Recent publication of the results of clinical trials in which lymph node dissection was not associated with any survival benefit in patients with sentinel node metastasis makes it necessary to reconsider the treatment of patients with melanoma. This article provides an update on the available evidence on the diverse factors (routes of metastatic spread, predictors, adjuvant therapy, etc.) that must be considered when treating patients with sentinel node-positive melanoma. The authors propose a decision-making algorithm for use in this clinical setting. The current evidence no longer supports lymph node dissection in patients with low-risk sentinel node metastasis (sentinel node tumor load ≤1mm).
La reciente publicación de los resultados de ensayos clínicos en los que la disección ganglionar no ha demostrado beneficio de supervivencia en pacientes con metástasis en el ganglio centinela plantea la necesidad de modificar el tratamiento del paciente con melanoma. El presente trabajo aporta una actualización de la evidencia sobre diferentes aspectos necesarios (vías de progresión metastásica, factores predictores, tratamiento adyuvante, etc.) para la toma de decisiones en el paciente con melanoma y metástasis en el ganglio centinela y plantea un algoritmo de toma de decisiones para este escenario clínico. La evidencia actualmente disponible respalda el abandono de la disección ganglionar en aquellos pacientes con metástasis de bajo riesgo en el ganglio centinela (carga tumoral en el ganglio centinela inferior o igual a 1mm).
The current protocols for cutaneous melanoma recommend primary tumor excision with safety margins and sentinel lymph node biopsy (SLNB) depending on Breslow thickness once diagnosis has been confirmed by anatomopathologic study.1,2 When metastasis is identified in the sentinel lymph node (SLN), the standard procedure to date has been full lymph node dissection in the region of the SLN metastasis, possibly with adjuvant therapy with interferon.
SLNB was introduced by Morton et al.3 as a minimally invasive prognostic procedure with fewer complications than elective lymph node dissection, the approach followed in all patients with melanoma up until then. The findings of the Multicenter Selective Lymphadenectomy Trial (MSLT)-I confirmed the advantage of SLNB for lymph node staging and raised the possibility that lymph node dissection could improve survival in those patients with SLN metastasis.4 Since then, SLNB and subsequent lymph node dissection in those patients with SLN micrometastases became the standard approach in patients with cutaneous melanoma.
More than 2 decades later, treatment of patients with melanoma is once again undergoing a major transformation. As before, the possibility is being considered of replacing lymph node dissection with a less invasive approach that has fewer side effects but does not compromise melanoma-specific survival.
Progression of Metastatic MelanomaFor decades, the accepted hypothesis has been sequential spread for primary melanoma whereby cells with metastatic potential spread to the lymph nodes and, from there, to the bloodstream and other organs. This Halsted hypothesis (Halsted 1907) was challenged in the 1970s by an alternative hypothesis in which lymph node metastasis was considered an indicator, rather than a trigger, of distant metastases.5 According to this alternative hypothesis, radical lymph node dissection would not be expected to improve patient survival. In addition to initial studies in breast cancer, other publications in the field of melanoma also support possible direct distant spread that does not require prior lymph node metastasis.6,7
A recent study of the German Central Malignant Melanoma Registry included 2299 patients with stage IA-IIC melanoma (seventh edition of the American Joint Committee on Cancer [AJCC]) who progressed to stage III and/or IV disease during follow-up. In this cohort study, 3 subgroups of patients were defined according to observed metastatic progression: patients with exclusively lymph node metastasis (38.4%), patients with distant metastasis only (16.2%), and patients with lymph node and distant metastases (45.4%).7 The distant metastasis-free survival, overall survival (OS), and melanoma-specific survival were the same, regardless of whether lymph node metastases were present prior to distant progression. With this observation, the authors concluded that both metastatic sites originated from the primary tumor in parallel rather than sequential fashion.7 This pattern of spread could also explain the absence of survival benefit observed after lymph node dissection in patients with positive SLNB.
