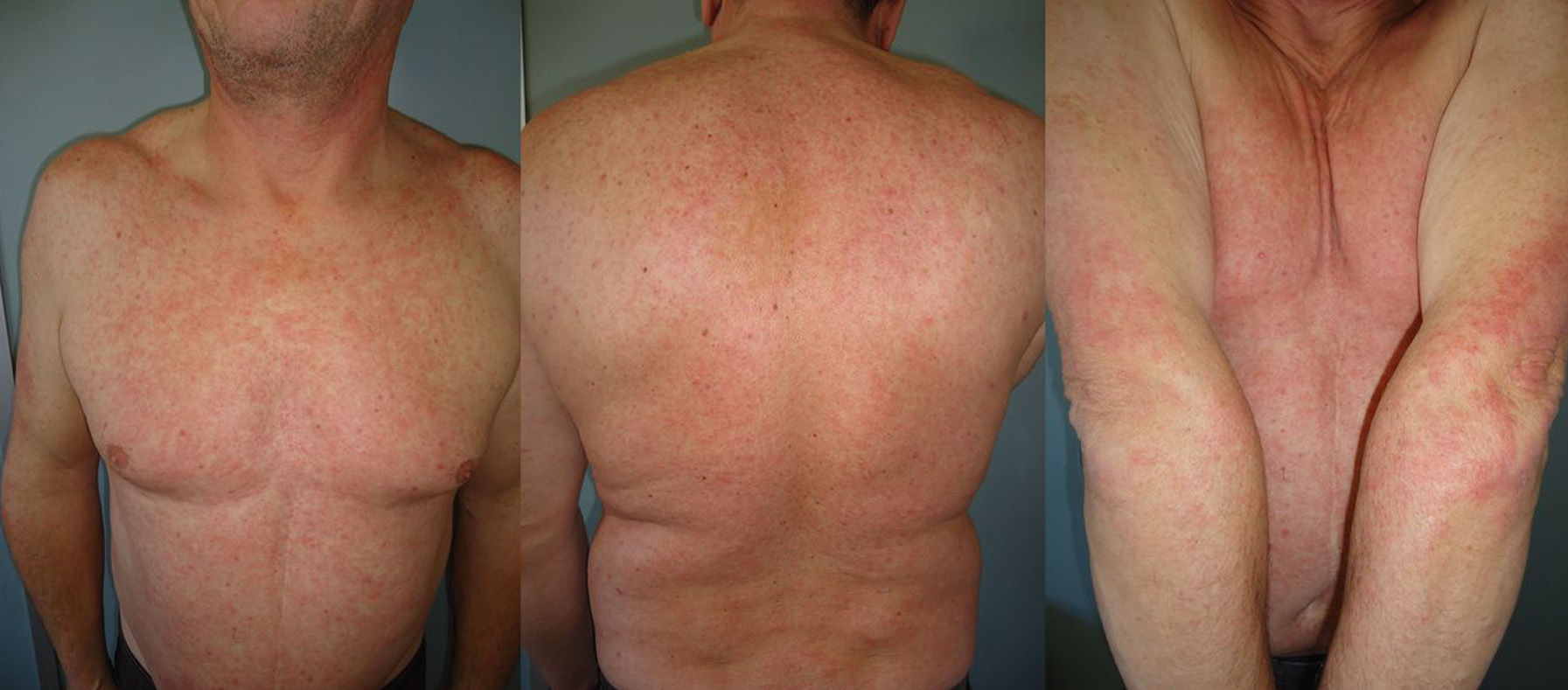
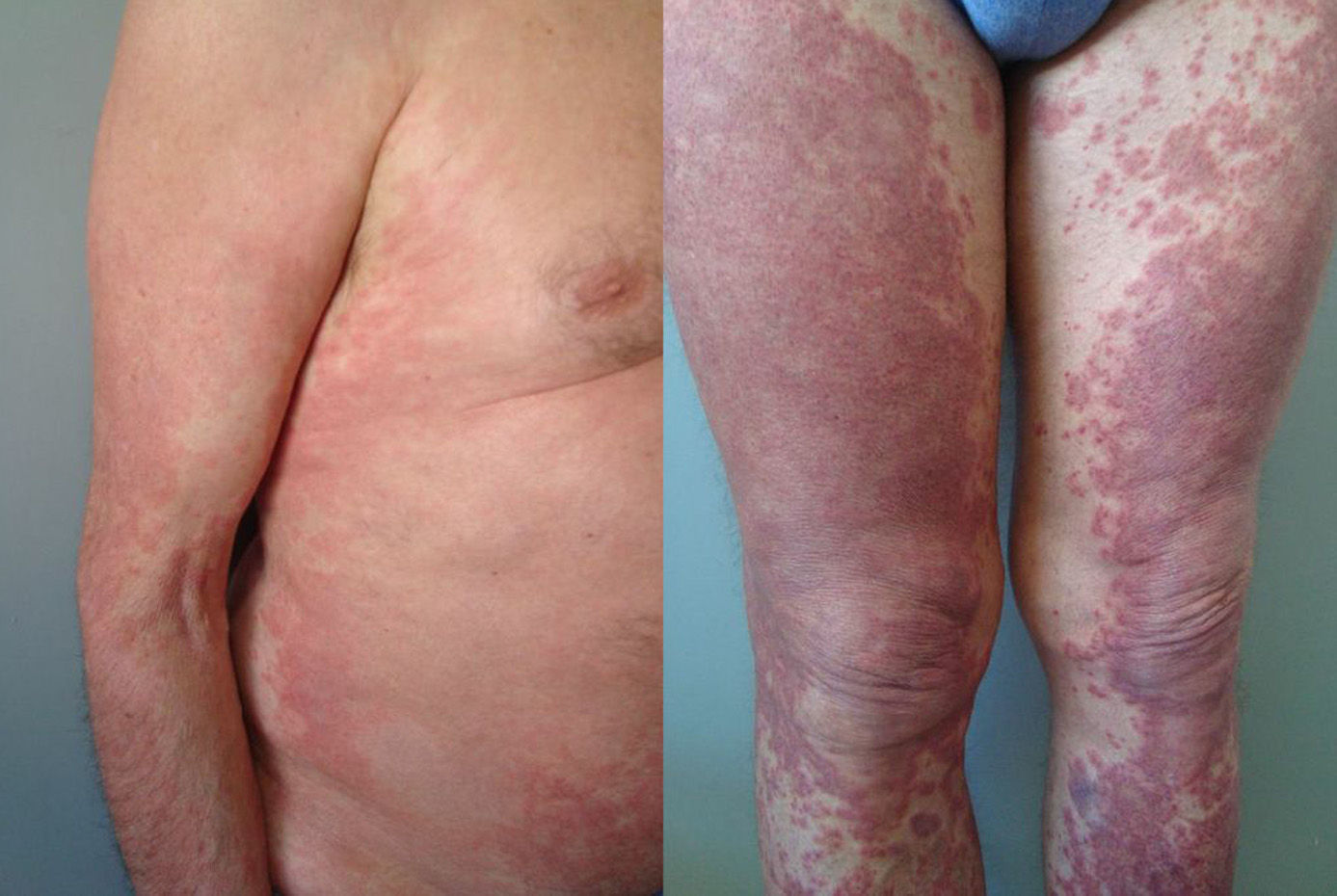
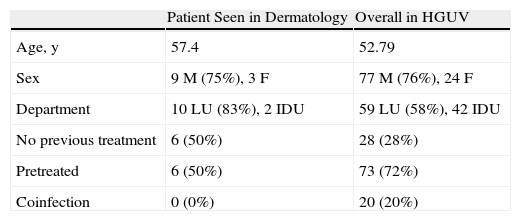
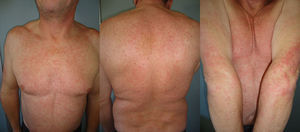
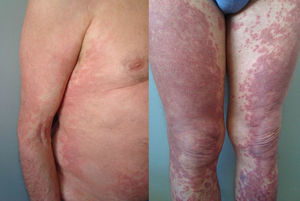
The duration of treatment with telaprevir before the first consultation in dermatology varied between 7 and 18 weeks. Two patients (11 and 12) were seen in weeks 16 and 18, respectively, of the regimen and had thus already completed the 12-week course of telaprevir, although the skin manifestations had started during treatment. Lesions were present on the trunk and limbs and were of 2 types: eczematous, erythematous papules that showed a variable degree of confluence, with marked xerosis and lichenification in some cases (Fig. 1); and more macular, erythematous-violaceous lesions that were purpuric on the lower limbs (Fig. 2). Over 50% of the body surface area (BSA) was affected in 7 of the 12 patients, but none presented systemic signs that would have suggested a serious drug reaction.
