Lymphogranuloma venereum (LGV) is an emerging disease in men who have sex with men (MSM): the incidence was 1.15 cases per 100 000 population in Spain in 2017. Patients with LGV characteristically have severe proctitis that can cause abscesses, fistulas, and anal stenosis. Genital ulcers and inflammatory inguinal adenopathy may occasionally be present. The aim of this study was to describe a series of patients with LGV treated in a public health service hospital in Andalusia, Spain.
Materials and methodsRetrospective, observational description of a series of patients diagnosed with LGV. We gathered epidemiologic, clinical, microbiologic, and treatment data. Patients’ sexual behaviors were also noted.
ResultsWe found 17 cases of LGV diagnosed in MSM between October 2016 and May 2019. Twelve of the patients were also infected with the human immunodeficiency virus, and 13 had severe proctitis with ulcers in the anal canal and rectum. Four patients had genital or inguinal manifestations. The following high-risk sexual behaviors were on record: a high number of sexual partners, receptive anal sex with strangers and without a condom, seeking sexual partners online, participation in group sex, and sex with partners from outside Andalusia. Chlamydia trachomatis serovar L2 was identified in all cases, and the infection responded well to oral doxycycline. Two patients with the most characteristic form of LGV required longer treatment cycles. Three required surgery.
ConclusionsWhen symptomatic proctitis is found in MSM who engage in high-risk sex, the LGV exudate should be sampled and the C trachomatis serovar identified. Genital ulcers or inguinal buboes are also highly suggestive of LGV. Clinical suspicion and early treatment are the keys to preventing complications and disease transmission.
El linfogranuloma venéreo (LGV) es una enfermedad emergente en hombres que tienen sexo con hombres (HsH), con una incidencia en España de 1,15 casos por 100 mil en el año 2017. Suele cursar como una proctitis severa que puede ocasionar abscesos, fístulas y estenosis anal. Raramente se manifiesta con úlceras genitales o adenopatías inflamatorias inguinales. El objetivo de este trabajo fue una serie en un hospital público de Andalucía.
Material y métodosEstudio observacional descriptivo de una cohorte retrospectiva de los casos diagnosticados de LGV. Se recogieron variables epidemiológicas, clínicas, microbiológicas, de tratamiento, así como los hábitos sexuales de los pacientes.
ResultadosSe seleccionaron los 17 casos diagnosticados entre octubre de 2016 y mayo de 2019 en HsH, 12 de ellos positivos para el VIH. Trece pacientes sufrieron una proctitis muy sintomática con ulceraciones en el canal anal y el recto. En 4 pacientes el LGV se manifestó en la forma genital o inguinal. Se identificaron prácticas sexuales de alto riesgo: un número elevado de parejas y sexo anal receptivo anónimo sin protección, búsqueda de sexo por internet y sexo en grupo o fuera de nuestra comunidad. LaChlamydia trachomatis L2 se identificó en todos los casos, con una buena respuesta a la doxiciclina oral. Dos pacientes con la forma clásica necesitaron ciclos más prolongados de tratamiento y en 3 de ellos fue necesario tratamiento quirúrgico.
ConclusionesAnte una proctitis sintomática en HsH con prácticas sexuales de alto riesgo debemos tomar muestras de exudado para identificar los serovares de LGV. Las ulceras genitales y los bubones inguinales son también muy sugerentes de la infección. La sospecha de LGV y el tratamiento precoz es fundamental para prevenir las complicaciones y la transmisión de la enfermedad.
Chlamydia trachomatis is an intracellular bacterium that is frequently involved in exudative infections of the mucosa of the urethra and cervix throughout the world. It is the main cause of pelvic inflammatory disease in women in developed countries.1 This pathogen is usually isolated in the anal canal in men and women who engage in receptive anal intercourse, although in most cases the infection goes unnoticed.2 Some serovars have a predilection for the lymph nodes and are responsible for lymphogranuloma venereum (LGV), which is a sexually transmitted infection (STI) that was recorded mainly in Africa, Asia, and South America and was extremely rare in our setting until recently.3 At the time of the HIV epidemic (late 1990s), new cases of anorectal LGV were recorded in men who have sex with men (MSM) in Europe4 and the United States.5 Clinically, these cases manifested as highly symptomatic proctitis with rectal tenesmus and bleeding but without the enlarged inguinal lymph nodes seen in the classic form. LGV is currently one of the main causes of severe proctitis in HIV-infected patients in our setting and has been associated with high-risk sexual practices. Onset of the classic form is generally as a painless papule that rapidly ulcerates, followed after a few weeks by genital lymphadenitis and enlarged suppurative inguinal lymph nodes. In the third stage, lymphatic obstruction can cause elephantiasis, swollen lymph node fistula and fibrosis, and chronic secondary ulceration.3 This form of genital LGV is rarely diagnosed in Europe, although it is almost limited to MSM.
