At birth, vernix caseosa can cover the whole body surface or accumulate only on the back and in the skin folds. Interest in its composition and function and its possible applications in adults has increased in recent years. The objective of this study was to determine the prevalence of vernix caseosa in newborn infants in the health care area of Ferrol, Spain, and to assess its relationship with neonatal and maternal factors. We performed a prospective study of 1000 newborns seen within the first 3days of life in our hospital. Vernix caseosa was observed in 42.9% of cases. The clinical profile associated with the presence of vernix caseosa was the following: healthy newborn girl with a high birth weight, born at term by normal vaginal delivery to a multiparous mother who had received medication and dietary supplements during pregnancy. The absence of vernix caseosa was associated with the presence of physiological scaling of the newborn and erythema toxicum neonatorum.
Al nacimiento la vérnix caseosa puede cubrir toda la superficie corporal o acumularse sólo en la espalda y los pliegues. En los últimos años ha aumentado el interés por su composición, funciones y aplicaciones en la edad adulta. Nuestro objetivo fue conocer la prevalencia de la vérnix caseosa en los recién nacidos del Área Sanitaria de Ferrol, y ver cómo repercutían los parámetros neonatales y maternos en su desarrollo. Realizamos un estudio prospectivo de 1.000 recién nacidos vistos en los primeros tres días de vida en nuestro hospital. Encontramos vérnix caseosa en el 49,2% de los neonatos. El perfil clínico de presencia de vérnix caseosa sería: recién nacido de sexo femenino, sano, a término, con peso elevado producto de una gestante no primigesta, con ingesta de fármacos y suplementos dietéticos durante el embarazo sometida a un parto eutócico. Existe relación entre ausencia de vérnix caseosa y la presencia de descamación fisiológica y de eritema tóxico neonatal.
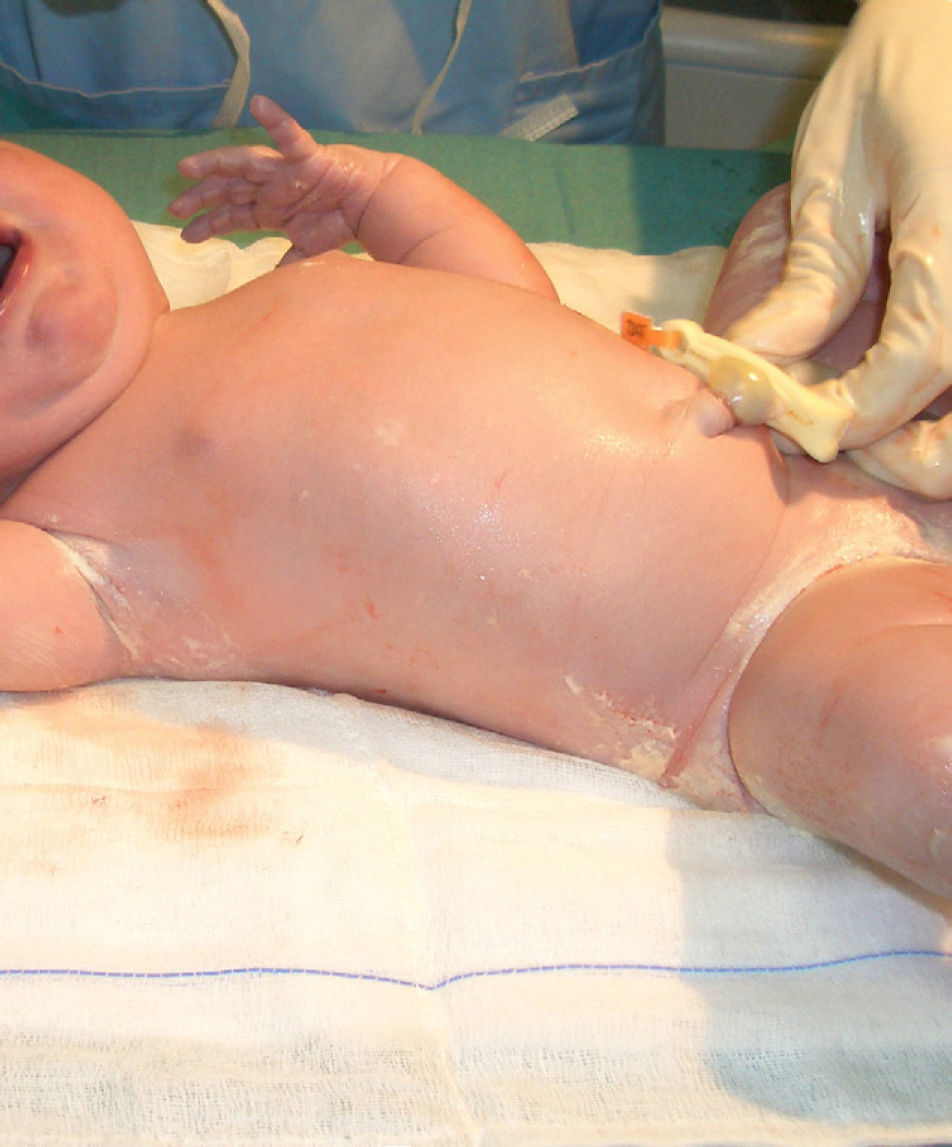
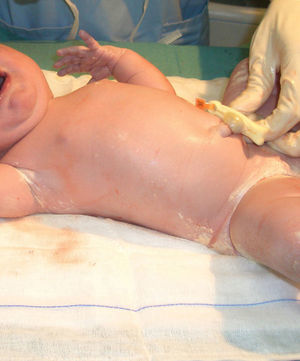
In the last trimester of pregnancy, the fetus becomes coated with a substance known as vernix caseosa, or simply vernix. This substance can cover the entire body or be found only on the back and in the skinfolds of newborn babies (Fig. 1).1,2 After several hours or days, it begins to dry and then spontaneously sheds. The color and odor of vernix can indicate intrauterine problems. A yellow color, for example, indicates hemolytic disease or a postterm infant while a yellow-brown color indicates contact with meconium; odor, in turn, is a sign of neonatal sepsis.3Vernix has attracted increasing interest in recent years, notably because of its composition, functions, and possible