The coronavirus 2019 (COVID-19) pandemic, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has had enormous health, economic, and social consequences. The clinical spectrum of cutaneous manifestations observed in patients with COVID-19 is both heterogeneous and complex. To date, reports have identified 5 main categories: acral lesions, vesicular rashes, urticarial rashes, maculopapular rashes, and livedoid and necrotic lesions. However, these will probably be modified as new information comes to light. Cutaneous manifestations associated with COVID-19 probably reflect the activation of pathogenic pathways by the virus or a response to inflammatory processes, vascular or systemic complications, or even treatments. Familiarity with the cutaneous manifestations of COVID-19 may enable early diagnosis or help guide prognosis.
La pandemia por SARS-CoV-2 ha causado un gran impacto desde el punto de vista sanitario, económico y social. La semiología dermatológica se ha demostrado heterogénea y compleja. En la actualidad se han definido cinco grupos principales de manifestaciones cutáneas asociadas a la COVID-19: lesiones acrales, exantemas vesiculares, erupciones urticariales, exantemas maculopapulares y lesiones livedoides/necróticas. Sin embargo, es probable que esta clasificación se modifique en el futuro. La clínica cutánea es probablemente el reflejo de distintas vías patogénicas con implicación variable de la infección vírica, del proceso inflamatorio, de las complicaciones vasculares o sistémicas de la enfermedad o incluso de los tratamientos administrados. El conocimiento de las manifestaciones cutáneas puede permitir un diagnóstico precoz o incluso servir como marcador pronóstico.
In December 2019, cases of pneumonia of unknown origin were reported in Wuhan, China. These were subsequently found to be caused by a new pathogen, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), isolated from the lower respiratory tract of affected patients.1
This is the third time in the last few decades that a coronavirus endemic in animal species has made the jump to humans.1 Prior to these jumps, coronaviruses (HCoVs) alfa (HCoV-229E and HCoV-NL63) and beta (HCoV-JHKU1, HCoV-OC43) had been identified as endemic in humans. In 2002, there was an outbreak in China of a coronavirus that caused severe acute respiratory syndrome (SARS). The virus was named SARS-CoV, and mortality was reported at around 10%. In 2012, a respiratory syndrome caused by a coronavirus from the Middle East (MERS-CoV) was identified in Saudi Arabia. In this case, the mortality was 35%. SARS-CoV-2 is the new member of this group. Disease associated with the virus is denoted coronavirus disease-19 (COVID-19), thus avoiding any geographical qualification. The virus propagated out of control and the outbreak was declared a pandemic by the World Health Organization (WHO) in March 2020.
The most frequent manifestations of infection with SARS-CoV-2 include fever, dry cough, and dyspnea; less frequent are odynophagia, gastrointestinal symptoms, and anosmia or ageusia. A high proportion of patients develop pneumonia, often bilaterally, and this may lead to respiratory failure and the need for respiratory support in more than 6% of cases.2 Around 30% will require admission to hospital, and 5% to 10% will need admission to intensive care units (ICUs). Mortality varies widely, from around 2% to somewhat greater than 10% in some countries,3 and it appears higher in older individuals and those with comorbidities and marked respiratory compromise.
Infection can also occur without symptoms or with very mild symptoms, and patients with these forms are probably potent vectors helping to spread infection.