Ten patients had been prescribed previous treatment for their dermatitis by their hepatologist or internist, the majority with topical corticosteroids and antihistamines (Table 1). During consultation in dermatology, emollients and antihistamines were added to the treatment, and oral corticosteroids were prescribed in 7 patients. We used prednisone, 0.5-0.75mg/kg/d, and, in 1 patient, dexamethasone at a maximum dose of 1.5mg/d.
The follow-up period was very variable, from 1 week to 12 months. Two patients (4 and 5) were lost to follow-up. In patients in whom follow-up was longer (patients 1, 2, and 6), oral corticosteroid treatment was continued for a maximum of 40 days, although further cycles of 2 to 3 weeks were required in some patients due to reappearance or poor control of their dermatitis. None of the patients interrupted their antiviral treatment because of skin toxicity. The viral load remained negative in all cases except one (patient 8), but that patient had already failed to respond prior to consultation in dermatology, and treatment was therefore discontinued. That patient did not require treatment with oral corticosteroids for the resolution of his dermatitis.
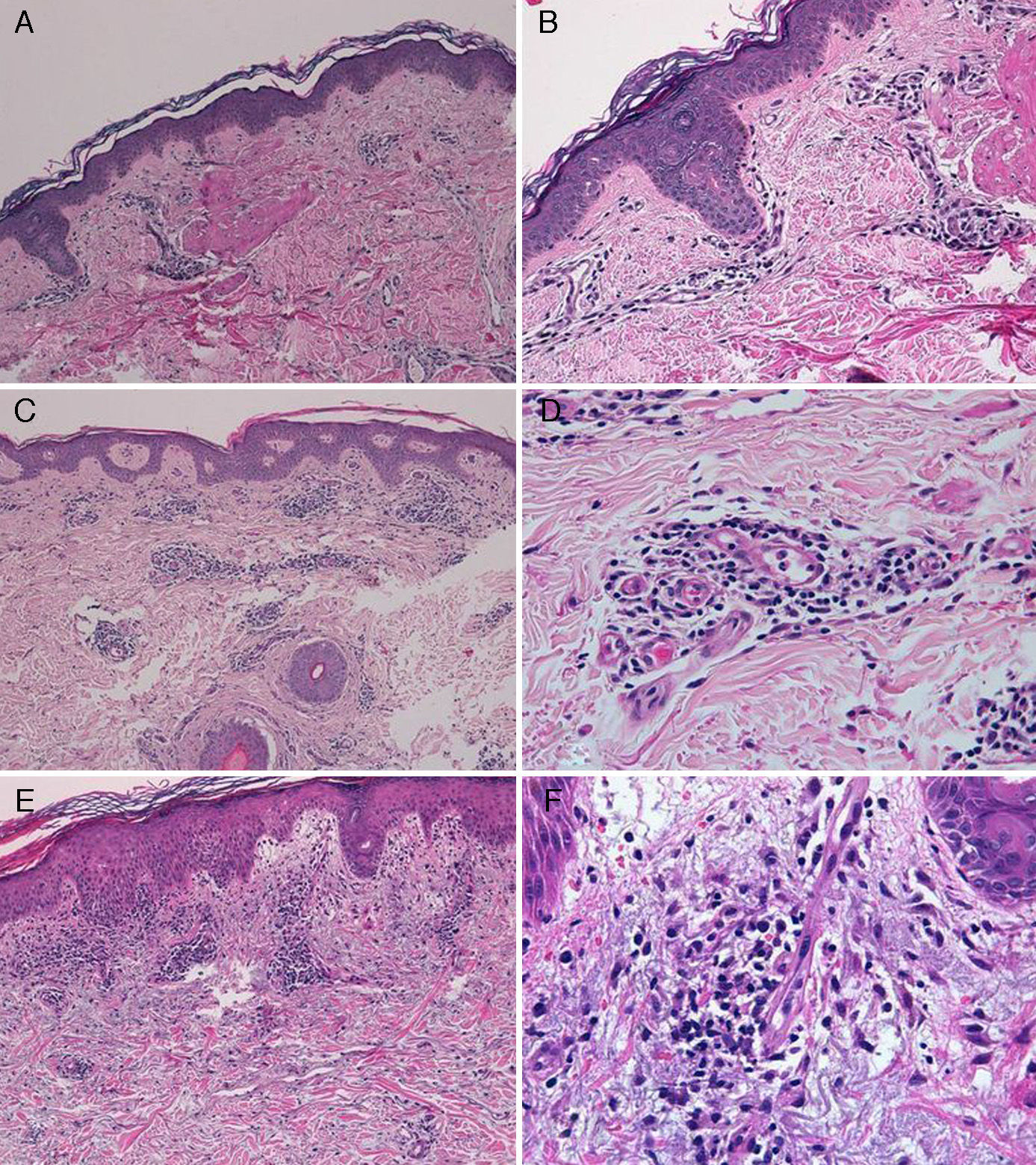
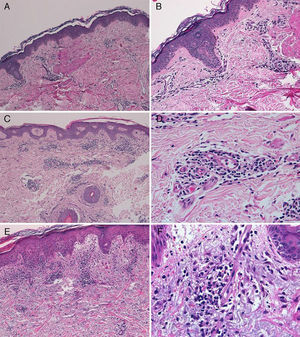
The diagnosis of telaprevir-induced dermatitis was based on the clinical manifestations and the temporal relationship with the introduction of the drug. Additional tests (biopsy, Fig. 3) were only performed in 3 patients (cases 1, 2, 9). Two patients (1 and 9) presented an eczematous rash and one (case2) presented a macular rash. In all 3 cases the biopsy revealed a superficial perivascular lymphohistiocytic dermatitis with minimal red-cell extravasation. The epidermis was affected in only 1 case (patient9), in which marked spongiosis and lymphocyte exocytosis were observed. There was no evidence of vasculitis or keratinocyte necrosis in any of the samples, and eosinophils were scarce or absent.