In the present study, we report cases of LGV diagnosed in an STI clinic. We recorded associated risk factors, clinical and epidemiological characteristics, response to treatment, and associated complications.
Material and methodsWe performed an observational retrospective cohort study of symptomatic patients diagnosed with LGV in the STI clinic of the dermatology department of a public institution, Hospital Costa del Sol, Marbella, Spain. We examined all cases recorded between October 2016 and May 2019. We recorded the patient’s personal details, as well as clinical, epidemiological, treatment, and microbiological data. The study population was fully screened for STIs according to risk practices, and serology test results were updated. Patients were questioned anonymously about specific aspects of their sexual behaviors.
Specific testing for Chlamydia was performed in patients with compatible symptoms suspected of having LGV. The microbiology workup consisted of a nucleic acid amplification test for C trachomatis (BD MAX CT/GC/TV) with samples of exudate, tissue, or both. The test has a sensitivity of 95.7% and a specificity of 99.2%. The results were subsequently confirmed in a reference laboratory using real-time polymerase chain reaction assay (PCR) that was specific for lymphogranuloma. Samples were extracted using an automated procedure (Maxwell Viral Total Nucleic Acid Purification Dit, Promega), followed by double PCR for the coding target of the outer membrane protein A gene (ompA). A genomic study was performed with sequencing of the 996-bp amplified region (ompA) using BigDye Terminator v3.1 (Life Technologies) and an ABI Prism 310 sequencer (Applied Biosystems). The technique has a sensitivity of 90% and a specificity of 100%, with a detection limit of 200 copies/mL.
Patients who underwent clinical imaging previously completed a specific informed consent document.
We performed a descriptive analysis using measures of position (median and interquartile range [IQR]) for quantitative variables and frequency distributions for qualitative variables. With the type of lymphogranuloma as the segmentation variable, the Fisher exact test was used to evaluate differences in distribution with respect to the qualitative variables, and the Mann-Whitney test was used for the quantitative variables. Statistical significance was set at P < .05.
ResultsA total of 17 MSM with a median age of 38 (16) years were diagnosed with LGV during the study period. Anorectal involvement was recorded in 13 cases, and the classic genital or inguinal form in 4. Patients were Spanish in 76.5% of cases, and 70.6% had completed their university education. In 88.2% of cases, patients had a history of other STIs, and 12 were HIV-infected. No other associated STIs were detected at diagnosis. Three patients with anorectal LGV had anal intraepithelial neoplasia associated with high-risk human papillomavirus, and 4 had a history of anogenital warts. Eight patients had a previous history of syphilis. All HIV-infected patients were in stage A and were receiving antiretroviral therapy, with a median CD4 count of 1014/mm3 (450.3/mm3). The viral load was undetectable in 10 cases at diagnosis (Table 1).
Personal Data of Patients With Lymphogranuloma Venereum: Clinical Characteristics, Diagnostic Aspects, and Treatment.