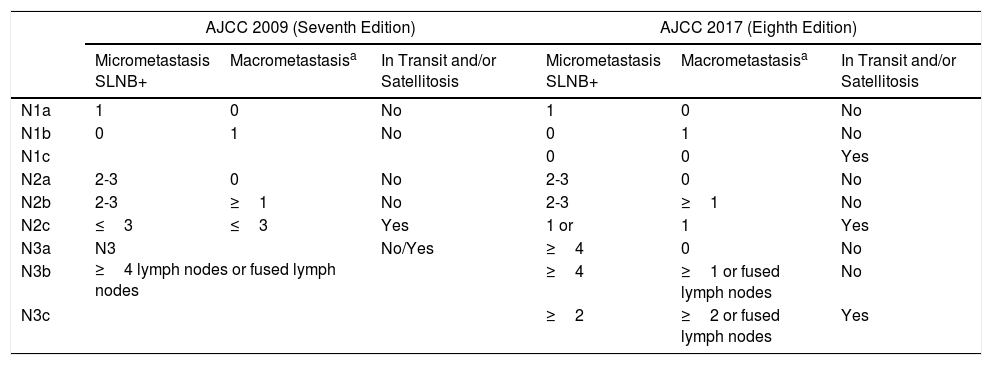
Factors Predictive of Survival in Patients with Sentinel Lymph Node Metastasis Stage III in the Eighth Version of the American Joint Committee on CancerThe eighth edition of the TNM classification of the AJCC maintains Breslow thickness and ulceration of the primary tumor as the strongest prognostic predictors in patients with localized melanoma.8 In patients with lymph node metastasis (stage III), this classification maintains presence of microscopic or macroscopic satellitosis (N1c, N2c, N3c), number of involved lymph nodes (N1-3), and tumor burden. With regards tumor burden, this edition introduces the concept of clinically occult metastasis, to refer to those patients with metastasis identified by SLNB and without clinical or radiological evidence of disease (N1a, N2a, N3a) (described as microscopic in the seventh edition of the AJCC) (Table 1).9 Those metastases identified by physical examination or imaging studies, and defined as macroscopic in the seventh edition are considered clinically apparent in the current edition (N1b, N2b, N3b). The survival curves of the AJCC show that those parameters allow stratification of patients with melanoma and locoregional metastases, with better survival in patients with clinically occult lymph node metastasis and fewer metastatic lymph nodes.8,9
Comparison of American Joint Committee on Cancer (AJCC) 2009 and 2017 TNM Staging for Cutaneous Melanoma.
| AJCC 2009 (Seventh Edition) | AJCC 2017 (Eighth Edition) | |||||
|---|---|---|---|---|---|---|
| Micrometastasis SLNB+ | Macrometastasisa | In Transit and/or Satellitosis | Micrometastasis SLNB+ | Macrometastasisa | In Transit and/or Satellitosis | |
| N1a | 1 | 0 | No | 1 | 0 | No |
| N1b | 0 | 1 | No | 0 | 1 | No |
| N1c | 0 | 0 | Yes | |||
| N2a | 2-3 | 0 | No | 2-3 | 0 | No |
| N2b | 2-3 | ≥1 | No | 2-3 | ≥1 | No |
| N2c | ≤3 | ≤3 | Yes | 1 or | 1 | Yes |
| N3a | N3 ≥4 lymph nodes or fused lymph nodes | No/Yes | ≥4 | 0 | No | |
| N3b | ≥4 | ≥1 or fused lymph nodes | No | |||
| N3c | ≥2 | ≥2 or fused lymph nodes | Yes | |||
Abbreviation: SLNB, sentinel lymph node biopsy.
The studies that analyzed survival in patients with SLN metastasis who either underwent or did not undergo lymph node dissection included, as would be expected, patients with a low tumor burden. In the DeCOG-SLT trial, 91% of patients in the observation group and 93% of those who underwent dissection had a single SLN metastasis (N1), whereas 9% and 7%, respectively, had metastases in 2 or more SLNs (N2 and N3). In addition, 68% of patients in the observation group and 63% of patients in the dissection group had SLN metastases of 1mm or less, with 25% and 26% of patients, respectively, with metastases greater than 1mm in each of the study groups.10 In this German trial, SLN tumor burden (≤1mm vs >1mm) was identified as an independent predictive factor for recurrence-free survival (RFS), distant RFS, and OS.10 However, the low proportion of patients with a tumor burden greater than 1mm (25%-26%) did not ensure sufficient statistical power to issue definitive recommendations to abandon lymph node dissection in patients with a tumor burden greater than 1mm. Similarly, in the Multicenter Selective Lymphadenectomy Trial-II (MSLT-II), 65.5% and 66.8% of patients in the observation and dissection groups, respectively, presented metastases of 1mm or less in the SLN, with a median diameter of 0.67 and 0.61mm, respectively.11 In the MSLT-II, tumor diameter greater than 1mm was not identified as an independent predictor of survival in either of the study groups (dissection or observation).11 Likewise, the number of SLN metastases, analyzed as N stage, was not predictive of survival in either of the clinical trials (DeCOG-SLT, MSLT-II). Of note, the extracapsular extension of SLN metastasis was not analyzed in the MSLT-II or DeCOG-SLT studies, as this criterion was considered as an exclusion criterion in both.11
A recent study sponsored by the EORTC and the Australian Institute of Melanoma based on a cohort of 1539 patients with SLN metastasis assessed the relevance of SLN tumor burden as a predictive factor using micromorphometric criteria: Rotterdam classification of maximum tumor diameter (<0.1mm, 0.1-1.0mm, >1.0mm), intranodal site according to the Dewar classification (subcapsular, not capsular), and SLN depth of invasion (Starz classification).12 The multivariate analysis identified nonsubcapsular metastasis site, tumor depth in the SLN >1mm, and maximum tumor diameter greater than 1mm as predictors of poor survival. The threshold of 1mm SLN tumor burden was the most consistent predictor of positive nonsentinel lymph nodes and reduced disease-free survival and melanoma-specific survival.12
Therefore, with regards SLN tumor burden as a factor for decision making, of note is the utility of this indicator as a marker of greater probability of regional and distant progression, and of worse melanoma-specific survival. From the point of view of therapeutic decisions, although lymph node dissection might provide better regional control, improved specific survival would not be observed, although this benefit might be observed with the new adjuvant regimens.
