We now have considerable experience in the use of biologic agents to treat psoriasis, but doubts about management arise in certain clinical settings. Surgery is one of them. Although treatment guidelines advise that biologics be suspended before major surgery, data about actual clinical practices and associated complications are lacking. We aimed to analyze current practice in the clinical management of these cases.
MethodsRetrospective study of cases in the Biobadaderm database. We analyzed the management of biologic therapy in patients with psoriasis who underwent surgical procedures.
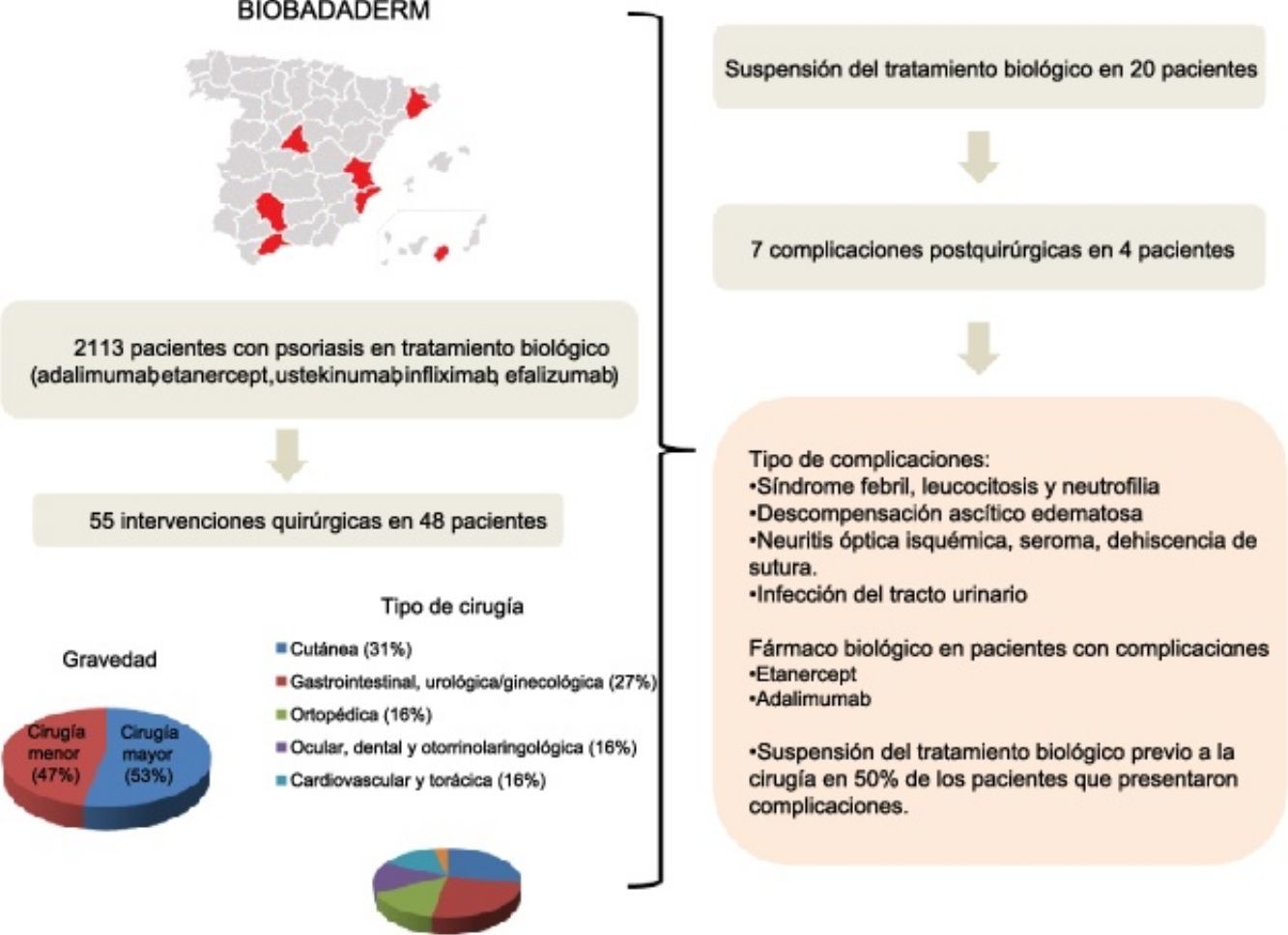
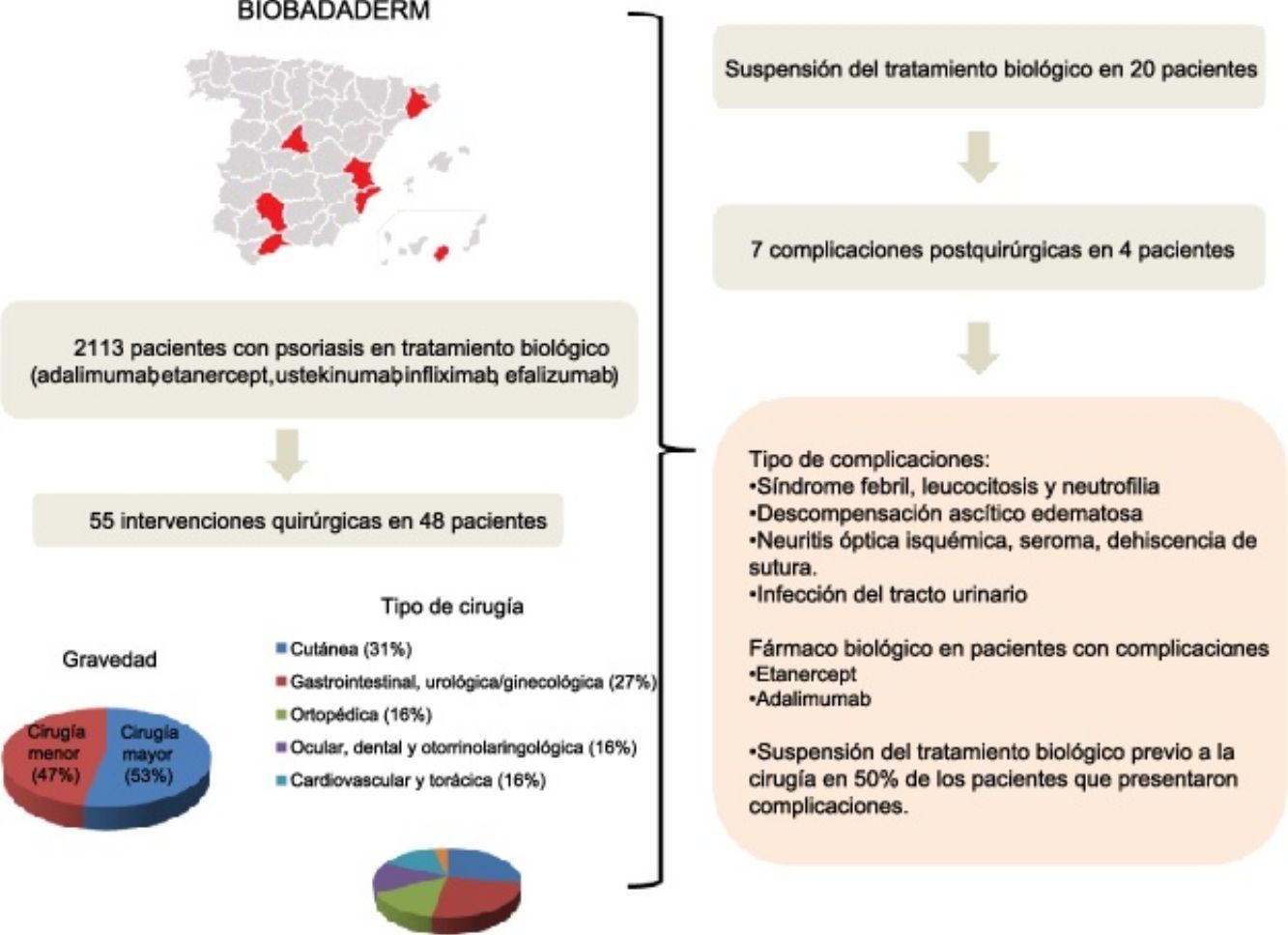
ResultsForty-eight of the 2113 patients registered in Biobadaderm underwent surgery. The largest percentage of procedures (31%) involved skin lesions. Biologic treatment was interrupted in 42% of the cases. No postsurgical complications were significantly related to treatment interruption. Likewise we detected no associations between treatment interruption and other variables, such as sex, age, or duration or severity of psoriasis.