PathogenesisFrom the pathogenic point of view, the immune response triggered by infection with SARS-CoV-2 may result in harmful effects, such as endothelial cell dysfunction and activation of coagulation pathways; this may explain the cardiovascular and thrombotic complications that affect a subgroup of patients.4
The corona (or crown) of this virus is formed from protruding glycoproteins that form spikes. These structures are what enables the virus to establish itself in the host. The infection process occurs by binding to receptors of angiotensin converting enzyme 2 (ACE2), a membrane protein expressed in the cardiovascular system, kidneys, gastrointestinal system, and lungs. This enzyme is implicated in activation of the renin-angiotensin-aldosterone system (RAAS). ACE2, in normal conditions, counteracts the activity of the ACE enzyme by reducing the amount of angiotensin II (vasoconstrictor) and increasing the vasodilatory metabolites of the RAAS. According to preliminary data, when SARS-CoV-2 infection occurs, ACE2 appears to be downregulated and this process is implicated in the development of acute lung lesions.5 Contact between these 2 proteins (spikes on the virus and ACE2) permits anchoring via the transmembrane protease, serine 2 (TMPRSS2) enzyme, setting in motion a molecular cascade by which the virus is able to enter the cells. The main target is the respiratory tract, and, in particular, the alveoli, where binding to the ACE2 receptors on pneumocytes occurs. Alveolar epithelial cells and macrophages release proinflammatory cytokines that attract neutrophils and macrophages. In normal conditions, these are components of immune protection. However, in some patients, the immune response is pathogenic or dysregulated, with exaggerated release of IL-1β, IL-6, and IFN-γ; epithelial cell apoptosis; and increased vascular permeability. These events may lead to the development of SARS, in which obliteration of the alveoli, formation of typical hyaline membranes, and hyperplasia of type II pneumocytes have been observed. The terms coined for this exaggerated inflammation are cytokine storm or cytokine release syndrome. Blood workup often shows lymphopenia, with elevated values for inflammatory indicators (C-reactive protein, ferritin, d-dimer, IL-6, and procalcitonin).2
In addition, the pattern of tissue damage observed in lung samples and some skin samples taken from patients with severe COVID-19 suggest occlusive microvascular damage mediated by complement activation of both the alternative pathway and the lectin-associated pathway. Capillary damage has been found with extensive deposition of the C5b-9 membrane attack complex, C4d, and mannose-binding lectin-associated serine protease 2 (MASP2) in the lungs. There have also been reports of a pattern of microvascular thrombotic disease mediated by complement in the skin of patients similar to that seen in livedo racemosa lesions and retiform purpura, with C5b-9 and C4d deposition. Activation of these mechanisms and interference in ACE2 function in target tissues due to viral action leads to an increase in angiotensin II, associated with greater inflammation and oxidative stress. The release of these reactive oxygen species and interference in antioxidant activity may increase complement activiation.6
Skin ManifestationsArticles published at the start of the pandemic in China considered skin manifestations as a minor and nonspecific sign, with rash reported in 0.2% of patients.7 However, this observation may presumably have been conditioned by the lack of dermatologists caring for patients affected by COVID-19 at the start of the outbreak. Recalcati,8 who was a dermatologist, observed skin manifestations in 20.4% of a group of 88 patients with COVID-19; in some cases, these manifestations were present from the start whereas others appeared during or after admission to hospital. Hedou et al.,9 in response to the aforementioned article, reported skin lesions attributable to COVID-19 in 4.9% of patients with a positive polymerase chain reaction (PCR) test result in a series of 103 patients. However, the real incidence of skin manifestations in infection by SARS-CoV-2 is not known, bearing in mind that at the time, the focus was on patients with severe signs or symptoms, and diagnosis was made in many patients with no or limited symptoms following a telephone call by the patient to the primary care physician, without any face-to-face visits, and therefore no skin examination (Table 1).
Characteristics of the Skin Manifestations Described in Association With SARS-Cov-2 Infection.
