Although there is no doubt that biologic agents are an effective alternative for the treatment of moderate and severe psoriasis, anti-tumor necrosis factor α therapy has been associated with reactivation of latent tuberculosis infection. Tuberculin skin testing (TST) is used to diagnose tuberculosis infection but it has low specificity in patients who have received the Mycobacterium bovis BCG vaccine and low sensitivity in patients with altered cell-mediated immunity. In vitro assays based on the detection of interferon γ released by T cells stimulated by specific Mycobacterium tuberculosis antigens have emerged as an option for the diagnosis of tuberculosis infection. The results to date show that they are a viable alternative to TST thanks to their higher specificity and sensitivity. Furthermore, these assays are also proving to have high negative predictive value, meaning that we might be able to use them without TST in the short to medium term.
Las terapias biológicas representan una alternativa de indudable eficacia en la psoriasis moderada y grave. Sin embargo, existe una asociación entre el tratamiento con anti-TNF-α y la reactivación de la infección tuberculosa. La tuberculina se utiliza como herramienta para el diagnóstico de la infección tuberculosa, pero presenta una baja especificidad en pacientes vacunados con la BCG (Mycobacterium bovis bacilo de Calmette-Guérin) y una baja sensibilidad en pacientes con alteraciones de la inmunidad celular. En este sentido se han desarrollado diferentes metodologías in vitro que incorporan antígenos específicos de Mycobacterium tuberculosis para estimular células T sensibilizadas y detectar posteriormente IFN-γ liberado para el diagnóstico in vitro de la infección tuberculosa. Los resultados obtenidos hasta ahora muestran a estas como una alternativa real a la tuberculina, ya que presentan una mayor especificidad y sensibilidad. Estas técnicas, además, están demostrando un elevado valor predictivo negativo que hace que nos podamos plantear a corto-medio plazo su utilización sin necesidad de combinarla con la tuberculina.
Psoriasis is a chronic inflammatory disease that affects a considerable proportion of the population. It can be severe and is an important cause of occupational disability in middle-aged patients, resulting in considerable personal and socioeconomic impacts. Biologic therapy, particularly that involving anti-tumor necrosis factor (TNF) α agents, has emerged as a viable alternative for the treatment of psoriasis in cases when conventional systemic agents are either ineffective or contraindicated.1
The use of biologic therapy, however, requires prior evaluation and monitoring of patients, as serious bacterial infections have been reported in association with anti-TNF-α therapy. Particular consideration should be given to the risk of tuberculosis (TB), as a clear link has been established between anti-TNF-α therapy and reactivation of latent TB infection.2,3 The main route of TB infection is respiratory. Mycobacterium tuberculosis is inhaled into the pulmonary alveoli, where it is phagocytosed by alveolar macrophages. M tuberculosis is an intracellular pathogen; it avoids destruction by preventing the fusion of phagosomes with lysosomes and it replicates within the macrophage, which is eventually completely destroyed. The infected macrophage releases cytokines that attract neutrophils, lymphocytes, and more macrophages, creating a focus of inflammation. Type 1 helper (TH1) cells are responsible for mounting the immune response, as they secrete both TNF-α, which attracts more macrophages, and interferon (IFN) γ, which activates infected macrophages. TNF-α plays a key role in forming and maintaining the tuberculous granuloma and hence in containing infection. It is essential to rule out latent TB infection and, of course, active TB, in all patients due to start anti-TNF-α therapy.4
Diagnosis of TB InfectionThe tuberculin skin test (TST) has been used for over 100 years to diagnose TB infection. Tuberculin is obtained by concentration of the sterilized filtrate of a culture of M tuberculosis. The test is currently conducted with tuberculin purified protein derivative (PPD), which is a mixture of over 200M tuberculosis proteins. In Spain, the recommendation is to use tuberculin PPD RT23 with Tween 80 (Statens Serum Institut). The standard dose is 2 tuberculin units per 0.1 mL, which is bioequivalent to the recommended 5-unit dose of the international standard tuberculin, PPD-S.5
The TST is performed using the Mantoux technique, which consists of injecting 0.1mL of solution containing the corresponding tuberculin dose into the anterior surface of the forearm, with reading of results at 48 to 72hours. Sensitized individuals develop a delayed hypersensitivity reaction, which produces an induration at the site of injection. This immune response is mediated by TH1 cells that migrate to the site of injection and trigger the release of several cytokines on recognizing the antigens presented by major histocompatibility complex class II molecules. The cytokines then activate the macrophages, causing a reaction characterized by erythema and induration.