ConclusionContinuity of biologic treatment and the risk of postsurgical complications were not associated in this study, although conclusions are limited by the small sample size.
Disponemos de una gran experiencia en el uso de los fármacos biológicos para el tratamiento de los pacientes con psoriasis, sin embargo, existen situaciones concretas, como la cirugía, en las que pueden surgir dudas sobre su manejo. Aunque las guías de tratamiento aconsejan su suspensión programada previamente a los procedimientos de cirugía mayor, no existe evidencia de cuál es la actitud habitual en la práctica clínica y su asociación a complicaciones. Nuestro objetivo fue analizar el manejo actual de esta situación en la práctica clínica habitual.
MétodosA través de un estudio retrospectivo de la base de datos Biobadaderm se analizó el manejo práctico de pacientes con psoriasis en tratamiento biológico que fueron intervenidos mediante algún procedimiento quirúrgico.
ResultadosDe los 2.113 pacientes incluidos en Biobadaderm, 48 fueron tratados con una intervención quirúrgica, de las que fueron mayoritarias las de tipo cutáneo (31%). El tratamiento biológico se suspendió en el 42% de los casos. No se observaron asociaciones estadísticamente significativas entre la aparición de complicaciones posquirúrgicas y la interrupción del fármaco. Tampoco se detectó asociación entre la interrupción del tratamiento con otras variables como el sexo, la edad, la duración de la enfermedad y la gravedad de la psoriasis.
ConclusiónNo se ha encontrado asociación entre la continuidad del tratamiento biológico y el riesgo de complicaciones posquirúrgicas, aunque el estudio presenta la limitación de tener un tamaño muestral escaso
Biologic agents approved for use in patients with moderate to severe psoriasis have been considered safe and well tolerated to date, with the exception of efalizumab, which has been withdrawn from the market.1
The arsenal of psoriasis treatment options has grown with the approval of new biologics and evidence of their efficacy and long-term safety. New evidence-based reviews to guide clinical decisions are therefore increasingly important.
We now have ample experience of using biologics to treat patients with psoriasis in most circumstances. An exception is their use during the period before and after surgery. The literature on this clinical context is limited and there is no evidence that allows us to draw unequivocal conclusions that can guide decisions.
In this study we analyzed practices in managing biologic therapy in surgical patients based on information in the Biobadaderm registry, which includes all psoriasis patients treated with biologic agents in Spain. We considered 2 types of biologic: inhibitors of tumor necrosis factor (anti-TNF agents, namely infliximab, adalimumab, and etanercept), and an inhibitor of interleukin 12/23, ustekinumab.
Our aim was to describe the perioperative management of biologic therapy in operated patients registered in the Biobadaderm database and to analyze surgical complications in relation to type of biologic, withdrawal of the drug or not, type of surgery, and other clinical and demographic variables.
MethodsBiobadaderm is the Spanish registry for adverse events that occur during systemic treatments for psoriasis. This system for collecting information prospectively about treatments and their adverse effects is a pharmacovigilance strategy. Twelve hospitals in several of the Spanish autonomous communities contribute data, which are uploaded to a web platform in real time as patients’ regimens are changed or an adverse event occurs. Information is checked continuously online by an auditor. Each center is also audited yearly to monitor input quality by checking the online data against patient charts. The Biobadaderm registry has been described previously.2
Between the registry's launch in October 2008 and data collection in November 2014, it received entries for 2113 patients. A total of 4450 treatment cycles and 4593 adverse events were on record. We reviewed a subset of patients on biologic therapy (adalimumab, etanercept, ustekinumab, infliximab, or efalizumab) who underwent surgery.
We extracted records of demographic, clinical, and treatment data—including length of time the biologic treatment was interrupted before surgery and when it was restarted. In addition we retrospectively collected information from patients’ charts regarding type of surgery and procedure used; whether the intervention was minor, major, and/or an emergency; and the type of anesthesia used. We also collected postoperative information, mainly about wound infections, delayed healing, and other possible complications.
Data for continuous variables were described with the mean (SD) if they were normally distributed and with the median and interquartile range (IQR) if nonnormally distributed. Qualitative variables were described with absolute frequencies and percentages. We used the χ2 test to explore factors influencing the decision to interrupt treatment or not.