| Acral or Acroischemic Lesions | Vesicular and Chickenpox-Like Lesions | Urticarial Rash | Maculopapular Rash | Livedoid or Necrotic Lesions | |
|---|---|---|---|---|---|
| Frequency | 19% | 9% | 19% | 47% | 6% |
| Site | Acral. AsymmetricFeet >hands5% other sites | Trunk (∼100%)±limbs (∼20%)No facial or mucosal involvement | Predominance on the trunk, proximal to the limbs.Symmetric | TrunkRegion proximal to the limbsNo mucosal involvement | Predominance of acral region and region distal to the legsRegions with greater hydrostatic pressure or lower regions |
| Symptoms | Asymptomatic (∼1/3), painful (∼1/3), or itchy (∼1/3) | Mild or asymptomatic pruritus (∼2/3), pain or burning sensation | Almost constant pruritus, of variable intensity | Pruritus (>2/3), mild to moderate | Pain, burning |
| Characteristics | Macules, papules, plaques, or nodulesErythematous to purpuric | Small-sized vesicles. DisperseMonomorphic | Erythematous and edematous papules and plaques | Erythematous macules and papules, often confluent | Ischemic or necrotic lesions, often diffuse |
| Possible subtypes and variants | 2 possible patterns:• Pseudochilblain lesions (>70%) in the digital region. Region distal from the fingers or toes. Edematous. Possible formation of vesicles, pustules, and scabs• Multiform erythema-like pattern (approx. 30%). Plaques (heels) and palms. Erythematous, confluent macules or papules, with possible blistering. No formation of typical targetoid lesions and less extensive than multiform erythema | • Generally, disperse lesions. Resemble other viral rashes• They may have hemorrhagic content, be of larger size, or show diffuse coverage of extensive areas• Less often, predominantly acral vesicles or pustules | Cases have been reported of acral and facial involvement | Forms have been described with perifollicular predominance, of the pityriasis rosea and flexural type that resemble SDRIFEIn some cases, craniocaudal progression has been reported | From forms that resemble livedo reticularis or racemosa to areas of retiform purpura, hemorrhagic blisters, diffuse ischemia, or gangrene involving the distal regions |
| Demographic profile | Adolescents or young adultsNo significant differences between sexes | Adults, middle age (30–40 years)No significant differences between sexes | Adults, middle age (40–50 years)No significant differences between sexes | Adults, middle age (30–50 years)No significant differences between sexes | Middle aged and elderly adultsNo significant differences between sexes |
| Lag with respect to respiratory or systemic manifestations | Late onset | Early onset. After a few days of respiratory and systemic manifestations (15% before other symptoms) | Early onset. Accompanies respiratory or systemic manifestations, or precedes them | Accompanies respiratory or systemic manifestations, or appears a few days later | Accompanies respiratory or systemic manifestations, or sometimes with late onset |
| Positive for PCR/serology | <50% (possibly associated with late onset)In favor: clinical and epidemiological evidence, positive contacts | Frequent, probably >50% | Frequent, probably >50% | Frequent, probably >50% | Very frequent, probably >75% |
| Mean duration (days) of the rash | 12.7 (±8) | 10.4 (±9.3) | 6.8 (±7.8) | 8.6 (±6.8) | 9.4 (±5.4) |
| Prognosis | It appears to be associated with milder disease (acral ischemic lesions in patients with DIC are excluded from these cases) | Moderate severityRecovery without any scarring | VariableCases ranging from mild-moderate severity to death as the outcome (2% mortality) | Severe cases, some with death as the outcome (10% mortality) | |
| Proposed treatments | Topical corticosteroids, alone or in combination with topical antibiotics | Watch-and-wait | Oral antihistamines | Topical corticosteroids, oral antihistamines, oral corticosteroids | Support measuresAnticoagulation measures |
| Main references | 11,13–18 | 11,19 | 11,21–23 | 11,25,30,31 | 6,34,35 |
Fernández-Nieto et al.10 reported difficulties facing dermatologists for taking samples and even clinical images in a pandemic that requires rigorous isolation measures, and this is a barrier to conducting reliable epidemiological studies. For these procedures, transparent bags can be considered both for transporting photographic equipment and the tools necessary for taking biopsies. The COVID-19 pandemic represents a completely new scenario for dermatologists, and this situation may be prolonged, depending on how the pandemic progresses.
