Lichen sclerosus is a chronic inflammatory disease that can progress to malignancy. The literature indicates an association with anogenital squamous cell carcinoma and verrucous carcinoma. Two pathogenic pathways, differentiated vulvar and penile intraepithelial neoplasias, which have recently been described in relation to squamous cell carcinoma, are both highly associated with genital lichen sclerosus independently of human papilloma virus (HPV) infection. Furthermore, tumor-promoting molecular changes unrelated to HPV infection have been demonstrated and may explain the malignant potential of lichen sclerosus. The possible relationship between HPV and genital lichen sclerosus currently remains open to discussion, and the prognostic importance of the overlapping of these 2 diseases is still unclear. This review considers the relationship between lichen sclerosus and squamous cell and verrucous carcinomas, the possible oncogenic mechanisms involved, and their possible association with HPV infection.
El liquen escleroso (LE) es una enfermedad inflamatoria crónica con un potencial maligno conocido. En la literatura se recogen datos de su asociación tanto con el carcinoma epidermoide (CE) como con el carcinoma verrucoso de localización anogenital. Recientemente se han descrito dos modalidades de neoplasias intraepiteliales de vulva y pene, el Vulva Intraepithelial Neoplasia (Neoplasia Intraepitelial Vulvar [VIN]) y el Penile Intraepithelial Neoplasia (Neoplasia Intraepitelial de Pene [PIN]) diferenciados, relacionadas con el CE genital. Ambas son entidades altamente ligadas al LE genital e independientes de la infección por el virus del papiloma humano (VPH). Además, se han demostrado alteraciones moleculares oncogénicas independientes de la infección por VPH que podrían explicar el potencial maligno del LE por sí mismo. La posible relación entre el VPH y el LE genital sigue siendo un tema controvertido en el momento actual, y se desconoce la implicación pronóstica cuando coexisten ambas entidades. En el presente artículo revisaremos la relación del LE con el carcinoma epidermoide y el carcinoma verrucoso, los mecanismos oncogénicos implicados, así como su posible asociación con el VPH.
Lichen sclerosus is a chronic inflammatory skin disease that is common in white postmenopausal women and affects between 1 in 1000 and 1 in 300 individuals in the general population.1 It presents on an anogenital site in 85%–98% of cases and on an extragenital site in only 15%–20%.2 The exact causes are not known, but autoimmune, genetic, hormonal, and infectious factors have been implicated. Clinically, lichen sclerosus presents as polygonal papules that coalesce to form pearly plaques. Sometimes, telangiectasias, fissures, ulceration, and internal erosion are associated with the papules. It can be asymptomatic or associated with pruritus and a local burning sensation. Histologic study shows orthokeratotic hyperkeratosis, hydropic degeneration of the basal cells, edema of the upper dermis, and homogenization of collagen associated with a predominantly lymphocytic inflammatory infiltrate. Treatment is with high-potency corticosteroids (level A evidence) and topical immunomodulators (level B and C evidence).3 Given the potential for long-term malignancy, check-ups at least every 6 months are recommended for patients with anogenital lichen scerlosus.4