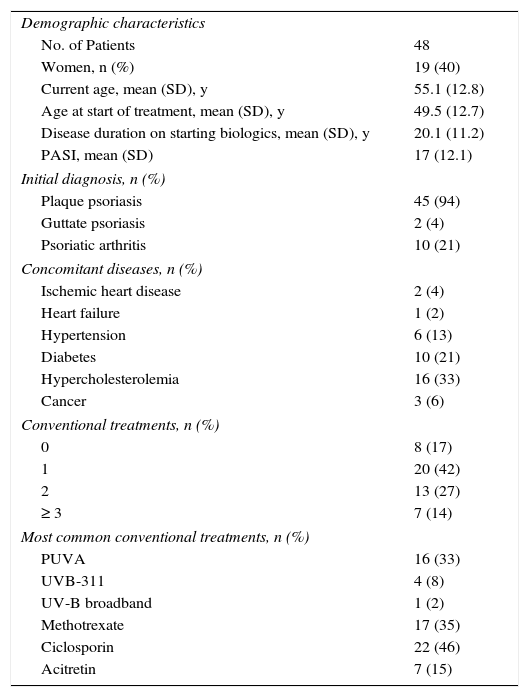
ResultsOf the total of 2113 cases in the Biobadaderm registry, 48 patients were on biologic therapy when surgery was scheduled or performed. Twenty-nine of the 48 (60%) were men and 19 (40%) were women. The mean (SD) age was 55 years. The mean time from onset of disease to start of biologic treatment was 20 (11) years (Table 1). Ninety-four percent had plaque psoriasis and 21% also had psoriatic arthritis. The mean Psoriasis Area and Severity Index (PASI) was 17 (12) at the start of biologic treatment (Table 1).
Demographic and Clinical Characteristics of Patients Who Underwent Surgery.
| Demographic characteristics | |
| No. of Patients | 48 |
| Women, n (%) | 19 (40) |
| Current age, mean (SD), y | 55.1 (12.8) |
| Age at start of treatment, mean (SD), y | 49.5 (12.7) |
| Disease duration on starting biologics, mean (SD), y | 20.1 (11.2) |
| PASI, mean (SD) | 17 (12.1) |
| Initial diagnosis, n (%) | |
| Plaque psoriasis | 45 (94) |
| Guttate psoriasis | 2 (4) |
| Psoriatic arthritis | 10 (21) |
| Concomitant diseases, n (%) | |
| Ischemic heart disease | 2 (4) |
| Heart failure | 1 (2) |
| Hypertension | 6 (13) |
| Diabetes | 10 (21) |
| Hypercholesterolemia | 16 (33) |
| Cancer | 3 (6) |
| Conventional treatments, n (%) | |
| 0 | 8 (17) |
| 1 | 20 (42) |
| 2 | 13 (27) |
| ≥ 3 | 7 (14) |
| Most common conventional treatments, n (%) | |
| PUVA | 16 (33) |
| UVB-311 | 4 (8) |
| UV-B broadband | 1 (2) |
| Methotrexate | 17 (35) |
| Ciclosporin | 22 (46) |
| Acitretin | 7 (15) |
Abbreviations: PASI, psoriasis area and severity index; PUVA, psoralen plus UV-A.
The agents prescribed for these patients before the surgical procedure in order of frequency were adalimumab (24 patients), etanercept (14), ustekinumab (4), and infliximab (3). Treatment was withdrawn before surgery in 20 patients (42%). The median (IQR) duration of treatment interruption was 3 weeks (IQR, 2–14.3 weeks). One patient who underwent oncologic surgery did not restart treatment. The median time until restart of treatment for the remaining patients was 2 weeks (IQR, 1–4.9 weeks).