A, Case 1. Lymphocytic perivascular dermatitis with no epidermal changes. Hematoxylin and eosin (H&E), original magnification ×10. B, Case 1. Detail of the mild perivascular and periadnexal lymphocytic inflammatory infiltrate. H&E, original magnification ×40. C, Case 2. Superficial perivascular dermatitis with no epidermal involvement. H&E, original magnification ×10. D, Case 3. Detail showing the lymphocytic perivascular infiltrate with the presence of a few plasma cells. H&E, original magnification ×40. E, Case 4. Superficial perivascular dermatitis with edema of the papillary dermis, spongiosis, and foci of lymphocyte exocytosis. H&E, original magnification ×20. F, Case 4. Detail showing the lymphocytic perivascular infiltrate with minimal red-cell extravasation and no vasculitis. H&E, original magnification ×40.
The addition of PIs to the treatment of HCV infection considerably increases therapeutic efficacy, but also toxicity, and the Spanish Ministry of Health and Consumer Affairs has established restrictive criteria for their use.5 In clinical trials with telaprevir, the incidence of rash and photosensitivity was found to be over 50% and was dose dependent. The diagnosis is eminently clinical, and we have found no reports in the literature of the use of additional tests. Roujeau et al.6 highlighted the differences with typical cutaneous drug reactions and stated that PI-related reactions affect a large number of patients, appear later (from days to weeks after the introduction of the drug), and resolve more slowly. Those authors reported resolution in a mean of 44 days, but with a range up to 504 days. In our series, some patients were followed up for 12 months and have occasionally required further courses of oral corticosteroids. We consider that persistence of the dermatitis, or even the appearance of new outbreaks, could be related to the treatment with IFN or ribavirin, which continue to be administered after completing the course of telaprevir. In support of this, 2 of our patients (11 and 12) first consulted after finishing the course of telaprevir, and although their lesions started during treatment with telaprevir, the consultation was mainly for persistence of the lesions, attributable in part to the use of IFN and ribavirin.