| Frequency | Percentage | |
|---|---|---|
| Age | ||
| Median (IQR): 38.0 (16) | ||
| Origin | ||
| Spanish | 13 | 76.5 |
| Non-Spanish | 4 | 23.5 |
| Educational level | ||
| Primary | 2 | 11.8 |
| Secondary | 3 | 17.6 |
| University | 12 | 70.6 |
| Previous STIs | ||
| Yes | 15 | 88.2 |
| No | 2 | 11.8 |
| Previous syphilis | ||
| Yes | 8 | 47.1 |
| No | 9 | 52.9 |
| Previous HPV | ||
| Yes | 7 | 41.2 |
| No | 10 | 58.8 |
| HIV | ||
| Yes | 12 | 70.6 |
| No | 5 | 29.4 |
| Time with HIV, y | ||
| Median (IQR), 10.0 (5.5) | ||
| HIV stage | ||
| A1 | 5 | 41.7 |
| A2 | 6 | 50.0 |
| A3 | 1 | 8.3 |
| Viral load, copies | ||
| Undetectable | 10 | 83.4 |
| 28 000 | 1 | 8.3 |
| 10 million | 1 | 8.3 |
| Nadir CD4 | ||
| Median (IQR), 318.5 (107.5) | ||
| Current CD4 | ||
| Median (IQR), 1014 (450.3) | ||
| Type of lymphogranuloma | ||
| Anorectal | 13 | 76.5 |
| Classic | 4 | 23.5 |
| Diagnostic delay | ||
| Median (IQR), 5.0 (3.5) | ||
| Sample | ||
| Exudate | 8 | 47.1 |
| Tissue | 6 | 35.3 |
| Both | 3 | 17.6 |
| Serovar | ||
| L2 | 16 | 94 |
| L2b | 1 | 6 |
| Serology | ||
| IgG | 13 | 76.5 |
| IgG/IgM | 1 | 5.9 |
| Not performed | 3 | 17.6 |
| Treatment | ||
| Doxycycline 3 wk | 15 | 88.2 |
| Doxycycline > 3 wk | 2 | 11.8 |
Abbreviations: HPV human papillomavirus; IQR, interquartile range; STI, sexually transmitted infection.
As for the microbiology workup, lymphogranuloma-positive samples during the study period accounted for 43% of all positive test results for C trachomatis in the anus (13/31). Gonorrhea was the second most common cause of proctitis (8 cases). Infections by the LGV serovar accounted for 16% of all C trachomatis infections at any site (17/106).
As for sexual behaviors, patients reported a lifetime total of 100 (200) sexual partners, with 6 (20.5) partners during the 6 months before onset of symptoms. Use of condoms was erratic in 17 patients (94.1%) during this period, and 14 (82.4%) had had unprotected receptive anal intercourse. Sexual relations were anonymous in 15 cases (88.2%), and in 16 cases (94.1%) the patients had had sex outside Andalusia or with men from other parts of Spain or abroad. Fifteen patients (88.2%) had arranged to meet for sex over the Internet or had used mobile applications. Twelve patients (70.6%) generally used anal douches before meeting for sex, and 9 (52.9%) had engaged in group sex in saunas or in darkrooms. The remaining sexual behaviors are shown in Table 2.
Sexual Behavior of Patients Diagnosed With Lymphogranuloma Venereum.
| Frequency | Percentage | |
|---|---|---|
| Lifetime sexual partners | ||
| Median (IQR), 100 (200) | ||
| Sexual partners during the 6 mo before onset of symptoms | ||
| Median (IQR), 6 (20.5) | ||
| Constant use of condoms during the previous 6 mo | ||
| Yes | 1 | 94.1 |
| No | 16 | 5.9 |
| Passive anal sex during the previous 6 mo | ||
| Yes | 15 | 88.2 |
| No | 2 | 11.8 |
| Unprotected passive anal sex during the previous 6 mo | ||
| Yes | 14 | 82.4 |
| No | 3 | 17.6 |
| Anonymous sex during the previous 6 mo | ||
| Yes | 15 | 88.2 |
| No | 2 | 11.8 |
| Group sex during the previous 6 mo | ||
| Yes | 5 | 29.4 |
| No | 12 | 70.6 |
| Sex at a sauna/darkroom during the previous 6 mo | ||
| Yes | 4 | 23.5 |
| No | 13 | 76.5 |
