A 36-year-old woman with no relevant personal history attended the clinic because of an asymptomatic lesion of 8 years duration on the left leg. The lesion was growing slowly, with constant scaling. The patient could not recall any trigger associated with this lesion.
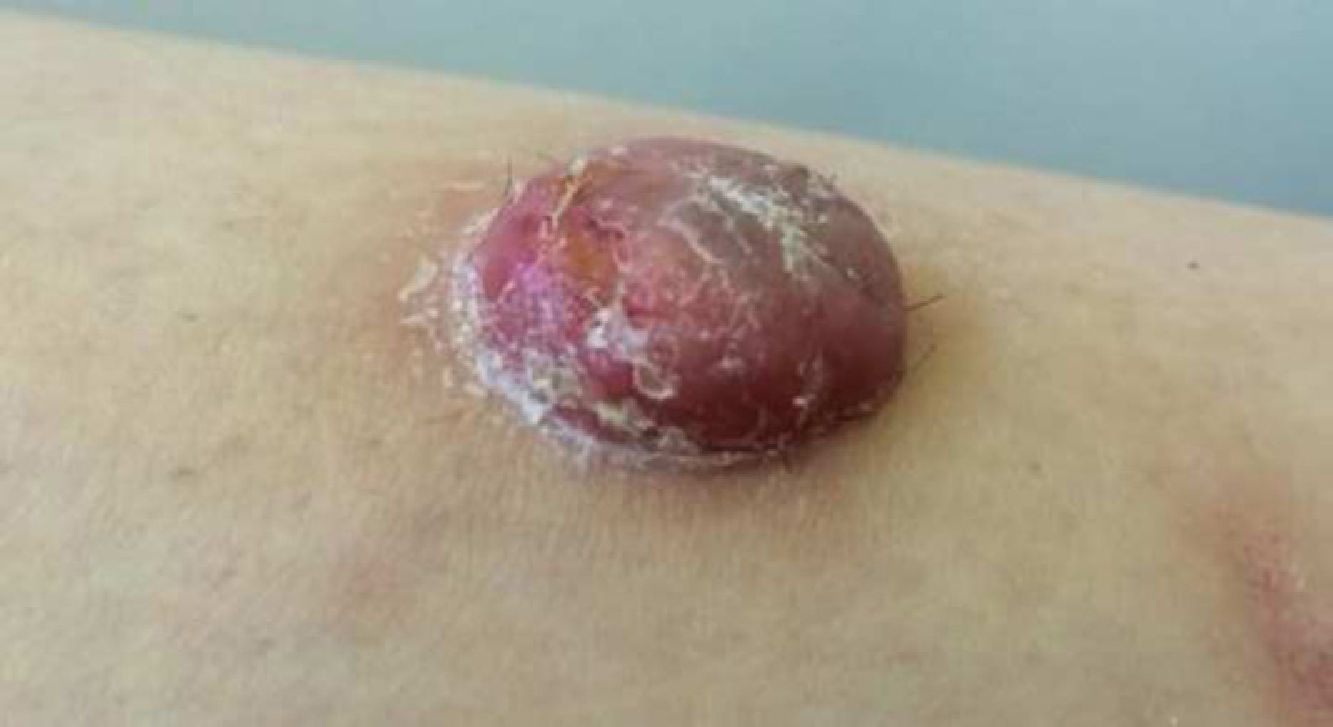
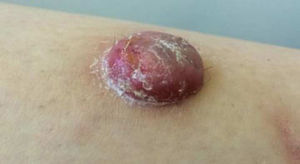
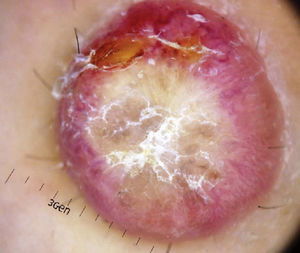
Physical ExaminationThe physical examination demonstrated the presence of a dome-shaped, erythematous lesion of hard consistency with superficial scaling, measuring 1.5cm, on the anterior aspect of the left leg (Figure 1A and B).