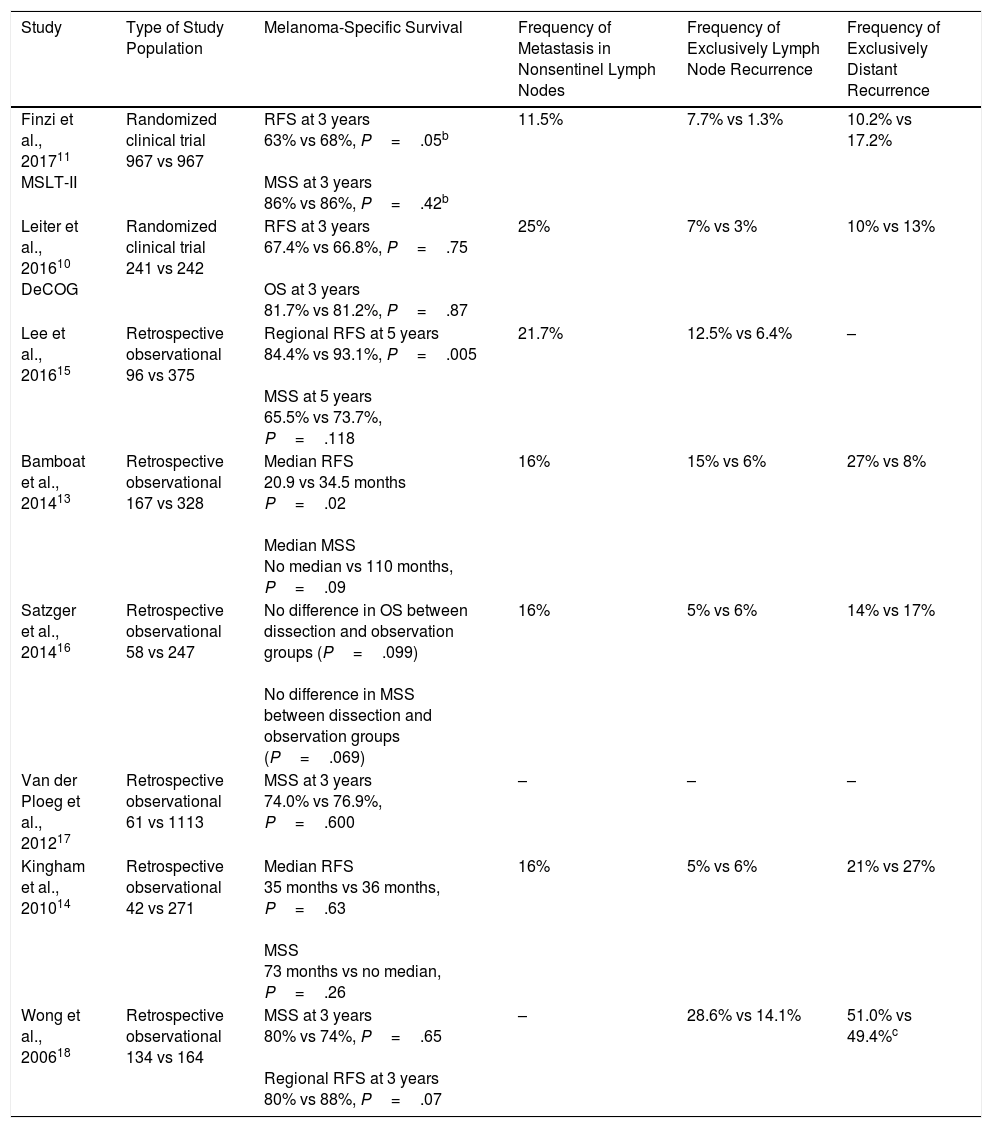
Lymph Node Dissection in Patients With Lymph Node Metastasis: Current EvidenceThe recent publication of the final results of the MSLT-II trial have opened a debate on whether the lymph node should be dissected in patients with microscopic melanoma metastases, that is, those identified by SLNB.11 This clinical trial shows that immediate lymph node dissection does not improve survival in this group of patients compared with observation and therapeutic dissection once the patient develops lymph node metastasis identified by physical exploration or imaging studies. These findings were preceded by similar results in the clinical trial sponsored by the German Cooperative Group (DeCOG-SLT),10 and in other retrospective studies with findings that do not support lymph node dissection in melanoma patients with SLN metastasis (Table 2).13–18
Studies That Analyze Outcomes in Patients with Sentinel Lymph Node Metastasis Who Underwent Observation or Immediate Lymph Node Dissectiona
| Study | Type of Study Population | Melanoma-Specific Survival | Frequency of Metastasis in Nonsentinel Lymph Nodes | Frequency of Exclusively Lymph Node Recurrence | Frequency of Exclusively Distant Recurrence |
|---|---|---|---|---|---|
| Finzi et al., 201711 MSLT-II | Randomized clinical trial 967 vs 967 | RFS at 3 years 63% vs 68%, P=.05b MSS at 3 years 86% vs 86%, P=.42b | 11.5% | 7.7% vs 1.3% | 10.2% vs 17.2% |