| Practicing or using prostitution during the previous 6 mo | ||
| Yes | 0 | 0.0 |
| No | 17 | 100 |
| Sex for money during the previous 6 mo | ||
| Yes | 0 | 0.0 |
| No | 17 | 100 |
| Sex outside or with people from outside Andalusia during the previous 6 mo | ||
| Yes | 16 | 94.1 |
| No | 1 | 5.9 |
| Searching for sex online or through applications during the previous 6 mo | ||
| Yes | 15 | 88.2 |
| No | 2 | 11.8 |
| Use of sex toys during the previous 6 mo | ||
| Yes | 2 | 11.8 |
| No | 15 | 88.2 |
| Anal douching before sex during the previous 6 mo | ||
| Yes | 12 | 70.6 |
| No | 5 | 29.4 |
| Use of enemas before sex during the previous 6 mo | ||
| Yes | 4 | 23.5 |
| No | 13 | 76.5 |
| Using drugs for sex drugs during the previous 6 mo | ||
| Yes | 7 | 41.2 |
| No | 10 | 58.8 |
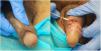
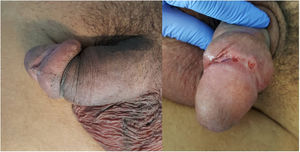
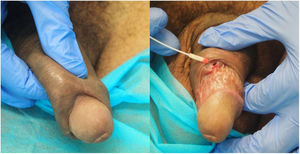
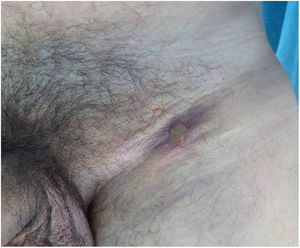
In 13 cases, the disease presented as highly symptomatic proctitis requiring a visit to the emergency department. Four patients consulted for genital lesions or inguinal masses. In one case, the disease manifested clinically as 2 ulcers on the prepuce with associated painful lymphedema (Fig. 1) and multiple enlarged lymph nodes in the pubic region. Another patient had a chronic abscess on the penis with a fistula to the skin (Fig. 2). In 2 patients, LGV manifested only as inguinal buboes, with secondary suppuration to the skin and a fistulous tract to the scrotum in 1 case (Fig. 3). The main symptoms in patients with anorectal forms were spontaneous pain and purulent anal exudate, although tenesmus and bleeding were recorded in other cases. The various diagnoses made in the anorectal forms of LGV were inflammatory bowel disease (4 patients), anal cancer (2 patients), and infectious colitis, fissures, anal fistulas, and hemorrhoids in the remainder. All patients had general malaise and low-grade fever, and 5 had lost weight. The results of the complete blood count and biochemistry were normal in all cases, and serology for C trachomatis was positive in the 14 patients who underwent testing (IgG in all cases and IgM in 1 case).
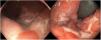
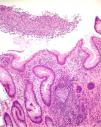
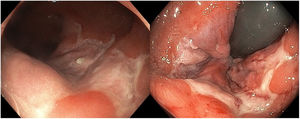
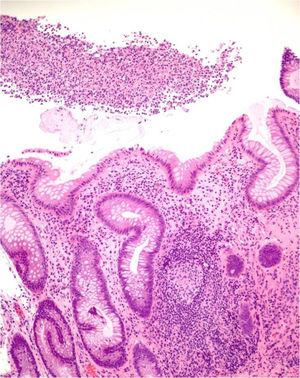
Nine patients with anorectal involvement underwent rectosigmoidoscopy, which revealed extensive superficial ulcers with geographic borders in the anal canal and rectal ampulla (Fig. 4). The main histopathology findings were granulomatous patterns with nonspecific chronic infiltrates associated with acute cryptitis in 3 cases (Fig. 5). No microorganisms were observed in the biopsy, and immunohistochemistry for cytomegalovirus and Treponema pallidum was negative. Patient 1 underwent magnetic resonance imaging, which revealed edema of the rectal mucosa with obliteration of the lumen extending into the mesorectal fat associated with multiple enlarged pararectal and hypogastric lymph nodes. In patients with classic LGV, ultrasound proved useful for demonstration and follow-up of the enlarged inguinal lymph nodes, abscesses, and fistulas. Serovar L2 of C trachomatis (GenBank reference CP002682.1) was identified as the etiologic agent in all cases.