Human papillomavirus (HPV) is a DNA virus of the Papovaviridae family. To date, more than 100 different types have been identified. Of these, 5 genera (α, β, γ, μ, and ν) only affect humans. α-HPVs (the mucosal form) are implicated in cervical cancer, genital carcinomas, and head and neck carcinomas while β-HPVs are associated with verruciform epidermodysplasia and nonmelanoma skin cancers such as squamous cell carcinoma (SCC) in particular. γ-HPV and ν-HPV are usually associated with benign lesions, although they too have been implicated in the development of different nonmelanoma skin cancers.5 The exact oncogenic mechanism of HPV has not yet been elucidated. Cellular abnormalities in the p53 gene and the retinoblastoma gene caused by viral E6 and E7 proteins, encoded by mucosal HPV, are thought to play a role in cellular immortality. In the case of subtypes with a lower oncogenic potential (β and γ types), environmental factors such as ultraviolet radiation are required to favor cell proliferation.5
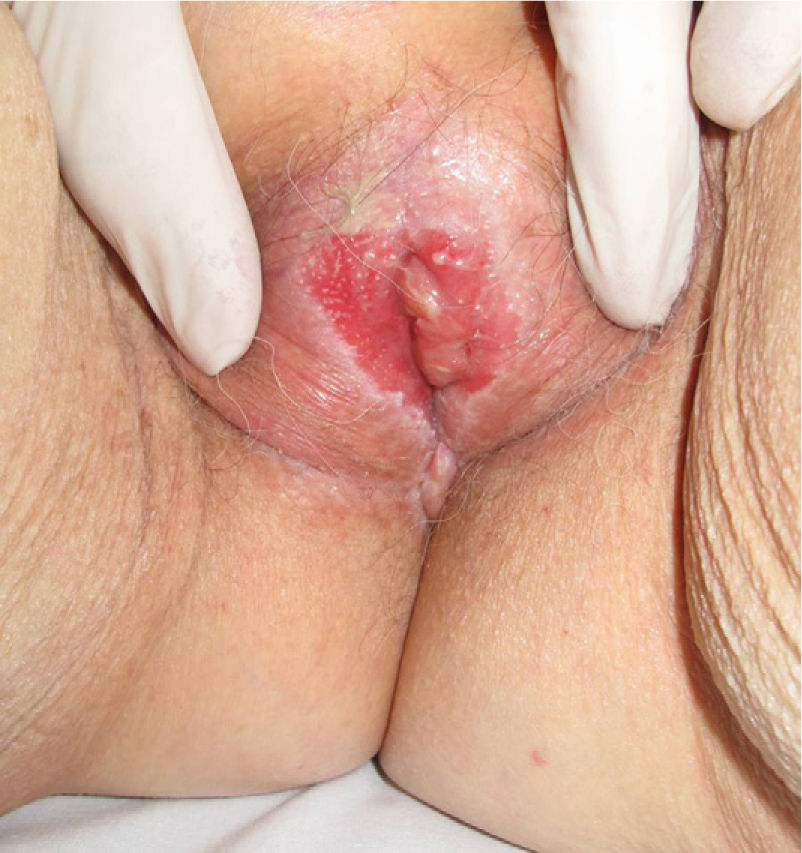
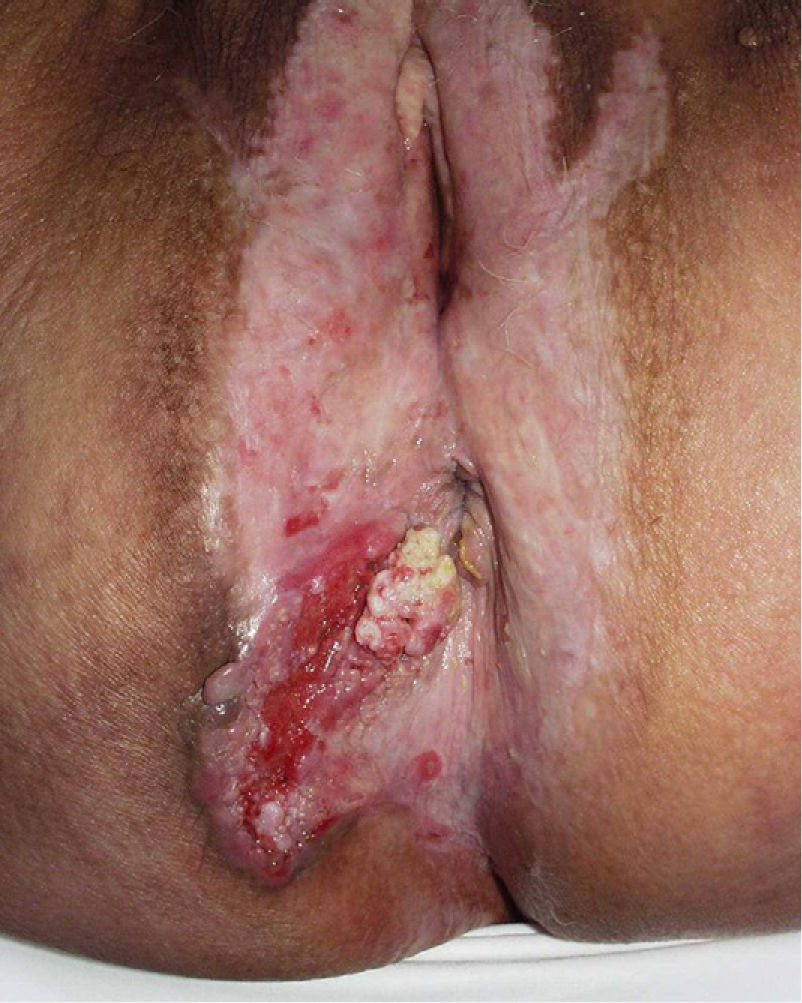
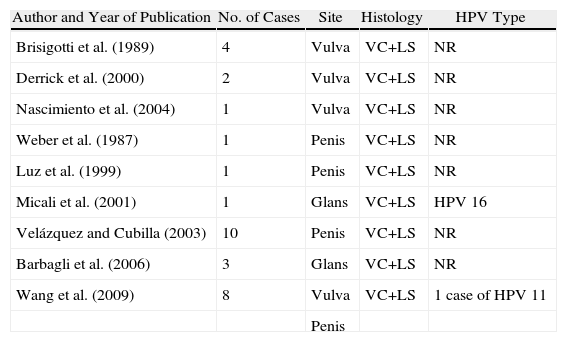
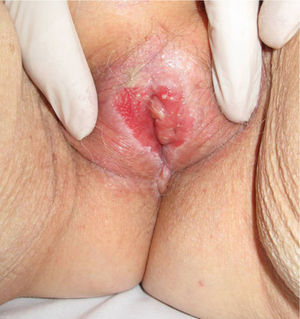
Association between Lichen Sclerosus and SCCLichen Sclerosus and SCC in WomenThe association between anogenital lichen sclerosus and SCC (Figs. 1 and 2) has been extensively reported in the literature. We only found 1 isolated report of SCC occurring on extragenital lichen sclerosus, so we cannot rule out the possibility that it was a chance finding.6
Between 3% and 7% of vulvar SCCs occur on vulvar lichen sclerosus.7 Careful histologic study of samples from patients with vulvar SCC shows evidence of lichen sclerosus in 60%.4 Derrick et al.8 published a retrospective observational study of 23 women with anogenital SCC, 8 (34%) of whom had lichen sclerosis of several years’ duration. An association between lichen sclerosus and vulvar SCC was found in 14 (31%) of the 44 patients studied by Chiesa-Vottero et al.,9 by 7 (50%) of the 14 patients studied by Poulsen et al.,10 and by 47 (61%) of the 78 patients studied by Leibowitch et al.11 In 1995, Carli et al.12 reported an incidence of vulvar SCC on lichen sclerosus of 1.4% (3/211) after 20 months of follow-up. Few other prospective studies on the topic have been published in the literature. Overall though, the evidence suggests that lichen sclerosus is an HPV-independent carcinogenic factor. This corroborates its potential for malignant transformation and the need for long-term follow-up in these patients.