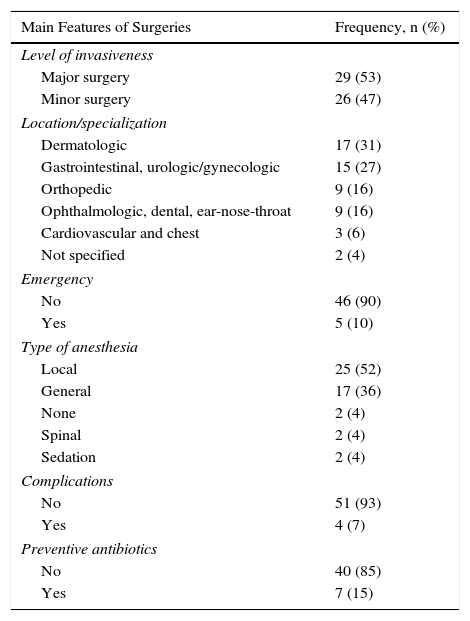
A total of 55 surgeries were performed in 48 patients; 29 (53%) of the procedures were major surgery. By organs and systems, 17 (31%) were dermatologic and 15 (27%) were gastrointestinal, urologic, or gynecologic (Table 2). Biologic treatment was interrupted most often (in 8 out of 24 [33%]) in gastrointestinal, urologic, and gynecologic surgery patients. No cases of wound infection or delayed healing were reported for patients whose treatment continued.
Characteristics of Surgeries.
| Main Features of Surgeries | Frequency, n (%) |
|---|---|
| Level of invasiveness | |
| Major surgery | 29 (53) |
| Minor surgery | 26 (47) |
| Location/specialization | |
| Dermatologic | 17 (31) |
| Gastrointestinal, urologic/gynecologic | 15 (27) |
| Orthopedic | 9 (16) |
| Ophthalmologic, dental, ear-nose-throat | 9 (16) |
| Cardiovascular and chest | 3 (6) |
| Not specified | 2 (4) |
| Emergency | |
| No | 46 (90) |
| Yes | 5 (10) |
| Type of anesthesia | |
| Local | 25 (52) |
| General | 17 (36) |
| None | 2 (4) |
| Spinal | 2 (4) |
| Sedation | 2 (4) |
| Complications | |
| No | 51 (93) |
| Yes | 4 (7) |
| Preventive antibiotics | |
| No | 40 (85) |
| Yes | 7 (15) |
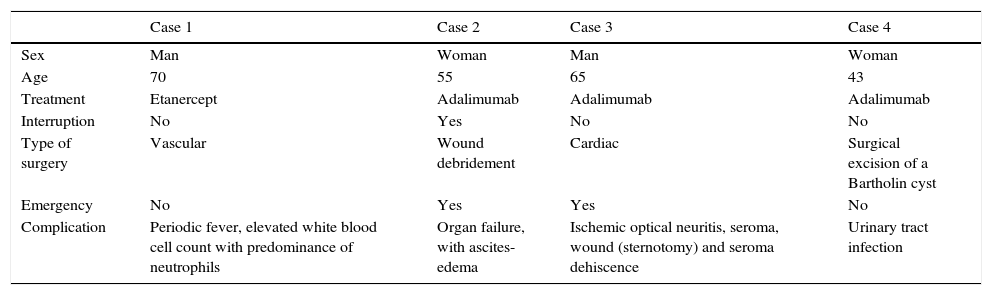
No statistically significant association was detected between the development of complications and treatment interruption (P=.59, χ2 test). Nor did we detect associations between treatment interruption and sex (P=.58), age (P=.53), disease duration (P=.06), or PASI (P=.16) (Table 3).
Complications.
| Case 1 | Case 2 | Case 3 | Case 4 | |
|---|---|---|---|---|
| Sex | Man | Woman | Man | Woman |
| Age | 70 | 55 | 65 | 43 |
| Treatment | Etanercept | Adalimumab | Adalimumab | Adalimumab |
| Interruption | No | Yes | No | No |
| Type of surgery | Vascular | Wound debridement | Cardiac | Surgical excision of a Bartholin cyst |
| Emergency | No | Yes | Yes | No |
| Complication | Periodic fever, elevated white blood cell count with predominance of neutrophils | Organ failure, with ascites-edema | Ischemic optical neuritis, seroma, wound (sternotomy) and seroma dehiscence | Urinary tract infection |
Patients on biologic therapy for psoriasis who are scheduled for surgery are seen increasingly often in routine practice. It is important to be aware of the possibility that patients on long-term therapies—such as biologics for psoriasis—will undergo scheduled or emergency surgery at some point, triggering doubts about whether to interrupt treatment. If treatment interruption is deemed necessary, we may further ask ourselves how long patients should remain off treatment before restarting.
Whether or not biologics alter the normal immune response necessary for proper recovery after surgery is being debated.3,4
Few studies have looked at postoperative complications in patients on biologics.