In order to reduce the risk of developing TB during treatment with biologic agents, current guidelines recommend that all information indicating past TB infection or recent contact with individuals with TB should be included in patients’ medical records. Other recommendations include the performance of a TST and a chest radiograph to check for signs of past infection or active disease. If the TST is positive (induration of ≥5mm at 72hours), the individual is considered to be infected. If an induration of less than 5mm is observed, a second test is performed 2 weeks later (to avoid the booster effect), and the result is considered positive for tuberculosis if the induration is 5mm or larger.5
The main disadvantage of the TST is that most of the proteins in the PPD are not specific to M tuberculosis. This reduces the specificity of the test, as individuals who have been exposed to nontuberculous mycobacteria (NTM) or who have received the Mycobacterium bovis BCG vaccine develop an immune response to PPD. Furthermore, the TST also has low sensitivity in patients with altered cell-mediated immunity, who are precisely the patients who need this test most due to their higher risk of developing active TB if infected. This reduced sensitivity is of particular relevance in patients with psoriasis who are candidates for biologic therapy as they will have been treated with immunosuppressives and may therefore have an altered immune response.
In Vitro Techniques Based on IFN-γ ReleaseImmunodiagnostic tests based on the in vitro quantification of the cellular immune response may provide an alternative for diagnosing TB infection. In recent years, a range of techniques have been developed to quantify this response using different mycobacterial antigens to stimulate sensitized T cells and detect the release of IFN-γ in vitro. In infected individuals, sensitized T cells release easily detectable quantities of IFN-γ when stimulated with M tuberculosis–specific antigens. The technology underlying these diagnostic methods is based on the principles of the enzyme-linked immunosorbent assay (ELISA) and the enzyme-linked immunospot (ELISPOT) assay, which, respectively, have given rise to 2 commercial IFN-γ release assays (IGRAs): QuantiFERON-TB Gold In-Tube test (QFT) (Cellestis) and T-SPOT.TB (Oxford Immunotec). Both tests have US Food and Drug Administration approval and use immunoassay technology to detect IFN-γ released by T cells stimulated with M tuberculosis–specific antigens. The success of the techniques depends on, among other factors, the antigens used. The use of region of difference (RD) 1 antigens, such as ESAT-6 and CFP-10, which are secreted by M tuberculosis complex and are not present in the BCG vaccine or NTM species, appears to be an extremely effective means of detecting TB infection.
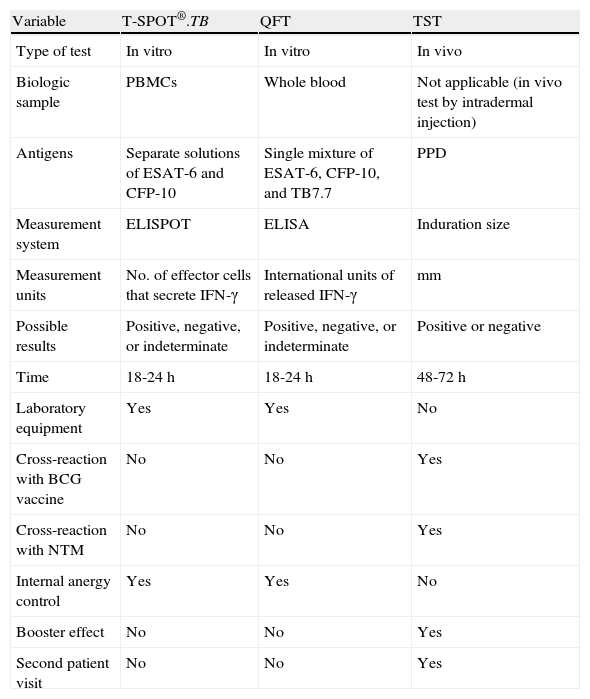
In QFT, IFN-γ release is measured by ELISA in whole blood samples, while in T-SPOT.TB, it is measured by ELISPOT in previously isolated peripheral blood mononuclear cells (PBMCs). One of the main differences between these 2 in vitro techniques is that in QFT, a single mixture of M tuberculosis–specific antigens (ESAT-6, CFP-10, and TB7.7) is used to stimulate whole blood, while in T-SPOT-TB, 2 separate mixtures (ESAT-6 and CFP-10) are used to stimulate PBMCs. The third antigen in QFT, TB7.7 (Rv2654), is an RD11-encoded antigen absent from the BCG vaccine and NTM species. Table 1 summarizes the main characteristics of QFT, T-SPOT.TB, and the TST.