Although the exact carcinogenic mechanism of lichen sclerosus is still under debate, several HPV-independent hypotheses have been put forward. Ueda et al.13 demonstrated cellular monoclonality in 6 patients with vulvar lichen sclerosus but no HPV infection or clinical manifestations indicative of associated SCC. Such an observation pointed to the malignant potential of lichen sclerosus in absence of clinical manifestations that might lead to suspicion of neoplastic proliferation. Rolfe et al.14 observed mutations in the p53 gene in 10 out of 12 patients with lichen sclerosus and in 7 of these 10, the mutation was located in the same codon as the one implicated in the SCC lesions. The authors therefore concluded that they were dealing with another carcinogenic mechanism, independent of HPV, for the development of anogenital SCC. Mutations in the PTEN gene in some differentiated vulvar SCCs and vulvar intraepithelial neoplasias (VINs) have also been observed, as well as microsatellite instability in 20% and 12% of differentiated VINs and lichen sclerosus lesions, respectively.15 Finally, in 2010, a study was published that demonstrated hypermethylation of genes such as RASSF2A, MGMT, and TSP-1 in SCC only when associated with lichen sclerosus.16 It seems that chronic inflammation associated with lichen sclerosus could lead to molecular abnormalities that in turn could facilitate neoplastic proliferation.