| Leiter et al., 201610 DeCOG | Randomized clinical trial 241 vs 242 | RFS at 3 years 67.4% vs 66.8%, P=.75 OS at 3 years 81.7% vs 81.2%, P=.87 | 25% | 7% vs 3% | 10% vs 13% |
| Lee et al., 201615 | Retrospective observational 96 vs 375 | Regional RFS at 5 years 84.4% vs 93.1%, P=.005 MSS at 5 years 65.5% vs 73.7%, P=.118 | 21.7% | 12.5% vs 6.4% | – |
| Bamboat et al., 201413 | Retrospective observational 167 vs 328 | Median RFS 20.9 vs 34.5 months P=.02 Median MSS No median vs 110 months, P=.09 | 16% | 15% vs 6% | 27% vs 8% |
| Satzger et al., 201416 | Retrospective observational 58 vs 247 | No difference in OS between dissection and observation groups (P=.099) No difference in MSS between dissection and observation groups (P=.069) | 16% | 5% vs 6% | 14% vs 17% |
| Van der Ploeg et al., 201217 | Retrospective observational 61 vs 1113 | MSS at 3 years 74.0% vs 76.9%, P=.600 | – | – | – |
| Kingham et al., 201014 | Retrospective observational 42 vs 271 | Median RFS 35 months vs 36 months, P=.63 MSS 73 months vs no median, P=.26 | 16% | 5% vs 6% | 21% vs 27% |
| Wong et al., 200618 | Retrospective observational 134 vs 164 | MSS at 3 years 80% vs 74%, P=.65 Regional RFS at 3 years 80% vs 88%, P=.07 | – | 28.6% vs 14.1% | 51.0% vs 49.4%c |
Abbreviations: MSS, melanoma-specific survival; OS, overall survival; RFS, recurrence free survival.