Patients with anorectal forms of the disease responded to a 21-day cycle of doxycycline 100 mg bid, with marked improvement in symptoms after the first week of treatment. One patient with an abscess on the penis and another with enlarged suppurative inguinal lymph nodes required longer courses of treatment, which were suspended when the symptoms resolved and cure was demonstrated by a fresh microbiology workup. The anal exudates taken in the anorectal forms 2 months after the diagnosis were negative in all cases. Sequelae included anal stenosis and abnormal healing with secondary ulceration in the area of the rectum adjacent to the pectinate line in 2 cases. Surgery was necessary to remove or drain the buboes in 3 cases and for treatment of a persistent abscess on the penis. The patients’ clinical characteristics are shown in Table 2. Comparison of sexual behaviors between patients with anorectal and classic LGV revealed that receptive anal intercourse and unprotected receptive anal intercourse were more common in the anorectal forms (P = .044 and P = .006, respectively). Other more common findings in patients with the anorectal form included anal douching before sex (P = .053), sex outside Andalusia or with persons from outside Andalusia (P = .235), and group sex (P = .261) (Table 3).
Sexual Behaviors of Patients with Lymphogranuloma Venereum: Comparison Between the Anorectal Form and the Classic Form.
| Anorectal Form | Classic Form | P | |
|---|---|---|---|
| Receptive anal intercourse during the previous 6 mo | 13 (100%) | 2 (50%) | .044 |
| Condomless receptive anal intercourse during the previous 6 mo | 13 (100%) | 1 (25%) | .006 |
| Anal douching before sex during the previous 6 mo | 11 (84.6%) | 1 (25%) | .053 |
| Sex outside or with people from outside Andalusia during the previous 6 mo | 13 (100%) | 3 (75%) | .235 |
| Group sex during the previous 6 mo | 5 (38.5%) | 0 (0%) | .261 |
We report data for 17 MSM with LGV, most of whom had anorectal disease. Two patients had exclusively inguinal disease, and a further 2 had lesions on the penis. While considered extremely rare in Europe, the classic forms are more difficult to manage and more likely to be affected by complications. In the present series, 5 cases were detected in non–HIV-infected patients with high-risk sexual behaviors for both infection by HIV and lymphogranuloma. This series, which is the second published series from the south of Spain, presents the first cases of classic forms of the disease.
Rectal forms of LGV that led to epidemics in gay communities in Holland4 and San Francisco5 began to be diagnosed after liberalization of sexual relations between men and the emergence of HIV. Of the 1693 cases reported in the European Community between 2002 and 2007, only 4 were in Spain,6 although this figure increased considerably in the following years. In Barcelona, 146 cases were reported between 2007 and 2011, and half of these were reported in 2011 alone.7 In Andalusia, the LGV serovar was identified in 13 anal samples (8 in patients with symptomatic proctitis) at 1 center between December 2013 and April 2015.8 In 2017, a total of 414 cases were reported in 6 Spanish Autonomous Communities, with an incidence of 1.15 per 100 000 inhabitants.9
Anorectal LGV mainly affects HIV-infected MSM who engage in high-risk sexual behaviors.10 In the present series, patients reported having had a high number of partners (many of whom were anonymous), inconsistent use of condoms, and sexual contacts outside Andalusia or with persons from outside Andalusia during the months before diagnosis. These factors are considered risk factors for contagion and spread of the infection. Ease of movement and contact through social networks on Internet and mobile applications have facilitated the spread of LGV by enabling contact between MSM from different areas of Spain and Europe. Patients with classic LGV frequently engage in active anal sex, thus explaining the portal of entry and location of the infection. Anal douching, which was reported by most of the patients in the present series, has also been considered to facilitate acquisition of Chlamydia, by altering the integrity of the anal mucosa.11 A case-control study of MSM in 6 British clinics showed that data such as anonymous sex, high number of partners, sex in darkrooms or in groups, and searching for sex online were significantly more frequent in patients diagnosed with LGV.12 Other risk factors recorded during the study included unprotected anal sex with HIV-positive men, previous anal douching, sex under the influence of drugs, and even sex games involving urine.