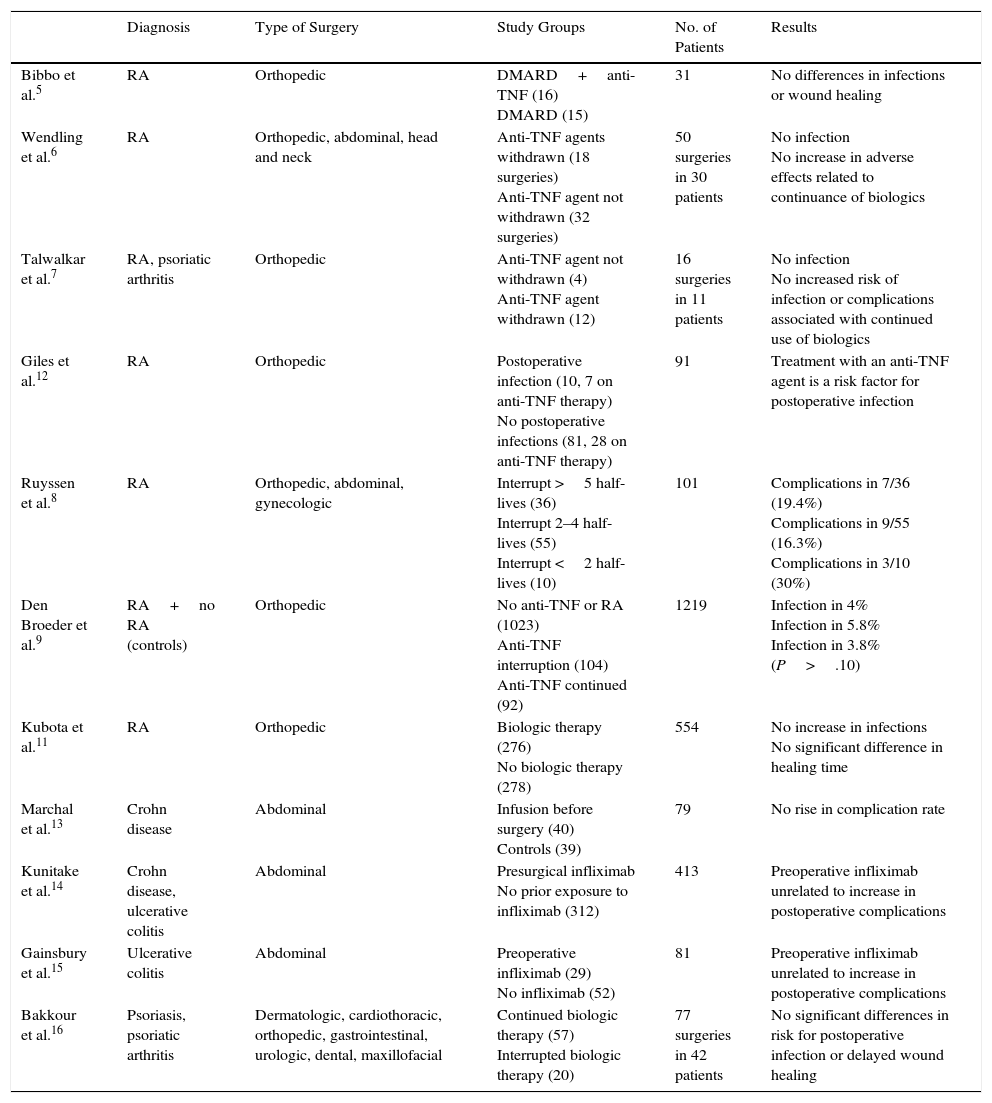
Table 4 shows the results of a literature search for relevant studies. Most included patients prescribed biologics for rheumatic diseases who were undergoing various types of surgery. Six studies found no significant differences in the rates of complications between patients whose biologic treatment was interrupted and those who continued5–11; the statistical power in these studies, however, was low. Only one study found that continuing an anti-TNF regimen conferred risk for infection after orthopedic surgery.12
Observational Studies of Surgical and Postoperative Complications in Patients on Biologic Therapy.