The precursor lesion in vulvar SCC is VIN. Traditionally, the literature described 3 grades of VIN: VIN 1, VIN 2, and VIN 3, according to whether the epithelial dysplasia was mild, moderate, or severe, respectively. This classification was used to ensure alignment with the well-known classification of cervical intraepithelial neoplasia (CIN). The VIN terminology was, however, modified in 2004, and the term VIN 1 fell into disuse. Currently, we only have 2 categories, VIN 2 and VIN 3, to refer to moderate and severe vulvar dysplasias.17
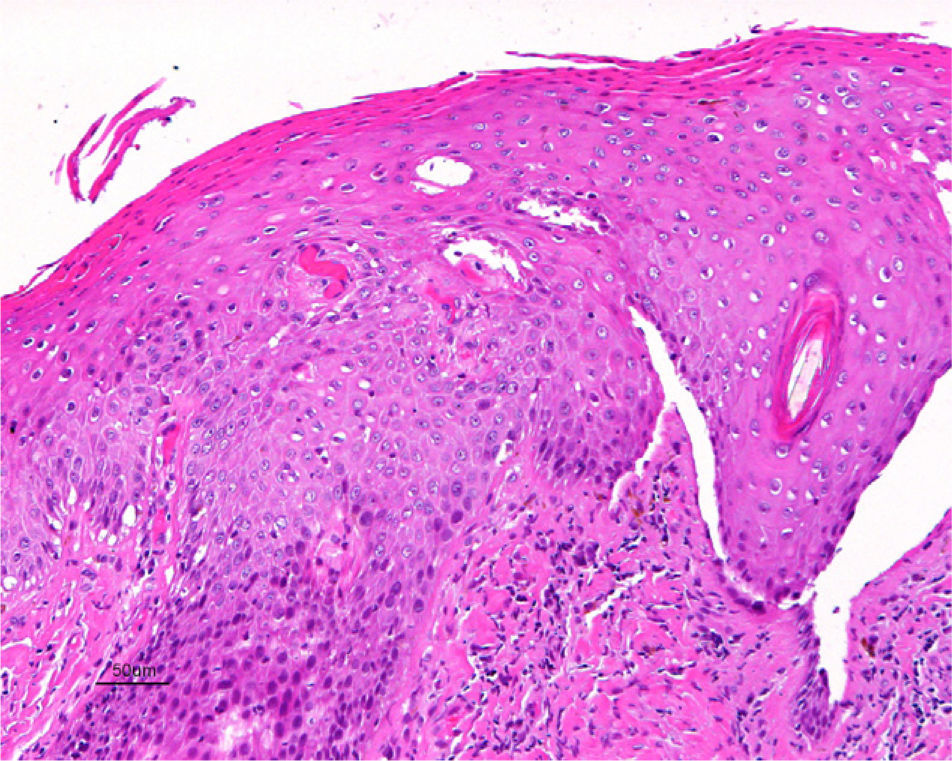
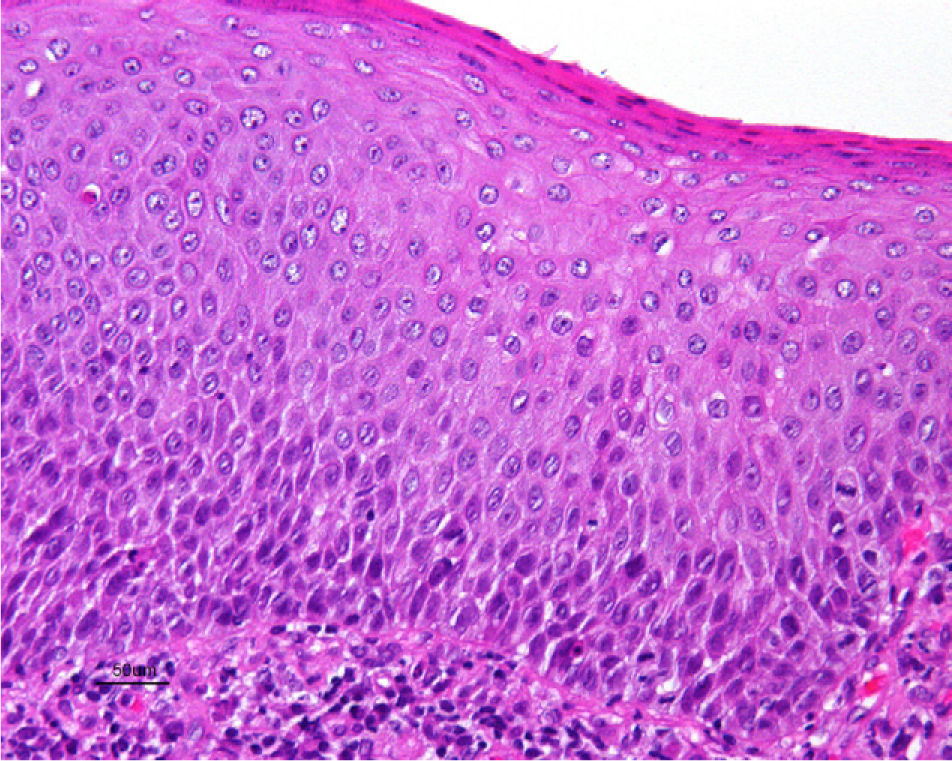
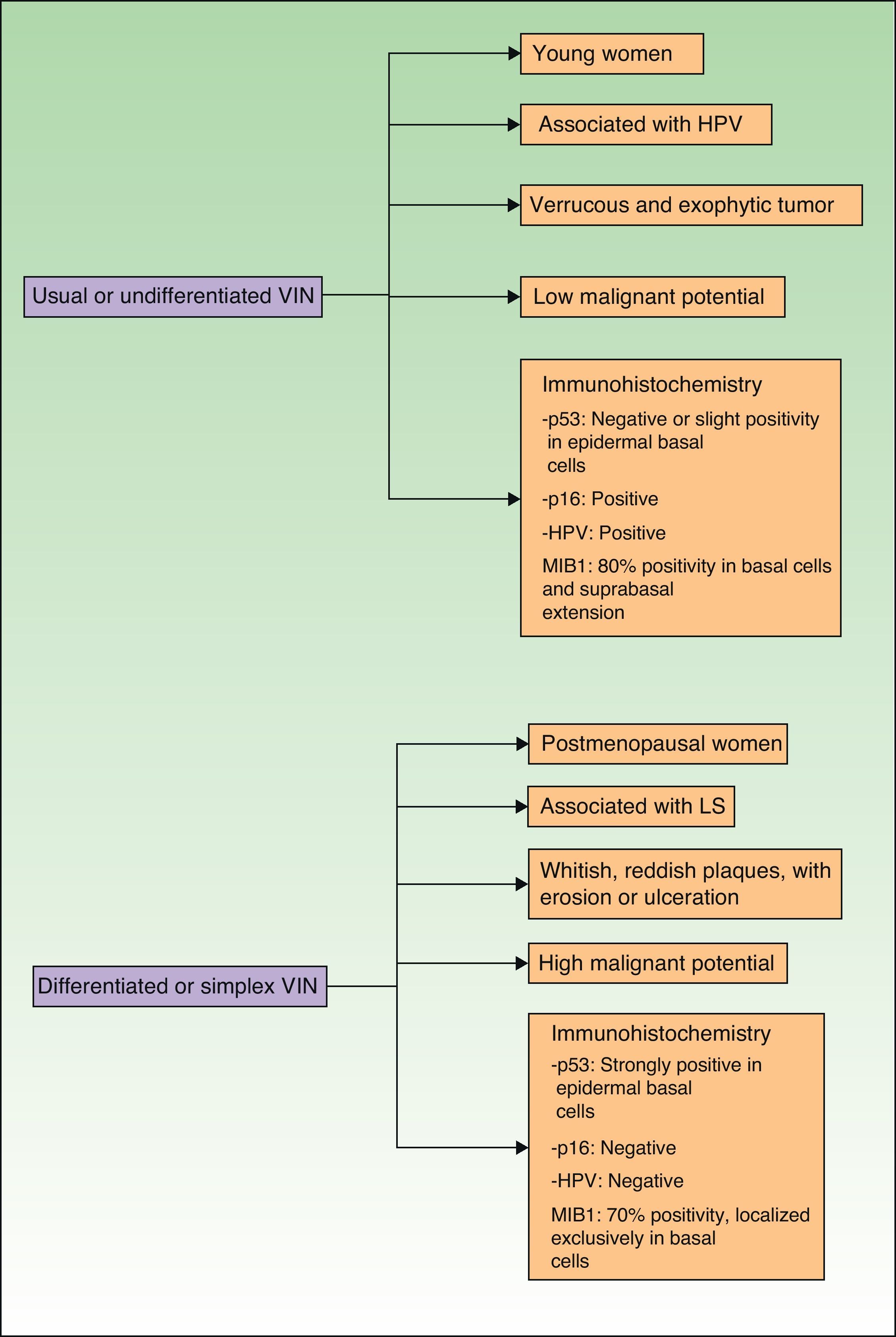
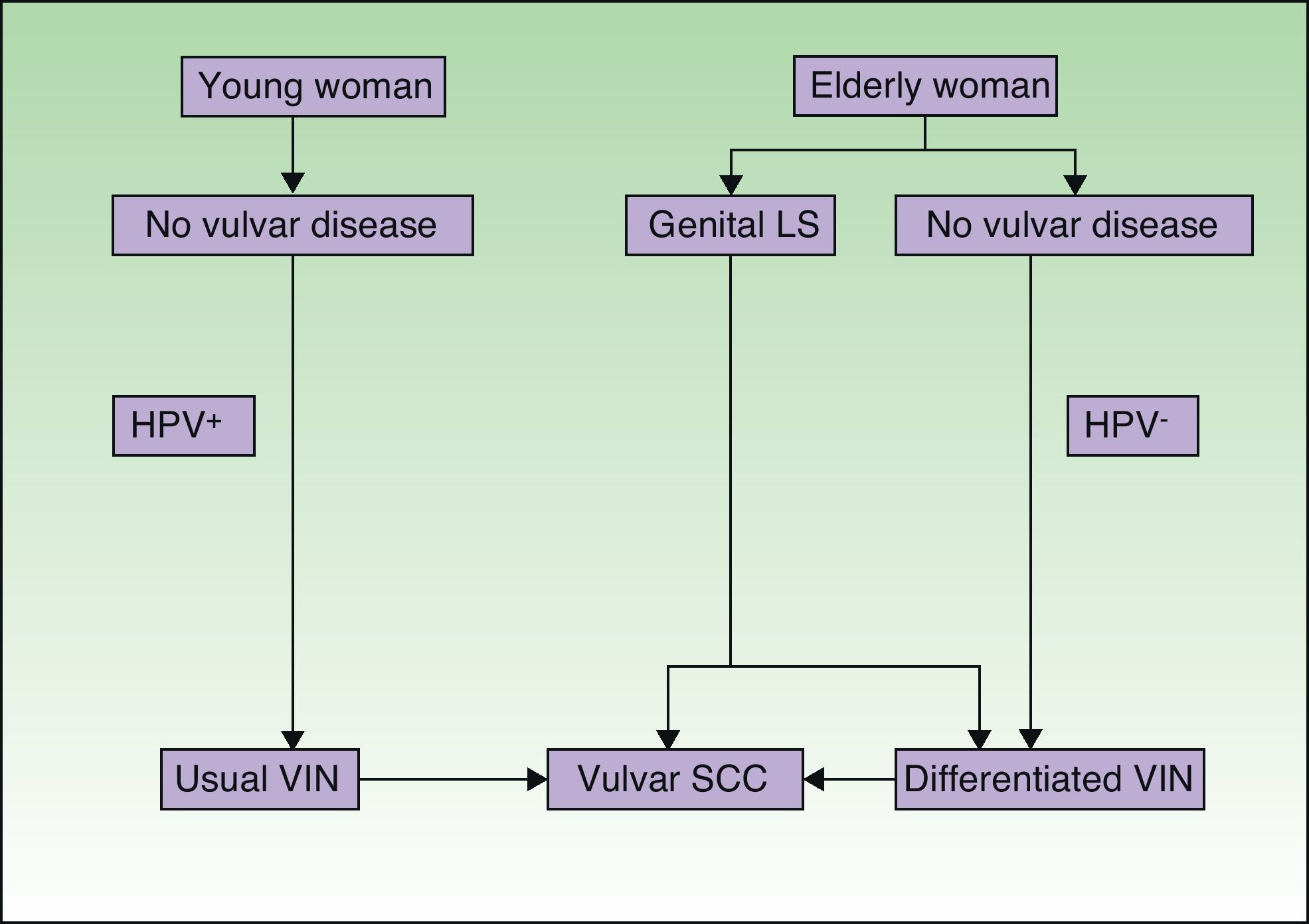
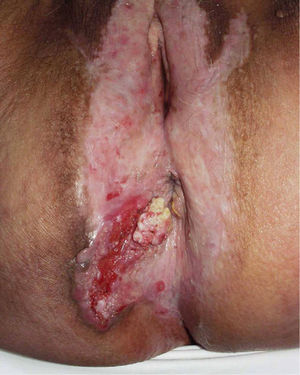
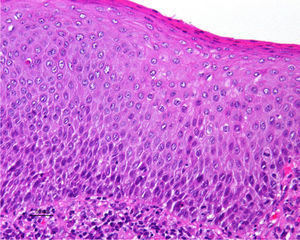
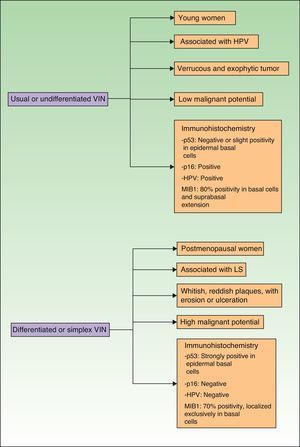
Jones et al.18 and Neill et al.19 established 2 clinicopathologic types of VIN: usual VIN (Fig. 3) and differentiated or simplex VIN (Fig. 4). The latter accounts for between 2% and 10% of all diagnosed VIN. Fig. 5 shows the clinical and immunohistochemical characteristics of each subtype.17,20 Differentiated or simplex VIN is the precursor of vulvar SCC associated with lichen sclerosus (Fig. 6).
Epidemiological, clinical, prognostic, and immunohistochemical characteristics of usual VIN and differentiated VIN. HPV indicates human papillomavirus; VIN, vulvar intraepithelial neoplasm; p53, p53 tumor suppressor gene; p16, p16 protooncogenINK4A; MIB1, monoclonal antibody against Ki67 nuclear antigen; LS, lichen sclerosus.
