Malassezia (Pityrosporum) species are lipophilic yeasts that are recognized as commensals of the skin of warm-blooded vertebrates.1 Aryl-hydrocarbon receptor (AhR) ligands produced by Malassezia species have been implicated in carcinogenesis, suggesting that this yeast may contribute to the development of skin cancer, particularly basal cell carcinoma (BCC).2 This hypothesis suggests that skin cancer treatments that combine antitumoral and antimicrobial effects may be particularly effective.
We investigated the effect of routine methylaminolevulinate photodynamic therapy (MAL-PDT) on Malassezia species present in the flora of peritumoral skin. The participants were patients with non-melanoma skin cancer who were treated with MAL-PDT (Metvix® in combination with Aktilite® red light illumination, 37J/cm2) in the Dermatology Department of the Hospital General San Jorge (Huesca, Spain) between September and November 2012.
Swab samples were taken from a 2-cm2 area of skin within 1cm of the lesion before and after MAL-PDT. Control swab samples were taken from an adjacent area of healthy skin before and after exposure to red light illumination only. The skin was not prepared before swabbing. Swabs were then used to immediately inoculate the entire surface of individual Dixon plates, which were incubated at 35°C.
Malassezia species were identified by micromorphology (morphological microscopy), cultural characteristics (effect of temperature, subculture on Sabouraud agar, growth and appearance on Leeming and Notman agar, Dixon modified media, CHROMagar, and Tween agar), biochemical characteristics (catalase production, bile esculin test), and the assimilation profile when grown on Tween agar.3
All data are presented as the mean and standard deviation (SD). Differences between groups were evaluated using the Mann–Whitney U test, Kruskal–Wallis test, and Wilcoxon matched-pairs signed ranked test, assuming a confidence level of 95% (i.e., P<.05). Analyses were performed using IBM SPSS software (Version 19.0). The study was approved by the regional ethics committee for clinical research.
Thirty patients (19 men and 11 women) participated in the study. Of these, 19 (63.3%) were treated for actinic keratosis, 10 (33.4%) for BCC, and 1 (3.3%) for Bowen disease. Lesions were most commonly located on the forehead (26.7%) and scalp (23.3%), followed by the nose (16.7%), back (10%), cheek (6.7%), neck (6.7%), chest (6.7%), and ear (3.3%).
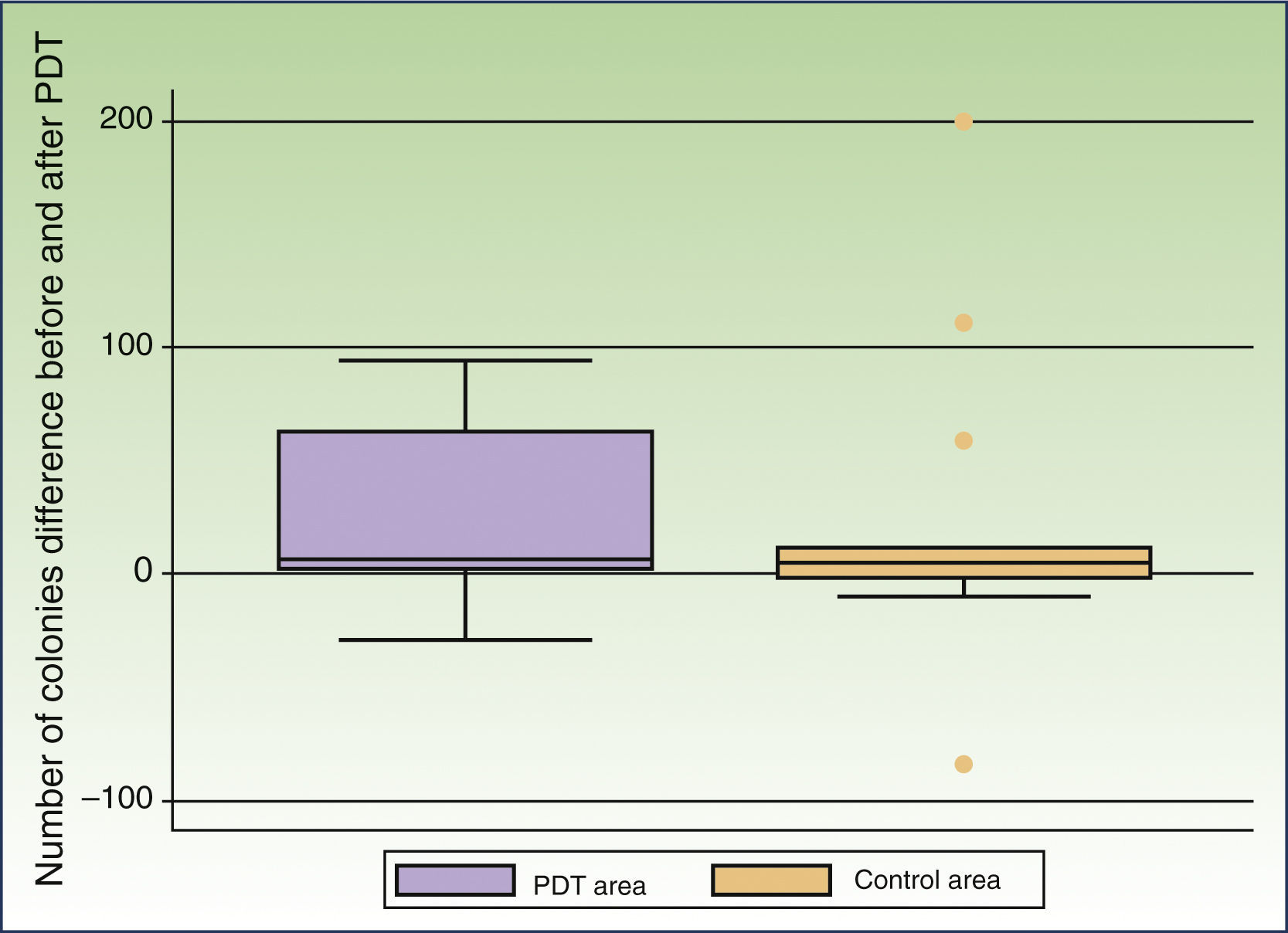
In 13 patients Malassezia species were detected in both the peritumoral and control areas before treatment. The most commonly found species was Malassezia globosa (10 patients), either alone or in combination with other species, followed by Malassezia sympodialis (3 patients). Treatment resulted in a decrease in the number of colonies in the peritumoral and control areas in 10 and 8 patients, respectively. The mean number of colonies observed before treatment was 46.00 (50.67) in the peritumoral area and 63.38 (119.44) in the control area. MAL-PDT resulted in a significant decrease in the number of colonies in the peritumoral area (20.46[33.94], P=.023); this effect was not observed in the control area after exposure to red light illumination (40.69[61.49], P=.22) (Fig. 1).