These studies analyzed other secondary endpoints of interest (Table 2). First, between 75.0% and 88.5% of dissections performed did not identify additional metastases in nonsentinel lymph nodes. Second, the relevant postoperative morbidity rate was significantly higher in the group of patients who underwent immediate dissection (24.2% vs 6.3% with lymphedema in the MSLT-II).11 On the other hand, both the MSLT-II and the DeCOG-SLT studies confirmed better regional control of the disease in patients who underwent immediate lymph node dissection (77% vs 92% at 3 years, P<.001 in MSLT-II).10,11
The most recent versions of the international clinical guidelines have still not included a clear recommendation for abandoning lymph node dissection in patients with positive SLN status. The most recent edition of the National Comprehensive Cancer Network (NCCN) guideline (v 1.2018; October 11, 2017) recommends careful follow-up of the lymph nodes in the affected region or lymph node dissection in patients with positive SLNB.19 However, this most recent edition of the NCCN guideline now provides information on the results of these 2 clinical studies on lymph node dissection (MSLT-II and DeCOG-SLT) and highlights the lack of survival benefit and greater surgical morbidity, although the advantage of dissection in terms of regional control and additional prognostic information is mentioned.19
In light of the DeCOG-SLT trial and despite its methodological limitations, the European Consensus-Based Multidisciplinary Guidelines already proposed in 2016 that the indication for lymph node dissection in patients with SLN metastases of less than 1mm should be critically discussed.20
The American Society of Oncology (ASCO) has updated the recommendations for treatment of patients with lymph node disease based on the results of the DeCOG-SLT and MSLT-II trials.21 This guideline differentiates between patients with low- and high-risk SLN micrometastases. High-risk situations are those in which some of the exclusion criteria applied in the MSLT-II trial are present: extracapsular extension, simultaneous microsatellitosis, and/or lymphovascular invasion in the primary tumor, more than 3 metastatic SLNs, more than 2 involved regional lymph nodes, and immunosuppression.11 Low risk situations are those defined by the absence of these criteria. With regards SLN tumor burden, the ASCO guideline points out that patients with a tumor burden less than 1.01mm are well represented in both clinical trials, with such patients accounting for 66% of the overall population.10,11 However, although one-third of patients enrolled with tumor burden greater than 1mm did not show any difference in survival between dissection and observation either, the relatively low number of patients in this subgroup makes it difficult to generalize the results.21 Based on these considerations, the ASCO guideline states that “complete lymph node dissection or careful observation are options for patients with low-risk micrometastatic disease, with due consideration of clinicopathological factors.” In patients at higher risk, “observation may be considered only after a thorough discussion with the patients about the potential risks and benefits of foregoing complete lymph node dissection.”21
Adjuvant Agents in Patients With Clinically Occult (Microscopic) Lymph Node DiseaseThe current clinical practice guidelines recommend assessment of adjuvant treatment in those patients with lymph node metastasis who undergo complete resection of the involved lymph node region and who are free of distant disease as well as in patients with a high-risk primary tumor (Breslow thickness greater than 4mm with ulceration).1,2 Interferon is still the only drug authorized as adjuvant therapy in Spain, based on the improvement shown in RFS. Meta-analyses performed of interferon outcomes in the adjuvant treatment of melanoma also show benefit of doubtful clinical relevance for OS,22–24 although the benefit for RFS and OS have been confirmed particularly in patients with primary ulcerated tumors.25
Ipilimumab at a dose of 10mg/kg is the first adjuvant immunotherapy regimen authorized by the United States Food and Drug Administration for patients with melanoma. The EORTC 18071 trial, which enrolled patients with stage III lymph node disease and intranodal tumor burden greater than 1mm, showed a 10% improvement at 5 years in RFS, distant RFS, and OS (RFS 40.8% vs 30.3%, distant RFS 48.3% vs 38.9%, and OS 65.4% vs 54.4%).26 However, substantial toxicity was reported for this regimen, with grade 3-4 adverse effects reported in 54% of patients treated and 1.1% of patients died due to immunologic treatment-related effects. In patients with clinically occult lymph node disease, primary tumor ulceration was a predictor of favorable response, as had already been observed with adjuvant interferon.26
Subsequently, the COMBI-AD trial, which compared dabrafenib-trametinib with placebo in patients with a BRAF mutation, stage III disease, and an intranodal tumor burden greater than 1mm, showed improved RFS (58% vs 39%) and OS (88% vs 77%) at 3 years.27 A phase III trial is currently ongoing to compare pembrolizumab with placebo in patients with lymph node metastasis and tumor burden greater than 1mm (EORTC 1325). Also in relation to new adjuvant regimens, of note is the advantage of nivolumab compared to ipilimumab in terms of RFS (65% vs 53% at 18 months)28; however, this trial recruited patients with resected stage IIIB and IV disease.