| Diagnosis | Type of Surgery | Study Groups | No. of Patients | Results | |
|---|---|---|---|---|---|
| Bibbo et al.5 | RA | Orthopedic | DMARD+anti-TNF (16) DMARD (15) | 31 | No differences in infections or wound healing |
| Wendling et al.6 | RA | Orthopedic, abdominal, head and neck | Anti-TNF agents withdrawn (18 surgeries) Anti-TNF agent not withdrawn (32 surgeries) | 50 surgeries in 30 patients | No infection No increase in adverse effects related to continuance of biologics |
| Talwalkar et al.7 | RA, psoriatic arthritis | Orthopedic | Anti-TNF agent not withdrawn (4) Anti-TNF agent withdrawn (12) | 16 surgeries in 11 patients | No infection No increased risk of infection or complications associated with continued use of biologics |
| Giles et al.12 | RA | Orthopedic | Postoperative infection (10, 7 on anti-TNF therapy) No postoperative infections (81, 28 on anti-TNF therapy) | 91 | Treatment with an anti-TNF agent is a risk factor for postoperative infection |
| Ruyssen et al.8 | RA | Orthopedic, abdominal, gynecologic | Interrupt >5 half-lives (36) Interrupt 2–4 half-lives (55) Interrupt <2 half-lives (10) | 101 | Complications in 7/36 (19.4%) Complications in 9/55 (16.3%) Complications in 3/10 (30%) |
| Den Broeder et al.9 | RA+no RA (controls) | Orthopedic | No anti-TNF or RA (1023) Anti-TNF interruption (104) Anti-TNF continued (92) | 1219 | Infection in 4% Infection in 5.8% Infection in 3.8% (P>.10) |
| Kubota et al.11 | RA | Orthopedic | Biologic therapy (276) No biologic therapy (278) | 554 | No increase in infections No significant difference in healing time |
| Marchal et al.13 | Crohn disease | Abdominal | Infusion before surgery (40) Controls (39) | 79 | No rise in complication rate |
| Kunitake et al.14 | Crohn disease, ulcerative colitis | Abdominal | Presurgical infliximab No prior exposure to infliximab (312) | 413 | Preoperative infliximab unrelated to increase in postoperative complications |
| Gainsbury et al.15 | Ulcerative colitis | Abdominal | Preoperative infliximab (29) No infliximab (52) | 81 | Preoperative infliximab unrelated to increase in postoperative complications |
| Bakkour et al.16 | Psoriasis, psoriatic arthritis | Dermatologic, cardiothoracic, orthopedic, gastrointestinal, urologic, dental, maxillofacial | Continued biologic therapy (57) Interrupted biologic therapy (20) | 77 surgeries in 42 patients | No significant differences in risk for postoperative infection or delayed wound healing |
Abbreviations: DMARD, disease-modifying antirheumatic drug; RA, rheumatoid arthritis.
Patients with inflammatory bowel disease who required abdominal surgery while on biologics did not have more postoperative complications than those whose biologic regimens were interrupted.13–15
The first study of patients with psoriasis or psoriatic arthritis on biologics enrolled 42 patients undergoing 77 different procedures.16 While patients who continued biologic therapy did not have higher rates of postoperative complications, those who did interrupt treatment found their underlying disease worsened. Most rheumatology associations recommend interrupting biologic therapy for at least 4 half-lives before scheduled major surgery; that criterion would involve going 4 to 6 weeks without infliximab, 6 to 8 weeks off adalimumab, 2 to 3 weeks off etanercept, and 12 to 25 weeks off ustekinumab before operations.17–19 The evidence supporting that recommendation, however, is weak; in fact, it is supported only by expert opinion.
The 2013 consensus statement of the Spanish Academy of Dermatology and Venereology (AEDV) proposed continuous treatment for psoriasis in general, but the experts did single out the case of surgical patients, who were thought to be candidates for treatment interruption.20
At present there are no guidelines or clear directives on the management of biologic therapy in surgical patients with psoriasis. Therefore, the decision to interrupt therapy or not must be an individual one that takes into consideration the following factors: type of surgery (clean procedures with low risk of infection vs surgery with risk of sepsis); type of patient (with a history of infection, a joint prosthesis, or on oral corticosteroids); and finally, the severity of psoriasis and the patient's response to treatment.