applications in adults.4 This natural barrier is formed by sebaceous secretions, the desquamation of epithelial cells, and the shedding of lanugo hair. It is mainly composed of water (80.5%), lipids (10.3%), and proteins (9.1%).5 In the uterus it plays an important role in preventing infections and amniotic fluid maceration and facilitating passage through the birth canal. In the infant's first days of life outside the womb, it helps thermoregulation, skin hydration, wound healing, and colonization of the skin by nonpathogenic bacteria; it also protects against fungi and bacteria, maintains pH balance, and acts as an antioxidant. Precisely because of these benefits, and because vernix does not cause hygiene problems, the general recommendation is not to remove it by wiping or bathing.4–8
In a recent study, we analyzed the prevalence of birth marks and transient benign skin lesions in newborns in our health area.9 For the current study, we decided to focus on vernix.
Case DescriptionsThe main aim of the present study was to determine the prevalence of vernix in newborns in the health area of Ferrol, in Galicia, Spain. A secondary aim was to analyze how the presence of vernix might be influenced by certain neonatal or maternal factors. We performed a prospective, descriptive study of 1000 neonates seen at the perinatology unit at Hospital Arquitecto Marcide, a secondary hospital in Ferrol.9 All infants from our health area and born in the hospital were seen during their first 3 days of life. In all cases, we investigated the presence of physiologic desquamation, erythema toxicum neonatorum (ETN), developmental abnormalities, and vernix. The physical examination was performed jointly by a dermatologist and a pediatrician. The newborns were fully naked (ie, not wearing a diaper) during the examinations, which were performed under adequate lighting. The neonatal cleaning and care protocol at our hospital recommends drying without wiping and advises against bathing neonates in their first 24hours of life.
We collected data on neonatal factors (gestational age, sex, race of parents, weight at birth, Apgar score at 1 and 5minutes, and presence of disease), maternal factors (age, number of previous pregnancies, and use of medication, dietary supplements, and toxic substances during pregnancy), type of delivery, and day of life on which the newborn was examined. The study was approved by the teaching and research council and the ethics committee of our hospital. Qualitative variables were expressed as percentages and analyzed using the χ2 test. Statistical analyses were performed using the statistical program SPSS, version 15. A P value less than .05 was considered statistically significant.