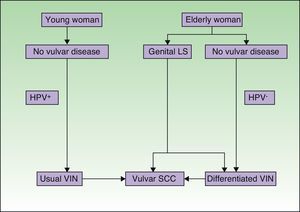
Pathogenic pathways implicated in vulvar squamous cell carcinoma according to Neill et al.8 LS indicates lichen sclerosus; HPV, human papillomavirus; VIN, vulvar intraepithelial neoplasm; SCC, squamous cell carcinoma.
In 2011, Van de Nieuwenhof et al.21 published a study showing that a high percentage of patients with lichen sclerosus that progressed to SCC should have been diagnosed initially with differentiated VIN. Of a total of 60 cases of lichen sclerosus associated with SCC, 42% were reclassified in a second histologic study as differentiated VIN. The majority of reclassified lesions had initially been diagnosed as lichen sclerosus associated with HPV-dependent lesions, as they showed signs of cellular atypia or epidermal hyperplasia. The authors observed that the time to progression to SCC was shorter when the precursor pathological process was differentiated VIN (28 months on average) compared to when it was lichen sclerosus (84 months on average). According to previous studies by the same authors, up to 33% of differentiated VIN progressed to SCC,21 in contrast to the percentage of 3%–7% cited earlier for lichen sclerosus. Moreover, these authors found that the histologic presence of dyskeratosis, hyperkeratosis, hyperplasia, and atypia in the basal keratinocytes of patients diagnosed initially with lichen sclerosus was associated with a greater risk of vulvar SCC.