The decrease in the mean number of colonies induced by MAL-PDT was significantly greater in patients that were positive for both M. globosa and M. sympodialis (76[15.06]) than in those with M. globosa alone (12.36[20.54]) or with other Malassezia species (P=.007). We found no association between the mean number of Malassezia colonies and patient gender (P=.70), tumor type (P=.95), or tumor location (P=.21).
Our results demonstrate that routine MAL-PDT attenuates the growth of Malassezia species on peritumoral skin. This observation is in line with previous reports describing the effectiveness of MAL-PDT in pityriasis versicolor,4 recalcitrant Malassezia folliculitis,5 and onychomycosis.6 The inhibitory effect of MAL-PDT on M. globosa was greater than that observed for M. sympodialis, suggesting that sensitivity to MAL-PDT varies among Malassezia species. In line with this view, differential sensitivity to PDT in vitro has been described for other yeast species, such as Candida.7 In most patients, we observed a decrease in the number of Malassezia colonies in the control area after exposure to red light illumination, in agreement with previous reports describing the inhibitory effects of a red light-emitting diode on the growth of bacteria and yeast.8
There are 2 main limitations to our study. First, statistical power was limited by the small sample size, although this effect was somewhat offset by using each patient as their own control. Second, the potential fungicidal effect of MAL in the absence of illumination was not evaluated. While neither aminolevulinic acid nor MAL exert cytotoxic effects when administered in vivo without illumination, high concentrations of aminolevulinic acid can be toxic to certain cell types in vitro.9
Bryld and coworkers10 reported no significant changes in bacterial flora after MAL-PDT. MAL-PDT may be a particularly attractive treatment option if it could effectively eliminate Malassezia species without damaging cutaneous bacterial flora, especially if the proposed role of Malassezia species in carcinogenesis is confirmed.
In summary, our findings demonstrate that MAL-PDT exerts a significant antifungal effect in vivo against commensal Malassezia species in non-melanoma skin cancer.
We thank Dr. G. Gaitanis for constructive comments and critical review of the manuscript.
Please cite this article as: Gilaberte Y, Aspiroz C, Alejandre C, Rezusta A. Crecimiento de Malassezia en Piel Peritumoral Tras Terapia Fotodinámica con Metil-5-aminolevulinato para Queratosis Actínica y Cáncer de Piel No Melanoma. Actas Dermosifiliogr. 2015;106:70–71.