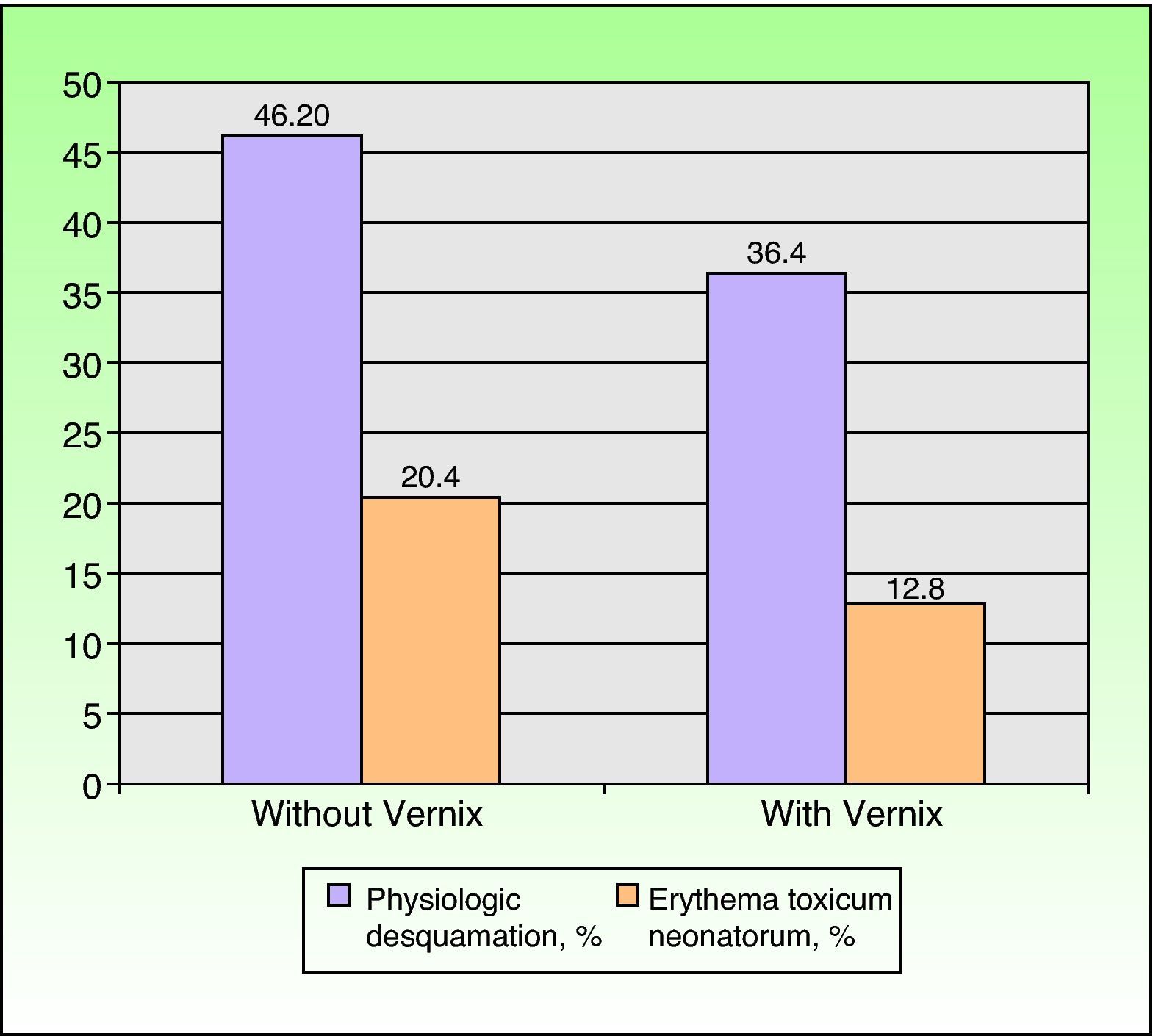
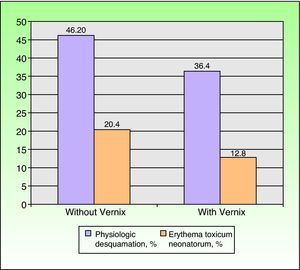
The infants included in the study were born between May 2008 and November 2009. Table 1 shows the prevalence of vernix according to the different factors studied. In total, 49.2% of the newborns had vernix, with a higher prevalence noted in a) newborns who were white or female or had been born at term and newborns who had a high birth weight, a high Apgar score, and absence of disease; b) multiparous women, women aged at least 30 years, and women who had taken medication, used toxic substances, or had a disease during pregnancy; c) vaginal delivery; and d) newborns examined on day 1. The differences were statistically significant for sex, gestational age, weight, Apgar score, maternal age, use of medication, type of delivery, and day of examination. The incidence of physiologic desquamation and ETN showed an inverse relationship with the presence of vernix (Fig. 2). Desquamation was observed in 46.2% of newborns with vernix and ETN in 20.4% compared to 36.5% and 12.8%, respectively, in those without vernix.