Characteristics of the T-SPOT.TB Assay, the QuantiFERON-TB Gold In-Tube (QFT) Assay, and the Tuberculin Skin Test (TST).
| Variable | T-SPOT®.TB | QFT | TST |
| Type of test | In vitro | In vitro | In vivo |
| Biologic sample | PBMCs | Whole blood | Not applicable (in vivo test by intradermal injection) |
| Antigens | Separate solutions of ESAT-6 and CFP-10 | Single mixture of ESAT-6, CFP-10, and TB7.7 | PPD |
| Measurement system | ELISPOT | ELISA | Induration size |
| Measurement units | No. of effector cells that secrete IFN-γ | International units of released IFN-γ | mm |
| Possible results | Positive, negative, or indeterminate | Positive, negative, or indeterminate | Positive or negative |
| Time | 18-24 h | 18-24 h | 48-72h |
| Laboratory equipment | Yes | Yes | No |
| Cross-reaction with BCG vaccine | No | No | Yes |
| Cross-reaction with NTM | No | No | Yes |
| Internal anergy control | Yes | Yes | No |
| Booster effect | No | No | Yes |
| Second patient visit | No | No | Yes |
Abbreviations: ELISA, enzyme-linked immunosorbent assay; ELISPOT, enzyme-linked immunospot assay; IFN, interferon; NTM, nontuberculous mycobacteria; PBMC, peripheral blood mononuclear cells; PPD, purified protein derivative.
IGRAs have been reported to be more specific than the TST in BCG-vaccinated patients and also to correlate better with the degree of exposure to M tuberculosis.6–10 Recent studies have shown that these assays have predictive value for the development of active TB,11–14 and there is increasing evidence that they are robust tools for diagnosing TB infection in individuals with a deficient cellular immune response,15,16 as can occur in children,8,17,18 patients coinfected with human immunodeficiency virus,19–23 and immunosuppressed patients.24–26
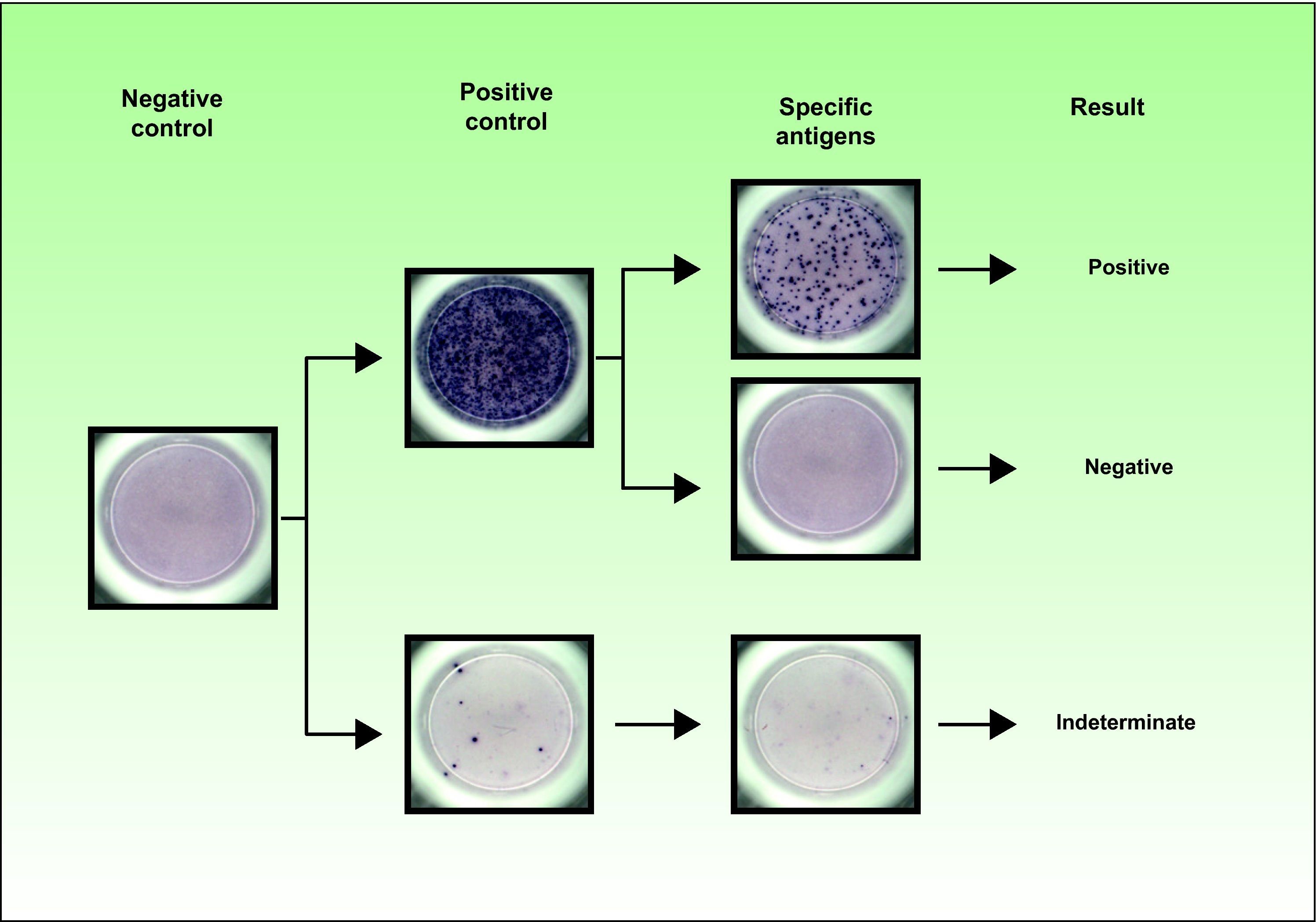
IGRAs can detect the absence of an immune response as they have internal controls to detect anergy. Accordingly, a negative mitogen (positive control) result and a negative antigen result must be interpreted as an indeterminate result, indicating the presence of an impaired T-cell response. A negative TST result in a patient with an indeterminate IGRA result could be a false negative TST. Indeterminate results have been associated with young age (< 5 years), immunosuppression, and negative TST results.8,27,28Figure 1 shows the range of results that can be obtained using the T-SPOT.TB test.