One argument in favor of dissection in patients with SLN metastasis is based the more detailed staging that this approach can offer compared with SLNB. Along these lines, there have even been proposals for extended SLNB that includes 4 to 5 lymph nodes. This approach is less invasive but may improve differentiation between patients with stage IIIA and stage IIIC disease, as these stages have significantly different prognoses.29 However, a recent retrospective study showed that the staging changed in fewer than 6% of patients with SLN metastasis who underwent lymph node dissection. The authors of the study suggested that, in absence of stronger predictors, the selection of patients for adjuvant therapy should be based on SLN tumor burden.30
With regards adjuvant agents in clinical practice guidelines, the most recent edition of the NCCN guideline (v 1.2018; October 11, 2017) already includes the tumor burden threshold greater than 1mm for high-dose adjuvant regimens with ipilimumab and a combined dabrafenib-trametinib regimen in patients with a BRAF mutation.19 Regarding adjuvant treatment with ipilimumab, the NCCN expert panel insists on finding an appropriate balance between expected benefit and toxicity, especially in patients with stage IIIA disease given that they usually have a favorable prognosis. In any case, the NCCN guideline recommends adjuvant treatment with a nivolumab regimen as the first option given its lower toxicity compared with ipilimumab and the benefit for RFS, although its impact on OS has not been clearly established. However, this adjuvant approach is restricted in this guideline to patients with at least stage IIIB disease.19
With regards interferon, the NCCN guideline maintains the option of high-dose interferon for patients with metastasis identified in the SLNB and highlight the benefit for RFS although not for OS.19 With regards the actual role of interferon as an adjuvant agent in melanoma, a recent review by Eggermont and Dummer29 on the topic concluded that use of the agent should limited to countries where nivolumab or dabrafenib-trametinib is not available and patients with ulcerated primary tumor. It is important to note that the European Medicines Agency has still not approved any of these adjuvant regimens, and so they are not available in Spain for clinical use outside the clinical trial setting.
Ultrasound Follow-up of Patients with Sentinel Lymph Node MetastasisClinical and ultrasound follow-up of regional lymph nodes corresponding to metastatic SLN yielded the same survival results as lymph node dissection after positive SLNB in the MSLT-II and DeCOG-SLT trials. To reproduce the results of a clinical trial in clinical practice requires use of the same interventions and procedures that were compared. In the MSLT-II trial, patients in both study groups were included in a follow-up program consisting of lymph node ultrasound in the SLN region every 4 months for the first 2 years and subsequently every 6 months up to the fifth year.11 In the DeCOG-SLT trial, follow-up included lymph node ultrasound every 3 months for the 3-year follow-up period.10 However, in the MSLT-II trial, in addition to careful ultrasound monitoring, participating sites could apply local imaging protocols (computed tomography, magnetic resonance imaging, positron-electron tomography-computed tomography).
Lymph node ultrasound is not a novel procedure in the care of patients with melanoma and some clinical practice guidelines had incorporated this technique years ago.1 In 2000, Blum et al.31 showed that ultrasound at a frequency of 7.5-10MHz improved diagnosis of lymph node metastasis compared with physical examination, with a sensitivity and specificity of 89.2% and 99.7%, respectively, for ultrasound, and of 71.4% and 99.7%, respectively, for physical examination. A set of validated criteria has been established for early detection of lymph node metastasis (balloon shape, hump structures, absence of central perfusion, presence of peripheral perfusion, loss of central echoes, echo-poor islands), and these have been widely applied and provide a sensitivity of 82% with a positive predictive value of 52%.32,33
Therefore, ultrasound represents a noninvasive technique for early diagnosis of lymph node metastasis. It has reproducible criteria, is not expensive, and represents a necessary complement in the follow-up of patients with SLN metastasis. Ultrasound also has the additional advantage that had can be performed at the point of care or patient's bedside, or as a complementary test performed by the radiology departments.34
Proposed Decision Making in Patients with Sentinel Lymph Note MetastasisAlthough adaptation of recommendations from other health care contexts can be problematic, it is of note that the ASCO guideline maintains the option of lymph node dissection both in low-risk and high-risk scenarios. However, the evidence currently available supports abandoning the use of dissection in situations that are well represented in clinical trials with the required statistical power.