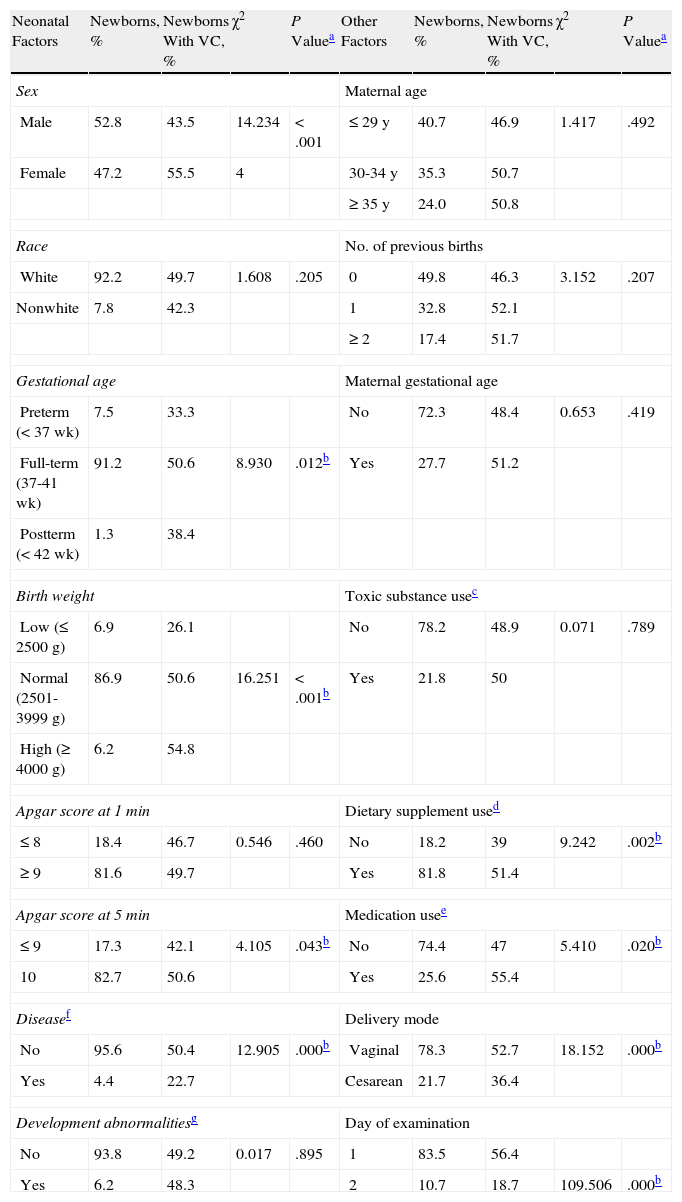
Prevalence of Vernix Caseosa (VC) According to Neonatal and Maternal Factors, Type of Delivery, and Day of Examination.
| Neonatal Factors | Newborns, % | Newborns With VC, % | χ2 | P Valuea | Other Factors | Newborns, % | Newborns With VC, % | χ2 | P Valuea |
| Sex | Maternal age | ||||||||
| Male | 52.8 | 43.5 | 14.234 | <.001 | ≤ 29 y | 40.7 | 46.9 | 1.417 | .492 |
| Female | 47.2 | 55.5 | 4 | 30-34 y | 35.3 | 50.7 | |||
| ≥ 35 y | 24.0 | 50.8 | |||||||
| Race | No. of previous births | ||||||||
| White | 92.2 | 49.7 | 1.608 | .205 | 0 | 49.8 | 46.3 | 3.152 | .207 |
| Nonwhite | 7.8 | 42.3 | 1 | 32.8 | 52.1 | ||||
| ≥2 | 17.4 | 51.7 | |||||||
| Gestational age | Maternal gestational age | ||||||||
| Preterm (< 37 wk) | 7.5 | 33.3 | No | 72.3 | 48.4 | 0.653 | .419 | ||
| Full-term (37-41 wk) | 91.2 | 50.6 | 8.930 | .012b | Yes | 27.7 | 51.2 | ||
| Postterm (< 42 wk) | 1.3 | 38.4 | |||||||
| Birth weight | Toxic substance usec | ||||||||
| Low (≤ 2500 g) | 6.9 | 26.1 | No | 78.2 | 48.9 | 0.071 | .789 | ||
| Normal (2501-3999 g) | 86.9 | 50.6 | 16.251 | <.001b | Yes | 21.8 | 50 | ||
| High (≥ 4000 g) | 6.2 | 54.8 | |||||||
| Apgar score at 1 min | Dietary supplement used | ||||||||
| ≤ 8 | 18.4 | 46.7 | 0.546 | .460 | No | 18.2 | 39 | 9.242 | .002b |
| ≥ 9 | 81.6 | 49.7 | Yes | 81.8 | 51.4 | ||||
| Apgar score at 5 min | Medication usee | ||||||||
| ≤ 9 | 17.3 | 42.1 | 4.105 | .043b | No | 74.4 | 47 | 5.410 | .020b |
| 10 | 82.7 | 50.6 | Yes | 25.6 | 55.4 | ||||
| Diseasef | Delivery mode | ||||||||
| No | 95.6 | 50.4 | 12.905 | .000b | Vaginal | 78.3 | 52.7 | 18.152 | .000b |
| Yes | 4.4 | 22.7 | Cesarean | 21.7 | 36.4 | ||||
| Development abnormalitiesg | Day of examination | ||||||||
| No | 93.8 | 49.2 | 0.017 | .895 | 1 | 83.5 | 56.4 | ||
| Yes | 6.2 | 48.3 | 2 | 10.7 | 18.7 | 109.506 | .000b | ||
The prevalence of vernix in our series, 49.2%, is much higher than the 14.2% reported by Boccardi et al10 in an Italian series. Although differences in practices regarding cleaning of vernix by nursing staff may partly explain this difference, we think it is mainly due to differences in time of examination. In our study, 83.5% of newborns were examined on the first day, and in the Italian study, 56.1% were examined on the second day.
Like Visscher et al,7 who analyzed the percentage of body surface covered by vernix, we found a higher prevalence of vernix in both white and female newborns, but unlike those authors, we also found a higher prevalence in infants born at term (vs preterm) and by vaginal delivery (vs cesarean section). We believe that vernix is less common in infants born by cesarean section than by vaginal delivery as in the first case there is a higher proportion of preterm and postterm births. Our data show that vernix is a marker of neonatal health as its presence was associated with a higher Apgar score and an absence of neonatal disease. In this respect, Visscher et al7 found an association between vernix and the absence of meconium.