The recent article by Galván et al.,11 supported by the Spanish Academy of Dermatology and Venereology (AEDV), reported heterogeneous and complex skin manifestations associated with COVID-19 infection. The authors studied a group of 375 patients prospectively enrolled in several Spanish hospitals for 2 weeks during the peak of the pandemic. They identified 5 main clinical patterns related to COVID-19: acral areas of erythema with vesicles or pustules (pseudochilblain pattern) (19%), vesicular rashes (9%), urticarial lesions (19%), maculopapular lesions (47%), and livedo or necrosis (6%).
The following paragraphs describe the most important clinical features and diagnostic and prognostic implications in patients with SARS-CoV-2 infection.
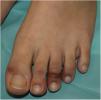
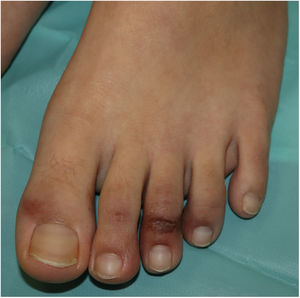
Acral or Acroischemic LesionsThe acral pseudochilblain pattern of lesions, reported by Galván et al.11 as acral, erythematous, and edematous lesions, with formation of vesicles and pustules, are perhaps the most characteristic skin lesions associated with the SARS-CoV-2 pandemic. The description and reporting of these lesions initially occurred outside academic circles in an informal fashion in medical or dermatological social media, based on shared images from family members, acquaintances, and colleagues. The first case published reported an adolescent aged 13 years who developed purpuric lesions on the feet prior to developing systemic symptoms such as fever, muscle pain, and headache, in a family with suspected cases of COVID-19 infection at the time the pandemic in Italy was spreading rapidly.12 However, no specific microbiological studies were performed. In the article, the authors mentioned the existence of an epidemic of similar lesions in children with suspected COVID-19 in Italy. In the series by Galván et al.,11 the pattern of pseudochilblain lesions was the second most common skin manifestation (19%). They developed in young patients, generally late on in the course of the disease, and were present for a period of 12.7 days, in general, in patients with mild disease or asymptomatic individuals. These lesions were described as painful (32%) or itchy (30%).
From the clinical point of view, the pseudochilblain pattern of lesions consists of macules, papules, or plaques, often about a millimeter in size, and usually with a clearly defined border in the metatarsophalangeal area, although the whole of the finger or toe can be involved (Fig. 1). Often, only some fingers or toes are affected while others completely lack any involvement, and at times, these lesions may be accompanied by lesions in the palmar and plantar region. They usually affect the feet and, to a lesser degree, the hands. Initially purpuric or bluish, they can form blisters or scabs during the course of the disease; at times, they may resemble multiform erythema13 or vasculitis; in fact, some authors recognize a multiform erythema type subgroup,13–15 with targetoid or atypical target lesions, which can be associated with lesions in extensor areas, such as the elbows. In cases with histological study, a lymphocytic infiltrate is reported in the superficial and deep dermis, of perivascular predominance, occasionally associated with edema and signs of endothelial activation.14 Keratinocyte necrosis and perieccrine reinforcement may be present.15
Alramthan and Aldaraji,16 reporting 2 clinical cases in young adults, proposed the hypothesis, not confirmed with histology in their own cases, that formation of hyaline thrombi in small caliber vessels of different organs observed in autopsies of patients could explain the pseudochilblain characteristics of the lesions.