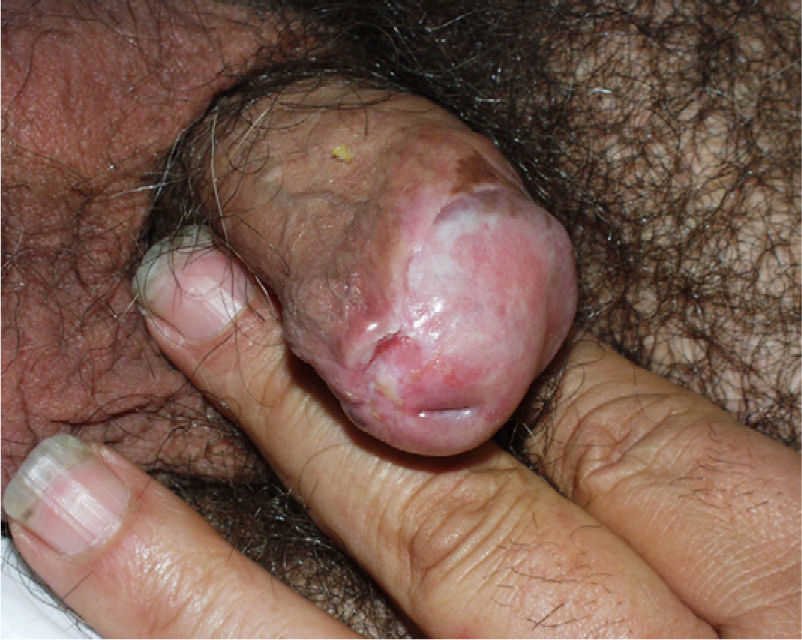
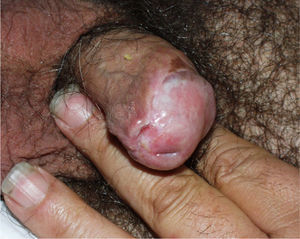
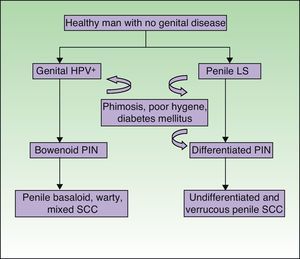
Lichen Sclerosus and SCC in MenThe association between lichen sclerosus and penile SCC is less extensively documented in the literature (Fig. 7). It has been postulated that concomitant factors such as diabetes mellitus, recurrent balanitis, phimosis, HPV infection, and poor hygiene could be etiopathogenic factors implicated in penile SCC.22
Data on penile SCC in the literature are variable and inconclusive, with 5.8%–30% of patients with lichen sclerosus having malignant disease.23,24 In a study of 130 men with genital lichen sclerosus, Barbagli et al.25 found that 8.4% of lesions were malignant (SCC, verrucous carcinoma) or premalignant (Queyrat erythroplasia). The time interval between diagnosis of lichen sclerosus and the development of SCC was 14–30 years. In their series of 54 patients with lichen sclerosus, Campus et al.26 reported 2 cases (3.7%) of penile SCC. Wallace27 described 2 patients out of 44 (4.5%) with lichen sclerosus who developed penile SCC. According to the above studies, the risk of developing penile SCC in patients with lichen sclerosus ranges from 4% to 8%. If we now analyze how many patients with penile SCC have histologic evidence of lichen sclerosus, the percentage is 32%–50%. Velazquez and Cubilla28 observed that 68 (32.8%) out of 207 of their patients with penile SCC had histologic evidence of lichen sclerosus. Perceau et al.29 found histologic evidence of lichen sclerosus in 8 (44%) of their 18 patients with penile SCC, while Powell et al.30 reported similar evidence in 8 of their 20 patients (40%).
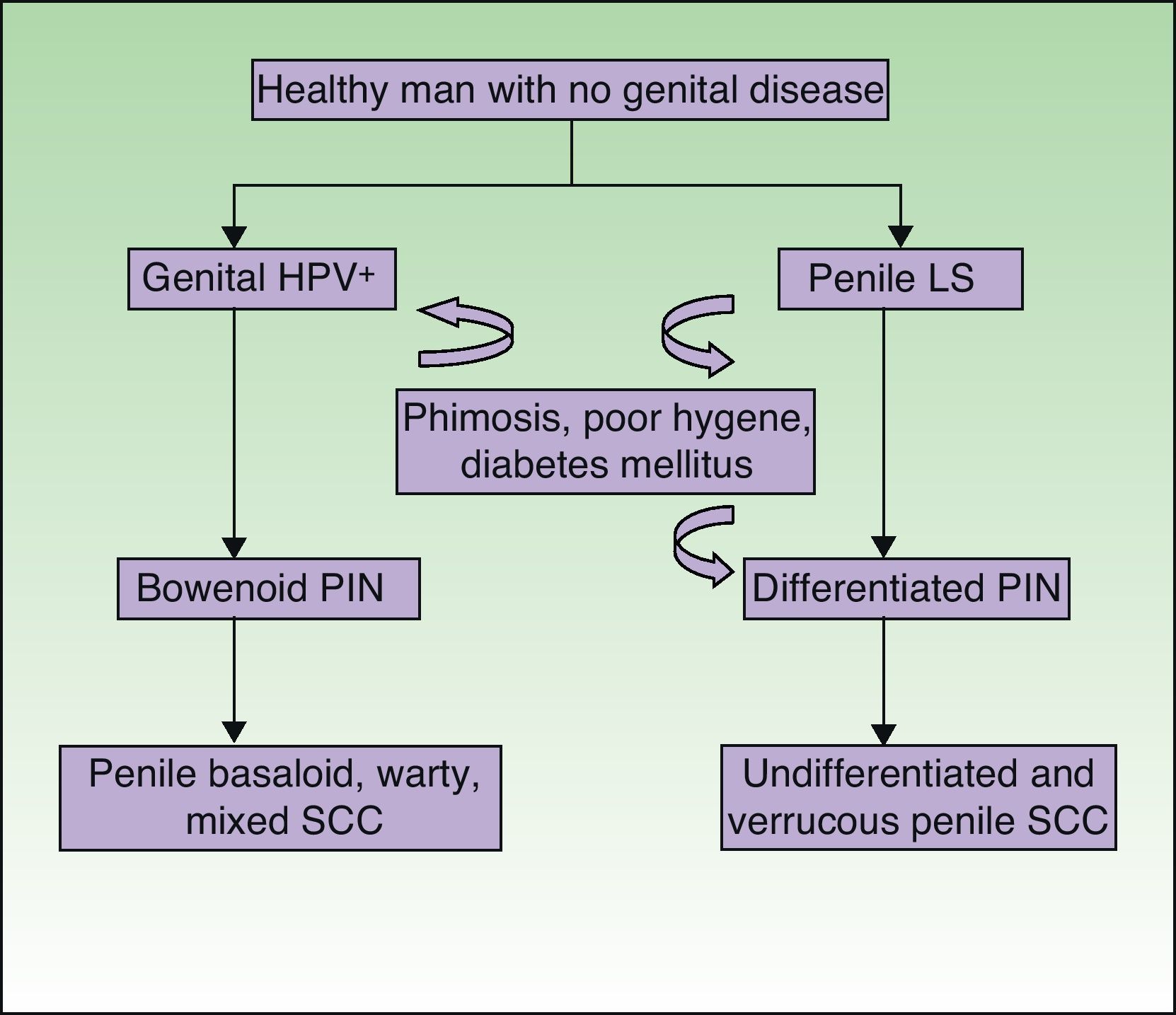
Recently, by analogy with the VIN categories, the grades PIN 2 and PIN 3 have been introduced to refer to penile intraepithelial neoplasm with moderate or severe dysplasia, respectively. Two forms of PIN have been described: differentiated PIN, equivalent to differentiated VIN and often associated with penile lichen sclerosus, and undifferentiated or Bowenoid PIN, equivalent to usual VIN and associated with HPV infection (Fig. 8). Renaud-Vilmer et al.31 performed a retrospective analysis of 53 patients with penile SCC with the aim of assessing the histologic abnormalities observed in lesions adjacent to the tumor, as well as the possible relation between these lesions and different types of SCC. HPV-associated SCCs were warty, basaloid, or mixed in nature, and accounted for approximately 45% (24 out of 53) of all penile SCCs in their series. They observed underlying Bowenoid PIN in all cases of these carcinomas. The etiopathogenic role of HPV in Bowenoid PIN was also demonstrated by Chaux et al.,32 who found that overexpression of the p16 geneINK4a—which