Anorectal LGV usually progresses as severe proctitis. The most common finding is anal exudate, which is sometimes bloody.13 Rectal tenesmus is one of the main symptoms and may be accompanied by general malaise and low-grade fever with weight loss if it persists over time.3 Pararectal lymph node involvement and the absence of inguinal node involvement make it impossible to demonstrate lymph node involvement in the physical examination. Magnetic resonance can demonstrate involvement of the rectal wall and surrounding tissues, as well as extension to the lymph nodes.4 Lack of knowledge of the syndrome on the part of clinicians may lead to confusion for radiologists, who may interpret the findings as originating from a tumor,14 as occurred in the first case we report. The magnitude of the symptoms obliges specialists not familiar with the condition to request endoscopy, where the images and histopathology findings mimic those of inflammatory bowel disease.15 Sampling for microbiology of the exudates or biopsy tissue and evaluation using nucleic acid sequencing techniques make it possible to identify lymphatic serovars of C trachomatis16; however, these techniques are only available in specialized laboratories. Taking a sample of exudate is mandatory in patients with symptomatic proctitis and a history of unprotected receptive anal intercourse, especially in the case of HIV-infected patients. Histopathology findings are indistinguishable from those of inflammatory bowel disease,15 although they do enable a differential diagnosis to be made with other types of infectious proctitis and anorectal carcinoma. The contribution of dermatologists to the diagnosis of LGV and the continuous training they can provide for other specialists who routinely treat affected patients are indispensable for correct management of the infection and to avoid unnecessary additional tests.
The genital and inguinal form of LGV is almost limited to MSM, although it is fairly more uncommon than the anorectal form,17 possibly because of the reduced integrity of the rectal mucous epithelium. Over a period of 6 years in France, 50 cases of classic LGV were confirmed (36 with enlarged inguinal lymph nodes and 14 with genital ulceration).18 In our series, 4 patients who had engaged in insertive anal intercourse had the classic form, 2 had ulcerated lesions or genital abscesses, and a further 2 had inguinal involvement. One of the patients was diagnosed based on a study of tissue after removal of an enlarged inflammatory inguinal lymph node with positive serology findings for C trachomatis in the absence of genital symptoms and with anal and urethral samples that were negative for the pathogen. Another patient with a history of urethritis after a risky sexual contact had bilateral fluctuant enlarged inguinal lymph nodes suppurating to the skin. The portal of entry is sometimes the urethra, and the disease progresses as urethritis with few and transient symptoms that may be associated with inguinal involvement.18 In the present series, LGV is an exceptional cause of genital ulceration caused by STIs, in contrast with other, much more frequent causes. During the study period, we found only 1 case, 83 genital ulcers caused by herpesvirus, and 70 ulcers caused by the agent responsible for syphilis.
If detected on time, the infection responds rapidly to tetracyclines,19 which must be started early and empirically in cases of clinical suspicion, while waiting for definitive confirmation with real-time PCR assay and sequencing.20 Diagnostic delays may lead to lymph node fibrosis and genital lymphedema, chronic fistula, and anal stenosis.13 In our series, clinical suspicion and a generic microbiology-based diagnosis of C trachomatis infection led us to start specific treatment with doxycycline at the recommended regimen, thus avoiding sequelae in most cases of anorectal involvement. Given that up to 27% of asymptomatic cases of proctitis caused by Chlamydia are associated with lymphatic serovars of the bacterium,15 it would be advisable to identify the subtype in order to adjust the duration of treatment. In 2 of the classic forms in the present series, the antibiotic cycles had to be extended because of the persistence of symptoms and infection. In the French LGV series (see above), antibiotic therapy had to be extended for more than 3 weeks in 8 patients, all of whom had inguinal abscesses.18 Complications had to be treated with surgery in 3 cases in the present series.
Our study focused on the symptomatic forms of LGV, since we did not routinely send generic anal Chlamydia samples from asymptomatic forms for serotyping. Current European guidelines recommend serotyping all positive anal Chlamydia species samples,21 since up to 35% of anorectal LGV infections are thought to be asymptomatic.
Anorectal LGV should be suspected with any type of symptomatic proctitis in men who engage in receptive anal intercourse, especially those who live with HIV. Acquisition of this infection is influenced by high-risk sexual practices. While classic LGV is extremely rare, it should be suspected in MSM who engage in risk practices and present with genital ulceration or painful enlarged inguinal lymph nodes that tend to suppurate. Suspicion and knowledge of the clinical forms of LGV should point us towards taking a sample of C trachomatis, identifying the serovar, and starting appropriate treatment early in order to avoid complications.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Repiso-Jiménez JB, Millán-Cayetano JF, Salas Márquez C, Correa Ruiz A, Rivas Ruiz F. Estudio clínico y epidemiológico del linfogranuloma venéreo en un hospital público del sur de España. Actas Dermosifiliogr. 2020. https://doi.org/10.1016/j.ad.2020.02.006