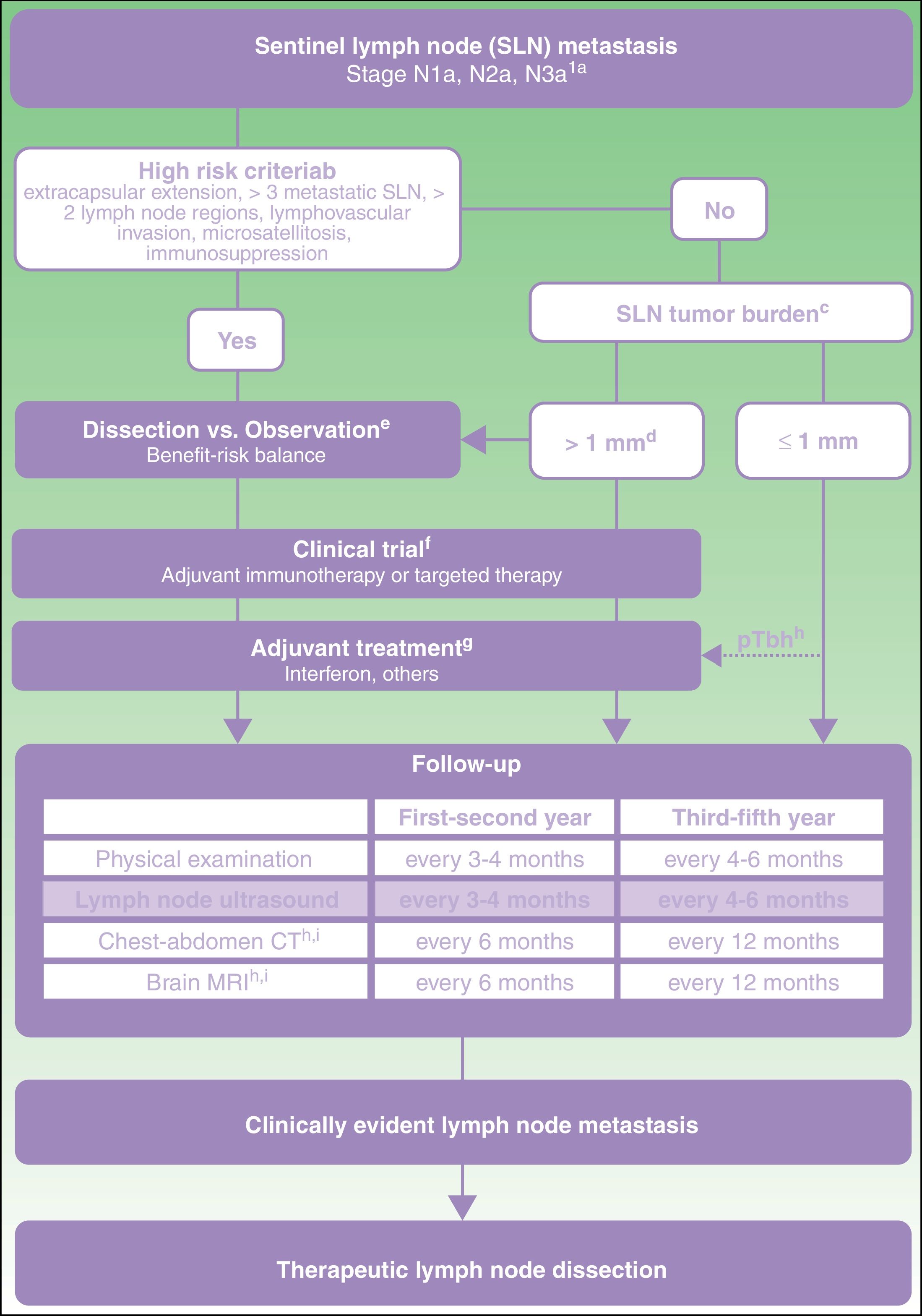
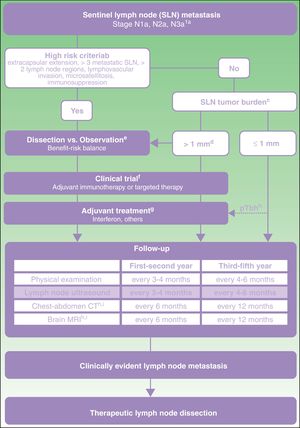
From this direct and indirect evidence (Table 3), an algorithm is proposed for decision making in patients with melanoma and SLN metastasis (Fig. 1). This decision making should start with an exhaustive assessment of clinical criteria and SLN pathology findings. In those patients without high-risk criteria and with SLN tumor burden less than 1mm, current evidence supports abandoning lymph node dissection. However, with a tumor burden greater than 1mm, and particularly tumor burden diameters less well represented in clinical trials, and so with lower statistical power, a more detailed assessment is required of risks and benefits expected with each of the options (dissection vs observation).
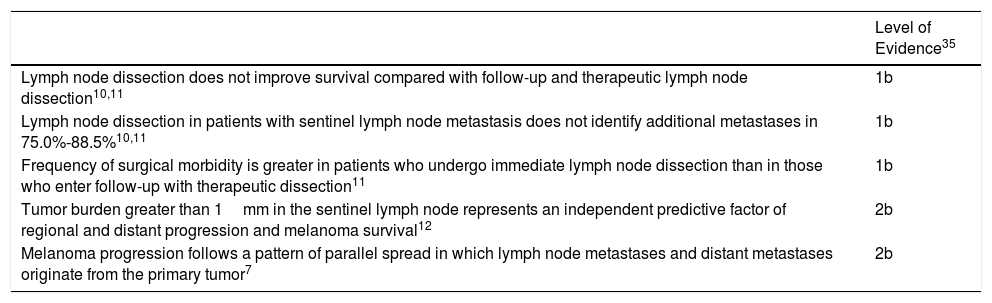
Evidence Related to Decision for Immediate Lymph Node Dissection in Patients With Sentinel Lymph Node Metastasis.