is strongly associated with HPV infection—was positive in penile basaloid and warty SCCs (P<.0001) but not in SCCs associated with differentiated PIN and lichen sclerosus. The histologic subtypes of SCC not associated with HPV infection are the usual and verrucous forms (well-differentiated variant of SCC with no evidence of associated koilocytosis) and accounted for 55% of all cases (29 out of 53) in the study by Renaud-Vilmer et al.31 Underlying lichen sclerosus was detected in 93% of these carcinomas. Likewise, no association has been found between HPV infection and sarcomatoid SCC of the penis in studies published to date.33 Renaud-Vilmer et al.31 also observed that the presence of acanthosis and orthokeratotic hyperkeratosis, the so-called squamous cell hyperplasia, was frequently associated with differentiated PIN and lichen sclerosus. Similar findings were also reported by Van de Nieuwenhof et al.21 in their study of vulvar SCC, where squamous cell hyperplasia was more frequent in patients with lichen sclerosus that progressed to SCC. Lichen sclerosus is an HPV-independent etiopathogenic factor in some types of usual and verrucous penile SCC, with differentiated PIN and squamous cell hyperplasia being the histological variants associated with and implicated in carcinogenesis.
Association Between Lichen Sclerosus and Verrucous CarcinomaAnogenital verrucous carcinoma, also known as Buschke-Löwenstein tumor, is a low-grade indolent tumor that rarely undergoes local metastasis.
There are 29 cases of anogenital verrucous carcinoma and lichen sclerosus reported in the literature (Table 1). Of these, 12 were on the vulva and 17 on the penis. Only 4 were tested for HPV infection, with positive results in 2 patients, 1 with type 16 HPV infection and the other with type 11 HPV infection. In a retrospective study by Wang et al.,34 the objective was to assess a possible association between lichen sclerosus and verrucous carcinoma. In total, 13 patients with genital and perianal verrucous carcinomas were included (5 with a vulvar lesion, 3 with a penile lesion, and 5 with a perianal lesion). None of the perianal verrucous carcinomas reported were associated with lichen sclerosus. The authors observed that the 5 patients with vulvar verrucous carcinoma had associated lichen sclerosus, whereas only 1 of the patients with penile verrucous carcinoma and none of those with perianal verrucous carcinoma had evidence of lichen sclerosus. Evidence of local recurrence was not observed in patients with no associated lichen sclerosus, whereas 3 (50%) of the 6 patients with lichen sclerosus had recurrence. According to the authors, the chronic inflammation and oxidative cell damage caused by lichen sclerosus could explain the propensity for local recurrence.