It is important to differentiate types of surgery. Major surgery is more complex and involves greater risk of perioperative complications than minor surgery. The duration of treatment interruption varies, but the reintroduction of biologics is recommended once the wound has closed if no signs of infection are present.21–23
In conclusion, we found no evidence of higher complication rates in patients who continue taking biologics when they undergo surgery, although the studies we found in the literature have low statistical power. Therefore, lacking guidelines at this time, the final decision on whether to interrupt biologic therapy before surgery or not should be taken after due assessment of the risks and benefits for the individual.
Ethical DisclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this investigation.
Data confidentialityThe authors declare that they followed their hospitals’ regulations regarding the publication of patient information and that written informed consent for voluntary participation was obtained for all patients.
Right to privacy and informed consentThe authors declare that no private patient data are disclosed in this article.
FundingThe Biobadaderm registry is a project of the Spanish Academy of Dermatology and Venereology (AEDV). It receives support from the Spanish Agency for Medicines and Health Care Products as well as pharmaceutical laboratories (Abbott/Abbvie, Pfizer, MSD, and Janssen). The collaborating laboratories contributed funds in similar proportions and did not participate in the analysis of data or the interpretation of results.
Authorship and CollaborationThis study was carried out within the Biobadaderm Group. The following members participated in collecting data and reviewing the manuscript: Cristina Carazo, José Bañuls, Juan Francisco Silvestre, Pilar Alvares, Isabel Betlloch, Montserrat Hernández, Patricia Guillem, Esther Margarit, Carlos Muñoz Santos, Sara Pedregosa, Lara Ferrandiz, and Ignacio García Doval.
Conflicts of InterestG. Carretero has consulted or carried out research for Abbott, Janssen-Cilag, MSD, and Pfizer. He has received fees from Abbott, Janssen, and Pfizer and equipment from MSD and Pfizer.
C. Ferrandiz has consulted for Abbott, Janssen-Cilag, and Almirall; received fees from Abbott, Almirall, Janssen-Cilag, and Pfizer; and spoken on behalf of Almirall and Janssen-Cilag.
F. Vanaclocha has given presentations sponsored by Abbott, Pfizer, MSD, and Janssen.
E. Daudén sits on the advisory board of and has consulted for, received grants and research support from, participated in clinical trials sponsored by, or received speaker fees from the following pharmaceutical companies: Abbvie/Abbott, Amgen, Janssen-Cilag, Leo Pharma, Novartis, Pfizer, MSD-Schering-Plough, Celgene, and Lilly.
E. Herrera Ceballos has served as a consutant or speaker for Abbvie, Janssen-Cilag, and Pfizer-Wyett.
I. Belinchón Romero has been a consultant for Pfizer-Wyeth, Janssen-Cilag, Almirall, and Leo Pharma and has spoken on behalf of Abbvie, Pfizer-Wyeth, Janssen-Cilag, and MSD.
J. L. Sánchez Carazo has consulted for Abbott, Janssen-Cilag, MSD, and Pfizer-Wyeth.
J. L. López Estebaranz has been a consultant for Abbott, Janssen-Cilag, MSD, and Pfizer and has spoken on behalf of Abbott, Janssen-Cilag, MSD, and Pfizer-Wyeth.
M. Alsina has been a consultant for Abbvie and Merck/Schering-Plough.
M. Ferrán has sat on advisory boards for MSD, Pfizer, Abbvie, and Janssen-Cilag; spoken on behalf of MSD, Abbvie, and Janssen-Cilag; and done research for MSD, Abbvie, Pfizer, and Janssen-Cilag.
J. M. Carrascosa has consulted for and spoken on behalf of Abbvie, Janssen-Cilag, MSD, Pfizer-Wyeth, Lilly, Novartis, and Celgene.
R. Rivera has sat on advisory boards for Abbvie, Janssen-Cilag, MSD, and Pfizer/Wyeth.
D. Ruiz Genao has spoken on behalf of Abbott, Pfizer, MSD, and Janssen.
P. de la Cueva has consulted for Janssen-Cilag, Abbvie, MSD, Pfizer, Novartis, Lilly, and Leo-Pharma.
The other authors declare that they have no conflicts of interest.
Please cite this article as: Galiano Mejías S, Carretero G, Ferrandiz C, Vanaclocha F, Daudén E, Gómez-García FJ, et al. Manejo de los tratamientos biológicos en pacientes con psoriasis moderada-grave sometidos a intervenciones quirúrgicas en el registro español Biobadaderm. Actas Dermosifiliogr. 2017;108:52–58.