Insufficient nutrient intake during pregnancy is associated with considerable fetal and perinatal morbidity. Nutritional deficiency increases the risk of low birth weight, premature delivery, malformations, and altered immune function (which, in turn, increases the risk of infection). The fact that an adequate intake of nutrients (such as iodine, iron, or folic acid supplements) is associated with an increased prevalence of vernix may partly explain why this substance was less common in low-birth-weight and preterm infants. A higher prevalence was also associated with maternal disease and use of medication during pregnancy; these associations were not found by Boccardi et al.10
We also detected an association between a loss of vernix and the development of physiologic desquamation and ETN. This might be explained as follows: if the amount of vernix decreases in utero, there will be greater maceration of the stratum corneum by amniotic fluid, and loss of the vernix in the first few days after birth will increase transepidermal water loss, leading to dehydration of the stratum corneum. These processes would trigger desquamation.7 As stated by Marchini et al,11 ETN is currently considered to be an immune response to microbial colonization of hair follicles, which is facilitated by the presence of vernix.6 The 2 processes might be consecutive, with ETN developing after the shedding of the vernix.
To conclude, we observed vernix in 49.2% of newborns in our health area. The prevalence of this protective barrier is influenced not only by gestational age and time of examination, but also by the type of delivery and other neonatal and maternal factors. Based on our findings, the clinical profile of a newborn with vernix would be a healthy, full-term female, with a high birth weight, born via vaginal delivery to a multiparous woman who took medication and dietary supplements during pregnancy. We also detected an association between an absence of vernix and desquamation and ETN.
One of the limitations of the present study is that we did not follow χ2 testing with multivariate analysis of data. Further studies are needed to explore the association between vernix and the neonatal and maternal factors analyzed; it would also be interesting to investigate the influence of other factors such as maternal weight gain during pregnancy and the relationship between maternal body mass index and the proportion of body surface covered by vernix at delivery. Monitoring newborn infants for several days after delivery would also shed light on whether there is a time sequence between the shedding of vernix and the appearance of physiologic desquamation and ETN.
Conflict of InterestsThe authors declare that they have no conflicts of interest.
Please cite this article as: Monteagudo B, et al. Influencia de los factores neonatales y maternos en la prevalencia de vérnix caseosa. Actas Dermosifiliogr. 2011;102:726-729.