The majority of patients presented a localized eczematous rash that could be controlled with emollient creams, topical corticosteroids, and antihistamines.6,7 López-Villaescusa et al.,8 on the other hand, described the appearance of annular urticariform lesions in a 58-year-old woman 4 weeks after starting the triple antiviral therapy. We observed 2 forms of presentation. One was a rash with an eczematous appearance, and the other was formed of confluent, violaceous macular lesions that were purpuric on the lower limbs, similar to the rash described by López-Villaescusa et al. The histologic changes were similar in both presentations: a superficial perivascular lymphohistiocytic dermatitis with a few foci of red-cell extravasation, no keratinocyte necrosis, and few or no eosinophils in the infiltrate. In 1 patient we observed spongiosis, lymphocyte exocytosis, and marked edema of the papillary dermis, which are findings consistent with eczema.
A considerable number of serious telaprevir-related cutaneous reactions have been described, including Stevens-Johnson syndrome, toxic epidermal necrolysis, and delayed drug hypersensitivity syndrome (DRESS),6 and there is also a case report of pityriasis rubra pilaris related to this treatment.9 We did not detect any serious drug reactions with telaprevir or with the traditional double therapy.
Comparing the characteristics of our patients with all patients who have been treated with PIs in our hospital (Table 2), it is interesting that no patients with HBV or HIV coinfection have been referred, despite accounting for 20% of the patients treated with PIs. Perhaps the immunosuppression of these patients leads to a lower incidence of cutaneous reactions. In comparison with all patients treated with PIs in this hospital, we saw a higher proportion of patients who had not previously received treatment for hepatitis C (50% vs 28%); however, we have found no data in the literature that compare the incidence of skin toxicity between previously treated and untreated patients.
Clinical and Epidemiological Characteristics of the Patients Seen in Dermatology and in All the Hospital Departments.
| Patient Seen in Dermatology | Overall in HGUV | |
| Age, y | 57.4 | 52.79 |
| Sex | 9M (75%), 3F | 77M (76%), 24F |
| Department | 10LU (83%), 2IDU | 59LU (58%), 42IDU |
| No previous treatment | 6 (50%) | 28 (28%) |
| Pretreated | 6 (50%) | 73 (72%) |
| Coinfection | 0 (0%) | 20 (20%) |
Abbreviations: F, female; HGUV, Hospital General Universitario de Valencia; IDU, infectious diseases unit; LU, liver unit; M, male.
Although the use of oral corticosteroids is not formally contraindicated in the telaprevir summary of product characteristics, their use was not permitted in patients in clinical trials due to their pharmacological interaction with the drug. The concentration and toxicity of both methylprednisolone and prednisone can be increased by the concomitant use of telaprevir, and dexamethasone is able to induce metabolism of the PI and reduce its efficacy.10 In our patients, given the extensive involvement, which exceeded 50% of the BSA in more than half of cases, and the fear of lesion progression or that the patient may stop taking therapy, both we and the hepatologists opted to continue antiviral treatment and add oral corticosteroids, with close follow-up of the clinical course. At the end of the study period, no patients had abandoned the treatment because of cutaneous toxicity, and the viral response remained negative in all patients who received oral corticosteroids.
In conclusion, the high prevalence of HCV seropositivity in our setting, the good results of treatment with the new antiviral agents, and the high incidence of associated cutaneous side effects make it likely that telaprevir-related dermatitis will be an ever more common cause for dermatologic consultation. It is therefore essential to know the presentation and management of this condition, whether in the form of mild rashes, or in the case of potentially severe cutaneous reactions. In this series we have described the use of oral prednisone to treat extensive, highly symptomatic rashes that had not responded to topical therapy and oral antihistamines, and we believe that this has contributed to compliance with the established triple antiviral therapy. Although, according to the literature, prednisone theoretically does not reduce the efficacy of telaprevir, it must be taken into account that its interaction with the PI can increase toxicity.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Garcias-Ladaria J, Pérez-Ferriols A, Ortega-García P, Diago M. Dermatitis por telaprevir: manejo con corticoides orales en casos refractarios. Actas Dermosifiliogr. 2014;105:e55–e60.