| Level of Evidence35 | |
|---|---|
| Lymph node dissection does not improve survival compared with follow-up and therapeutic lymph node dissection10,11 | 1b |
| Lymph node dissection in patients with sentinel lymph node metastasis does not identify additional metastases in 75.0%-88.5%10,11 | 1b |
| Frequency of surgical morbidity is greater in patients who undergo immediate lymph node dissection than in those who enter follow-up with therapeutic dissection11 | 1b |
| Tumor burden greater than 1mm in the sentinel lymph node represents an independent predictive factor of regional and distant progression and melanoma survival12 | 2b |
| Melanoma progression follows a pattern of parallel spread in which lymph node metastases and distant metastases originate from the primary tumor7 | 2b |
Proposed care for patient with melanoma and sentinel lymph node metastasis.
a American Joint Committee on Cancer 2017 TNM classification for cutaneous melanoma.8
b Characteristics of high risk based on exclusion criteria applied in clinical trials of dissection vs observation.11,21
c Sentinel lymph node (SLN) tumor burden measured by maximum tumor diameter of the largest tumor (Rotterdam criteria).36
d In the MSLT-II trial, the dissection and observation groups included 33.2% and 34.5% of patients, respectively, with SLN metastasis greater than 1mm. The 75th percentile of tumor burden was 1.32 and 1.381mm in each of the study groups, respectively. In the DeCOG-SLT trial, 7% of the patients enrolled had SLN metastasis greater than 2mm and 2% had metastasis greater than 51mm.10,11
e Discussion with the patient should include information on the expected risk of complications and expected survival benefit with each of the options (dissection vs observation).
f Committee assessment of melanoma for possibility of entering a clinical trial of an adjuvant agent.
g Currently, the only adjuvant agent approved in Spain for patients with melanoma is interferon. Any adjuvant agent authorized for use in clinical practice should be assessed at the same point in the algorithm.
h pTb, ulcerated primary tumor. Maximum benefit of interferon as an adjuvant has been observed in patients with an ulcerated primary tumor.22–24
i In patients with stage IIIB-IIIc disease and high risk criteria, the recommendation of positron-electron tomography-computed tomography every 6 months during the first 2 years and annually between the third and fifth year can be considered.37
Regardless of the decisions about dissection or observation, those patients with a tumor burden greater than 1mm should be considered candidates for clinical trials of adjuvant agents because of the greater risk of regional and distant recurrence (Fig. 1). If no clinical trials are available, and until one of the adjuvant regimens with immunotherapy or targeted therapy is authorized in Spain, treatment with interferon should be offered, particularly in patients with an ulcerated primary tumor. All these patients should undergo a program of intensive ultrasound follow-up regardless of whether they receive adjuvant therapy or not. In addition, with the aim of early identification of possible systemic progression, it is recommended to continue applying imaging protocols indicated in the clinical guidelines and current protocols (computed tomography, magnetic resonance imaging, positron-electron tomography-computed tomography) (Fig. 1).
Therapeutic lymph node dissection should be reserved for those patients who develop lymph node metastasis during follow-up, as identified by physical examination or imaging studies (Fig. 1).
ConclusionTreatment of patients with melanoma is undergoing a paradigm shift that offers the opportunity of longer survival with lower morbidity as procedures become less invasive. From this new perspective, current evidence demands a reflection about abandoning lymph node dissection in patients with positive SLN status in order to generate homogeneous and consensus decisions in a greater number of melanoma units. As concluded by a renowned oncology surgeon in the editorial that prefaced the results of the MSLT-II trial in the New England Journal of Medicine, “If this aggregate of data is insufficient to extinguish the enthusiasm for complete lymph node dissection, then it is unclear what more is required.”38
Conflicts of InterestThe authors declare that they have no conflicts of interest.
We thank Dr. Sergi Vidal Sicart of the Nuclear Medicine Department of the Hospital Clínic in Barcelona, Spain, coauthor of the MSLT-II publication, for critically reviewing the manuscript.
Please cite this article as: Moreno-Ramírez D, Boada A, Ferrándiz L, Samaniego E, Carretero G, Nagore E, et al. Disección ganglionar en el paciente con melanoma y metástasis en el ganglio centinela: propuesta de decisión basada en la evidencia actual. Actas Dermosifiliogr. 2018;109:390–398.