The T-SPOT.TB technique uses isolated peripheral blood mononuclear cells to detect interferon γ released by stimulated T cells using the enzyme-linked immunospot assay. The cells are stimulated by the Mycobacterium tuberculosis-specific antigens ESAT-6 and CFP-10, applied separately. The appearance of spots in the well indicates the presence of reactive T cells. These spots are then counted manually or with an automated plate reader. The result is interpreted as positive if there are at least 6 more spots in either or both of the ESAT-6 or CFP-10 panels than in the nil control panel; the spot count in the antigen panels must also be twice that of the nil control panel. The result is considered indeterminate if there are fewer than 20 spots in the positive control panel and fewer than 6 spots in the antigen panels.
Several studies have assessed the value of IGRAs in the diagnosis of M tuberculosis infection in patients with a chronic inflammatory disease being considered for biologic therapy.26,29–36 The populations analyzed, however, were quite heterogeneous (mixture of patients with rheumatoid disease, digestive disorders, and dermatologic conditions) and had different degrees of drug-induced immunosuppression. Furthermore, some of the patients had already been treated with anti-TNF-α agents and several of the studies used earlier versions of QFT. These differences make it difficult to accurately assess the utility of IGRAs for the diagnosis of TB infection in psoriasis patients scheduled for biologic therapy.
A number of recent studies have analyzed the value of IGRAs in psoriasis,37–40with results confirming that these assays are less influenced by the BCG vaccine than the TST.38 In one of these studies, De Andrade Lima et al.39 reported a much higher frequency of positive TST results and induration size in controls (patients with common dermatologic disorders not including psoriasis) than in patients with untreated moderate to severe psoriasis. The authors, however, found no significant differences between the groups in terms of the frequency of positive T-SPOT.TB results or the number of spots elicited by the test. They concluded that the T-SPOT.TB test performed better than the TST in diagnosing latent TB infection in patients with psoriasis and suggested that this was because the in vitro test was less influenced by the immune dysregulation that characterizes psoriasis.