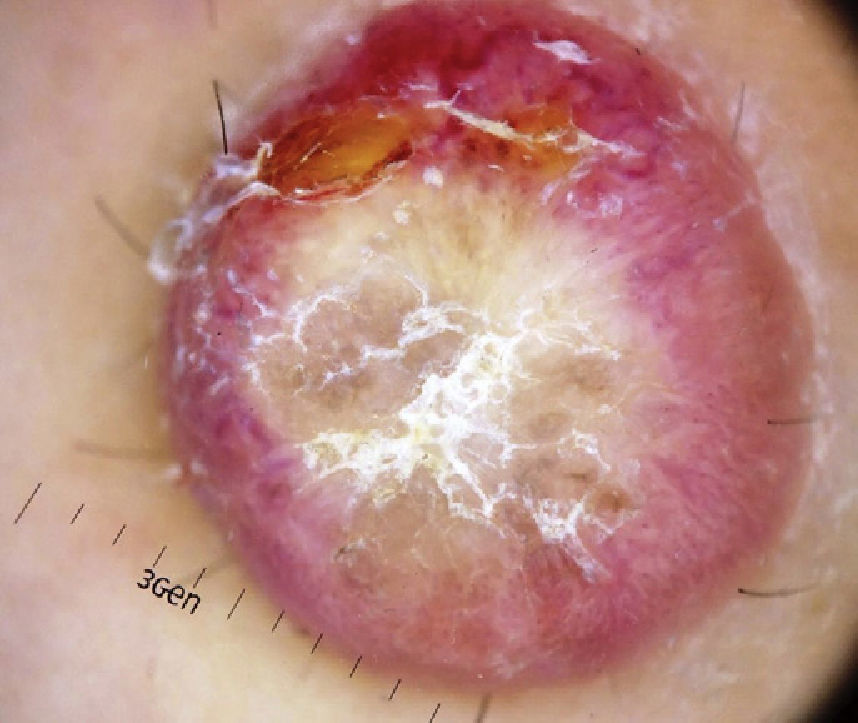
Dermoscopy examination revealed a central whitish-yellow, scaly lesion with traces of irregularly distributed pigment, surrounded by a diffuse, vascularized erythematous area, with some polymorphous-atypical vessels (Figure 2). There were no additional findings in the physical examination.
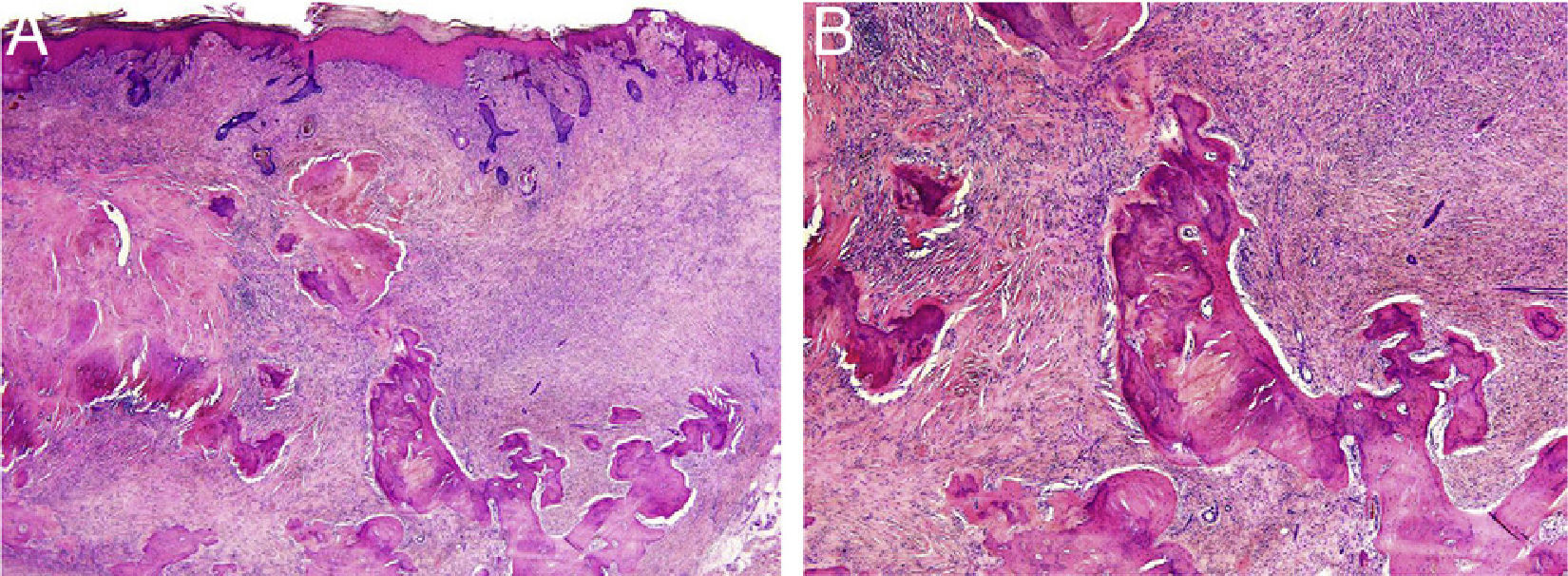
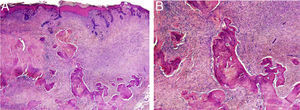
HistopathologyThe histopathology study showed an epidermis with focal ulceration, parakeratosis, and acanthosis with follicular induction. A tumor was located in the dermis, composed of spindle cells with vacuolated eosinophilic cytoplasm, arranged in a storiform pattern, embedded in a collagenous stroma with multiple foci of lamellar bone spicules but no osteoblasts or osteoclasts (Figure 3A and B).