Published Cases of Verrucous Carcinoma (VC) Associated With Genital Lichen Sclerosus (LS).
| Author and Year of Publication | No. of Cases | Site | Histology | HPV Type |
| Brisigotti et al. (1989) | 4 | Vulva | VC+LS | NR |
| Derrick et al. (2000) | 2 | Vulva | VC+LS | NR |
| Nascimiento et al. (2004) | 1 | Vulva | VC+LS | NR |
| Weber et al. (1987) | 1 | Penis | VC+LS | NR |
| Luz et al. (1999) | 1 | Penis | VC+LS | NR |
| Micali et al. (2001) | 1 | Glans | VC+LS | HPV 16 |
| Velázquez and Cubilla (2003) | 10 | Penis | VC+LS | NR |
| Barbagli et al. (2006) | 3 | Glans | VC+LS | NR |
| Wang et al. (2009) | 8 | Vulva | VC+LS | 1 case of HPV 11 |
| Penis |
Abbreviations: HPV, human papillomavirus; NR, not reported.
The exact mechanisms responsible for the association of lichen sclerosus and verrucous carcinoma are not clear, but factors such as HPV infection, mutations in the p53 gene, chronic inflammation, and oxidative stress could all play a part.34
The prevalence of HPV infection in patients with lichen sclerosus is still subject to much debate, and a wide range of percentages can be found in the literature. In 18%–67% of patients, the most common genotypes found are 6 and 11, both of which are implicated in the pathogenesis of verrucous carcinoma.34 Prolonged treatment with the highly potent topical corticosteroids prescribed for lichen sclerosus could favor reactivation of latent HPV infections and increased carcinogenis.35 On the other hand, it has been demonstrated that mutations in the p53 gene in patients with vulvar lichen sclerosus36 and greater oxidative tissue damage resulting from impaired enzymatic antioxidant defense could explain the greater oncogenic risk.37
Case reports have been published of basal cell epitheliomas, melanomas, and Merkel cell carcinomas located on anogenital lichen sclerosus, but lichen sclerosus has not been shown to be associated with a greater incidence of these malignancies or to be implicated in their development.4
Association between Lichen Sclerosus and HPVThe prevalence of HPV infection in patients with lichen sclerosus varies from 1% to 21%.38 With the aim of investigating whether the prevalence of HPV infection was greater in patients with lichen sclerosus than in the healthy population, Nasca et al.38 studied 46 men with lichen sclerosus and compared them with a control group. With polymer chain reaction techniques to identify HPV infection, they found that 17.4% of patients with lichen sclerosus tested positive for HPV (75% of them with type 16 HPV) compared to 8.7% for the control group (odds ratio, 2.55). In a previous study of 86 patients with lichen sclerosus and penile SCC, the authors observed that 8 (9.3%) of the 86 patients were HPV-16 positive and the latency time between lichen sclerosus and the onset of SCC was less (average 15 years) in HPV-positive patients than in HPV-negative ones (average 23 years).39 The authors suggested that infection with HPV could accelerate the malignant process.
Vignale et al.40 reported that clinical improvement in their 2 HPV-positive patients with lichen sclerosus was only achieved after adding imiquimod for 3 months to topical corticosteroid treatment. The authors proposed 2 possible explanations for this observation: either the virus might be directly linked to development of lichen sclerosus or it was a contaminant unrelated to development of the disease.
Different hypotheses have been put forward to explain the association between lichen sclerosus and HPV. Patients with lichen sclerosus might have a greater risk of HPV infection as a result of genetic predisposition for defective viral clearance.30 On the other hand, prolonged use of potent topical corticosteroids could reactivate latent opportunistic high- and low-risk mucosotropic HPV infection.35 Finally, other authors suggest that patients with lichen sclerosus are more susceptible than healthy individuals to the development of malignancies caused by oncogenic HPV types.30
In contrast to the 2 studies commented earlier, Aidé et al.41 investigated the presence of HPV in 34 patients with vulvar lichen sclerosus, and made a comparison with 17 patients without vulvar disease (control group). None of the samples from patients with lichen sclerosus had concurrent HPV infection, although such infection was found in 23.2% of samples from the control group.
In short, there are studies that suggest that concurrent infection with HPV in patients with lichen sclerosus could be an extra oncogenic factor for the development of anogenital SCC. Currently, the data are still subject to debate. Larger studies with longer follow-up are needed to clarify whether this association is a chance occurrence or whether HPV may really have an etiopathogenic role in anogenital lichen sclerosus.
Conclusions- 1.
Anogenital lichen sclerosus should be considered as a condition with potential for malignant transformation to SCC. We should therefore ensure that these patients receive long-term follow-up.
- 2.
Although the risk of developing penile SCC is 4%–8% in patients with lichen sclerosus according to published series, there is histologic evidence of lichen sclerosus in between 32% and 50% of samples from patients with penile SCC. Similar findings have been reported for the association between vulvar SCC and lichen sclerosus. Thus, although widely accepted figures put the percentage of SCCs occurring on lichen sclerosus at 3%–7% of cases, histologic evidence of lichen sclerosus can be found in up to 60% of vulvar SCC studies.
- 3.
HPV-independent oncogenic molecular changes have been shown that could explain the malignant potential of lichen sclerosus in its own right.
- 4.
There are 2 clinicopathologic forms of vulvar SCC, usual VIN, which is associated with HPV infection, and differentiated VIN, which is the precursor for malignant transformation in cases of lichen sclerosus.
- 5.
By analogy with VIN, 2 variants of PIN have been described recently, the undifferentiated or Bowenoid form associated with HPV infection and differentiated PIN, which is the premalignant precursor of penile SCC in patients with lichen sclerosus.
- 6.
We do not have sufficient evidence of an association between HPV and lichen sclerosus, as the studies in the literature are contradictory.
The authors declare that they have no conflict of interest.
Please cite this article as: Gutiérrez-Pascual M, et al. Liquen escleroso y carcinoma escamoso. Actas Dermosifiliogr. 2012;103:21–8.