Fewer than half the cases had microbiological or serological test results available (specific PCR or rapid antibody test for IgM/IgG). In general, when assessed, laboratory abnormalities or elevations have generally been lacking in measures of inflammation such as D-dimer, C reactive protein (CRP), or lactate dehydrogenase (LDH), indicative of poor prognosis for this disease. The strongest evidence of association with viral infection was therefore that presentation occurred during the pandemic (and in a different context to that of chilblains, in the warmer months than when they usually appear) and a history in the family environment or personal situation consistent with infection. The authors suggested these negative results were due to the late development of these lesions in the course of the disease; the low sensitivity of the tests used, which has led to mass recall of some batches; and the rapid disappearance of antibodies.11,15
Docampo-Simón et al.,17 in the first prospective study of skin manifestations, found a positive PCR result in only 1 of the 38 samples tested. In addition, due to the aforementioned technical reasons (time since infection, false negatives), the authors considered the possibility that this manifestation simply has nothing to do with COVID-19. Other possible explanations could be a traumatic origin during lock-down or even the concurrent expansion of another virus, such as parvovirus B19. This possible relation with the lock-down period, and not necessarily with COVID-19, has also been suggested in a recent series with similar results.18
During the worst weeks of the pandemic, we saw patients with these characteristics almost on a daily basis in our clinics. From both the clinical and epidemiological point of view, the characteristics are consistent with those described above. Most of our patients were young, asymptomatic or with mild symptoms, and not one of them had a serious complication associated with COVID-19.
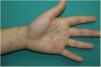
Vesicular and Chickenpox-Like LesionsIn 8 Italian centers, clinical data were collected from patients with COVID-19, with microbiological confirmation by RT-PCR from a nasopharyngeal swab and with no history of medications that could have triggered chickenpox-like lesions. In total, the study included 22 patients, mainly males, with a mean age of 60 years. The lag from the onset of symptoms of COVID-19 to development of skin manifestations was relatively short, 3 days (range, 2 to 12 days). Most patients presented with the full clinical manifestations of the disease, with general and respiratory manifestations.19
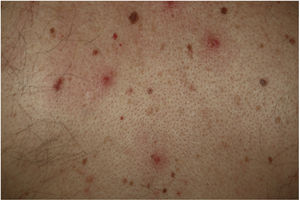
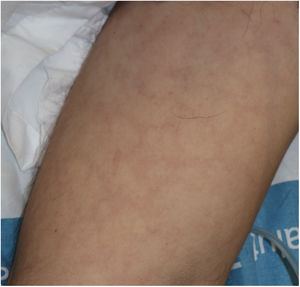
Vesicular lesions, usually monomorphic, appear early on and may at times precede other symptoms (in 15% of patients),11 although in most cases, up to 79.2% in a series of 24 patents reported by Fernandez-Nieto et al.,20 they occur at the onset of other symptoms. There is involvement of the trunk in almost all cases and in 20% the limbs are also affected (Fig. 2). Exceptionally, facial and mucosal involvement have been reported. The skin lesions are barely symptomatic, but when they do cause symptoms, these are usually mild itching and, to a lesser extent pain or burning sensation.11,19 Although the lesions may be disperse, an extensive disseminated pattern is more frequent, 75% according to Fernandez-Nieto et al. In some patients, the legs may be affected, or the lesions may present with hemorrhagic content or be large, with a diffuse distribution. The mean duration of skin symptoms is 10.4 days (±9.3).11 In cases in which a biopsy was obtained, the findings were described as consistent with viral infection, showing vacuolar abnormalities and abnormal maturation of keratinocytes, as well as larger, multinucleated keratinocytes, and dyskeratosis.19 There have been no reports of presence of SARS-Cov-2 confirmed with positive PCR in biopsies of skin lesions.
Urticarial RashHenry et al.21 reported the case of a female patient who developed an urticarial rash, accompanied by odynophagia and arthralgia, before developing the full clinical manifestations of COVID-19. Van Damme et al.22 reported a further 2 cases of urticarial rash as the first clinical manifestation of COVID-19, although microbiological confirmation of infection was only obtained in 1 of those, and in both cases the skin manifestations preceded pyrexia. One of the patients developed severe respiratory failure, leading to death.