What was the diagnosis?
DiagnosisDermatofibroma with metaplastic bone formation on the leg.
Course and TreatmentThe lesions were completely excised and, given their benign nature, the surgical margins were not extended.
RemarksDermatofibroma is a complex entity with a wide range of clinical, dermoscopic, and histopathologic features, probably as a result of a series of as yet unknown triggers. The case presented is the second report of a dermatofibroma with metaplastic bone formation without osteoclast-type giant cells.1 Recognition in the histopathological study is very important, as identification of metaplastic bone formation in the skin opens up a wide range of differential diagnoses, from inflammatory-reactive processes to benign and malignant skin tumors.
Usually, dermatofibromas present as a solid papule or nodule of red or coffee color with a smooth or keratotic surface. The dimple sign can be observed with lateral compression, but this is not exclusive to dermatofibroma and may not be very evident. In the dermoscopy examination, the most frequent pattern is a central white scar-like patch, with a fine peripheral pigment network.1–3 This pigmented network is not often distributed irregularly throughout the lesion (2.7%).2 The presence of vascular structures has been reported in 50% of cases.2,3 Several dermoscopic findings and atypical patterns have been described. These are uncommon and simulate other tumors. Findings include homogenous or irregular pigmentation, multiple whitish patches, whitish network, coffee spots, crusty scabs, ulcers, fissures, irregular linear and polymorphic vessels, and globular structures.2 Differential diagnosis is therefore usually broad and the lesions are sometimes difficult to distinguish from melanoma, basal cell carcinoma and spindle cell carcinoma, dermatofibrosarcoma protuberans, Kaposi sarcoma, morphea, neurofibroma, or adnexal tumor.3 In these cases, surgical excision is necessary for pathology study to clarify diagnosis.
The key pathological findings are presence of a spindle-cell dermal tumor with fibrohistiocytic differentiation, without atypia, with trapped hyalinized collagen bundles in the periphery, variable hemorrhage and hemosiderin deposition, and epidermal changes (acanthosis and follicular induction). There are several histopathological variants according to the predominant microscopic component, with fibrocollagenous, histiocytic, sclerotic, cellular, hemosiderotic, and cobblestone subtypes, among others.3 The presence of metaplastic bone formation in dermatofibroma is rare, with few cases reported and no dermoscopic description of the lesion.4–6 In general, osteoclast-like giant cells are present.
The presence of bone tissue in the skin is uncommon, and can be explained by a metaplastic process in response to trauma or local inflammatory processes. The most common cutaneous tumors with metaplastic bone formation are melanocytic nevi, basal cell carcinomas, and pilomatrixomas.4 The presence of metaplastic bone tissue in the dermis and hypodermis may be a distraction during pathology study and mask the main lesion, particularly when present in substantial amounts, as in the case presented. Metaplastic bone formation may correspond to the main component in certain tumors or be a process secondary to the main lesion. Reactive processes with metaplastic bone tissue include scarring and panniculitis ossificans. It is the main component of osteomas, osteosarcomas, ossifying fibromyxoid tumor, and osteogenic melanoma, or may be a secondary process in pilomatrixoma, melanocytic nevi, trichilemmal or pilar cysts, and hemangiomas, among others.5–7
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Abarzúa-Araya A, Ortiz-Lazo E, González-Bombardiere S. Tumor eritemato-descamativo en la pierna de largo tiempo de evolución. Actas Dermosifiliogr. 2017;108:153–154.