Value of IGRAs in Predicting Disease ProgressionIf IGRAs are to be incorporated into routine clinical practice, it is crucial to establish the safety of not prescribing TB prophylactic treatment in patients with a positive TST and a negative IGRA result (regardless of BCG-vaccination status) due to start immunosuppressive therapy. Very little is known about screening for TB infection during anti-TNF-α therapy or about the value of IGRAs in predicting disease progression. At the present time, it would therefore seem wise to use both the TST and an IGRA in such cases and to consider a diagnosis of TB infection when a positive result is observed with either of the tests. Nevertheless, there are preliminary results suggesting that IGRAs might be used as a stand-alone tool in the not so distant future.37,38,40
Laffitte et al.,38 for example, used the TST and T-SPOT.TB to test 50 patients with psoriasis before they were due to start anti-TNF-α therapy. Twenty-eight patients had a negative result on both tests, 12 had a positive TST result and a negative T-SPOT.TB result, and 10 patients had a positive T-SPOT.TB result (8 of these also had a positive TST). Thirty-eight patients with a negative T-SPOT.TB result did not receive prophylactic treatment and none developed TB in 4 years of follow-up. Garcovich et al.37 also screened for TB infection with the TST and QFT in 50 patients about to start anti-TNF-α therapy. None of the patients with a negative QFT result and a normal chest radiograph received prophylactic treatment, and none of them developed TB in a follow-up period of 18 months. The authors also performed screening tests during the first 12 months of biologic therapy and observed 5 QFT conversions (3 at 6 months and 2 at 12 months). TB prophylaxis was prescribed in 4 of these patients, none of whom had developed active TB at follow-up. Finally, Chiu et al.40 used QFT to screen for latent TB infection in 100 patients with psoriasis receiving biologic therapy and observed 12 positive results. For different reasons, prophylactic treatment was prescribed in just 4 of these patients. Of the 100 patients analyzed, only 1—a patient with a positive QFT result who refused prophylaxis—developed active TB.
Indeterminate and Discordant ResultsLittle is known about the effect of immunosuppressive drugs on IGRA results, although Soborg et al.30 showed that corticosteroid treatment was associated with an increased risk of an indeterminate QFT result and a lower frequency of positive TST results. In our experience, patients with psoriasis have a higher rate of positive T-SPOT.TB and QFT results and a lower rate of indeterminate results than patients with Crohn disease.41 The differences are probably due to the different immunosuppresive agents that patients had received before starting anti-TNF-α therapy.
Most studies attribute discordant results between IGRAs and the TST to the BCG vaccine, but positive TST and negative IGRA results have also been observed in patients who have not been vaccinated. One possible explanation for this discordance is sensitization to NTM. In fact, our group has shown that NTM exposure might explain discordant results between IGRAs and the TST.17,42
Future DevelopmentsOne of the challenges facing IGRA testing is to improve sensitivity without loss of specificity and to reduce the number of indeterminate results. Recent studies analyzing new biomarkers for the diagnosis of latent TB infection and active TB could open the door to the use of new antigens43,44 and testing for multiple cytokines in future generations of IGRAs.45–47
ConclusionsIn brief, IGRAs are a promising alternative to the TST for the diagnosis of TB infection. They are not affected by the BCG vaccine or sensitization to NTM and therefore have higher specificity and sensitivity. Furthermore, they are less influenced by prior treatment with immunosuppressive agents. However, because safety must be a top priority, we recommend that patients currently being considered for anti-TNF-α therapy should be tested with both an IGRA and the TST. Nevertheless, given that both in vitro tests have high negative predicitive value, in the not so distant future, it is foreseeable that IGRAs will be used as a stand-alone test. Further studies are needed to improve our understanding of these in vitro techniques and to help in the design of individualized follow-up strategies.
Ethical DisclosuresProtection of Human and Animal SubjectsThe authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of DataThe authors declare that no patient data appear in this article.
Right to Privacy and Informed ConsentThe authors declare that no patient data appear in this article.
FundingThis article was funded by the Spanish Health Research Fund (FIS, PI 10/00214). J Domínguez is a researcher funded by the Miguel Servet program of the Instituto de Salud Carlos III (Spain).
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Domínguez J, et al. Técnicas basadas en la detección de IFN-γ en el diagnóstico de la infección tuberculosa en pacientes con psoriasis candidatos a terapias biológicas. Actas Dermosifiliogr. 2012;103:880–6.