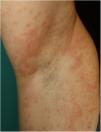
Urticarial rash accounts for 19% of skin manifestations in COVID-19. In general, these skin manifestations become evident more or less at the same time as other general and respiratory symptoms. Lesions occur predominantly on the trunk, and involvement of the face and hands is frequent, with resolution in approximately 7 days.11 In one of the cases reported, histological study showed edema of the upper dermis and perivascular lymphocytic infiltration, with some eosinophils present.10 Although such lesions may be associated with worse prognosis in some patients,11 this is a nonspecific rash, and it has been reported in patients with a favorable clinical outcome and those with limited symptoms.23
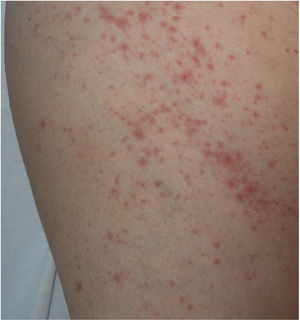
There is probably a certain degree of variability in the way in which rashes are reported as urticarial maculopapular because, in the cases described, it is not specified whether the lesions follow a transient course or not. These are nonspecific rashes in which it is difficult to establish a solid relationship with viral infection, bearing in mind that the patients who present with these lesions have often received a range of treatments such as antiviral agents, antibiotics, hydroxychloroquine, anticoagulants, and support treatments, and these could also trigger skin reactions (Fig. 3). In fact, in a histological study, some cases of vacuolar interface dermatitis were observed with occasional necrotic keratinocytes, findings more reminiscent of a multiform erythematous pattern.24
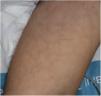
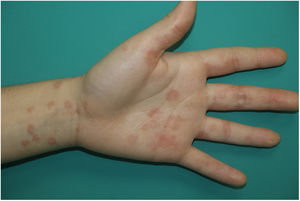
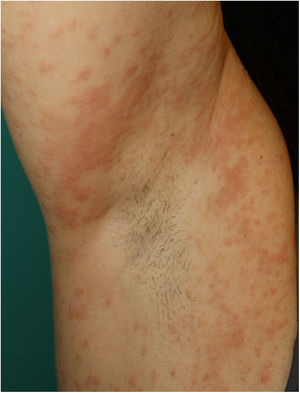
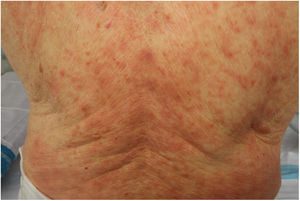
Maculopapular RashThis group of lesions includes a heterogeneous group of rashes that, considered together, and given the difficulty of further subclassification, account for 47% of the skin manifestations in patients with COVID-19.11 At times, these rashes may be accompanied by a petechial component or with macules or more extensive areas with a purpuric appearance. In other cases, the lesions have a markedly perifollicular distribution (Fig. 4) with variable degrees of scaling, some of which have been reported as similar to pityriasis rosea.25 Infiltrated papules have also been observed; these are pseudovesicular lesions or similar to erythema elevatum diutinum or multiform erythema, and they may occasionally be pruritic (Fig. 5).26 A markedly craniocaudal development has also been reported, with involvement of the folds but without the palmoplantar region or the mucosa being affected.27,28
Jimenez-Cauhe et al.29 reported the case of a female patient who developed a coalescent erythematous-purpuric rash about a millimeter in size, with a flexural distribution predominantly in the periaxillary region. The authors considered the difficulty of associating the skin manifestations with viral infection in view of its nonspecific appearance and concomitant medication use. Other authors have reported the appearance of rash that resembles the typical skin involvement of symmetrical drug-related intertriginous and flexural exanthema (SDRIFE), possibly associated with viral infection, given that the rash resolved despite continued use of the drug.30 We have also observed a similar distribution in our own patients (Fig. 6). From the histological point of view, a range of features have been reported within the group of maculopapular rashes, such as a perivascular inflammatory infiltrate with discrete lymphocytic exocytosis, marked vessel dilatation in the superficial and medial dermis, as well as lymphocytic vasculitis.31,32
There is limited additional information in these patients regarding the timing with respect to other clinical manifestations or its prognostic or diagnostic value. In most cases, maculopapular rashes appear either at the same time as the characteristic respiratory symptoms or a few days later.11,28,32,33 However, in our hospital, we have detected more or less generalized maculopapular rashes, in some cases similar to multiform erythema, in young patients with a history of mild or even no symptoms, although with epidemiological evidence of infection with SARS-CoV-2 and these resemble those that can be seen in other viral infections.
Livedoid or Necrotic LesionsLivedoid or necrotic lesions are relatively uncommon, accounting for 6% of skin lesions in the series reported by Galván et al.11 These are lesions usually reported in elderly patients with prior comorbidities and with severe forms of COVID-19 infection. They are considered secondary to vascular micro-occlusion and acral ischemia due to general deterioration in the patient's state and/or the coagulation disorders attributed to COVID-192,5 (Fig. 7).8 However, in some patients, episodes have been reported of patchy livedo reticularis that presented over the course of minutes or hours, of an uncertain nature and a benign course.35
In the histological study of cutaneous purpuric lesions, a pauci-inflammatory thrombogenic vasculopathy has been found, with C5b-9 and C4d deposits, and localization of viral particles, leading to suspicion of the presence of a catastrophic microvascular lesion caused by complement activation.6
ConclusionsThe SARS-CoV-2 pandemic has had a major impact from the healthcare, economic, and societal point of view, and will probably lead to lasting changes in our generation. Considered initially as of little relevance, the dermatological manifestations have proved to be varied and complex. Recent efforts to characterize cutaneous involvement in patients with COVID-19, implemented in a study conducted quickly and rigorously in a full health emergency, has identified 5 main groups of lesion (acral, vesicular, urticarial, maculopapular and livedoid/necrotic lesions).11 Although these manifestations are considered a reflection of different pathogenic pathways, with a variable implication of the viral infection, inflammatory processes, and vascular and systemic complications of the disease, there is a substantial knowledge gap in many aspects. Thus, it cannot be ruled out that acral lesions, reported as characteristic given the epidemiological evidence rather than microbiological tests in most cases, may not be directly related to COVID-19. Extensive urticarial or maculopapular rashes, often described in symptomatic or even hospitalized patients, may be linked in many cases to drugs able to trigger them, such as hydroxychloroquine or antibiotics, administered during the COVID-19 pandemic despite limited evidence of their effectiveness. Finally, the heterogeneous set of maculopapular rashes consistent with viral infection, multiform erythema, or SDRIFE, may be associated with other etiologic agents neglected during the pandemic, as in many cases, microbiological or serologic confirmation of SARS-CoV-2 infection is lacking. Thus, in the panorama of lesions associated with COVID-19, for the most part, priority in the international literature during the peak of the pandemic was given to rapid publication, even though the description, support, or methodological rigor were not ideal. Familiarity with the skin manifestations may allow not only greater investigation into aspects still little known in COVID-19, but also may help a more rapid diagnosis and even serve as a prognostic marker.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
We would like to thank Doctors Aram Boada, Isabel Bielsa, María Blanco, Ferran Ballescà, Juli Bassas, Elena del Alcázar, Gonzalo Castillo, Carlos Ferrándiz, María José Fuente, Adrià Plana, Nina Richarz, Verónica Mora, Arantxa Arrieta, and Ane Jaka, with whom the authors have worked and shared knowledge during the peak of the COVID-19 pandemic.
Please cite this article as: Carrascosa JM, Morillas V, Bielsa I, Munera-Campos M. Manifestaciones cutáneas en el contexto de la infección por SARS-CoV-2 (COVID-19). Actas Dermosifiliogr. 2020. https://doi.org/10.1016/j.ad.2020.08.002