Inflammatory bowel disease (IBD) is a complex entity that includes Crohn disease and ulcerative colitis. It is characterized by a chronic proinflammatory state of varying intensity that often leads to considerable morbidity. In the last decade, several therapeutic targets have been identified that are susceptible to the use of biological agents, including anti-tumor necrosis factor alpha antibodies, which are associated with paradoxical psoriasiform reactions in 5% of patients. Decision-making in the management of these cases requires close collaboration between the dermatologist and gastroenterologist. Inflammatory bowel disease is also associated with various other dermatologic and rheumatologic manifestations, and presents a genetic and pathogenic association with psoriasis that justifies both the interdisciplinary approach to these patients and the present review.
La enfermedad inflamatoria intestinal es una entidad compleja que incluye la enfermedad de Crohn y la colitis ulcerosa, y se caracteriza por un estado proinflamatorio crónico con un curso oscilante y que en muchas ocasiones conlleva una gran morbilidad a estos pacientes. En la última década se han identificado distintas dianas terapéuticas que permiten el uso de fármacos biológicos, en particular los anticuerpos dirigidos contra el factor de necrosis tumoral alfa, que se asocian en un 5% de los casos con reacciones paradójicas psoriasiformes, que requieren una estrecha colaboración entre el dermatólogo y el gastroenterólogo en la toma de decisiones. La enfermedad inflamatoria intestinal se asocia, asimismo, a otras diversas manifestaciones dermatológicas y reumatológicas, y presenta una asociación genética y patogénica con la psoriasis, que justifica tanto el abordaje interdisciplinario de estos pacientes como la presente revisión.
The term immune-mediated inflammatory disease (IMID) refers to entities that share common pathogenic pathways. Although the organs involved may differ, these diseases are characterized by chronic inflammation and also by response to treatment with anti-tumor necrosis factor (TNF) agents. IMIDs include skin conditions such as psoriasis, rheumatic conditions such as rheumatoid arthritis and a range of spondyloarthropathies, and digestive diseases such as inflammatory bowel disease (IBD), a term which encompasses both Crohn disease (CD) and ulcerative colitis (UC). Taken together, IMIDs affect between 5% and 7% of the population in western countries. These diseases share a genetic predisposition, and so more than one may occur in the same patient.
Psoriatic arthritis and the appearance of paradoxical reactions to anti-TNF treatment provide grounds for collaboration between dermatologists and rheumatologists, as well as for integrated care for more complex patients. In the case of CD and UC, given the frequent occurrence of skin manifestations (erythema nodosum, neutrophilic dermatoses, and hidradenitis, for example) and paradoxical reactions, along with the possible development of rheumatological manifestations, close collaboration is also justified between specialists in dermatology, rheumatology, and digestive diseases, with particular dedication to these diseases and experience in the use of immunosuppressants and biologic agents.
On November 21, 2014, in the Hospital de la Santa Creu i Sant Pau, Spain, the first meeting of gastroenterologists, rheumatologists, and dermatologists experienced in IMIDs took place. The different specialists, from leading hospitals in Aragon, the Balearic Isles, and Catalonia met with the aim of discussing topics of common interest. Topics debated in a workshop format included common and differential pathogenetic aspects of IBD and psoriasis; management of other inflammatory manifestations generally associated with IBD activity (erythema nodosum, pyoderma gangrenosum, arthritis); paradoxical manifestations of anti-TNF treatment; and current and future strategies in the treatment of IBD. We considered it of interest to publish the content of these discussions, given the limited literature on the topic.
Etiopathogenesis, Genetics, and Comorbidities: Parallels Between the Gastrointestinal Tract and the SkinThere is a marked parallel between the digestive tract and the skin, as both structures have extensive interfaces in permanent contact with different antigens and an individual microbiome whose disturbances may have pathogenic implications. Both systems also have a complex associated immune system in which both innate and adaptive immunity play important roles.
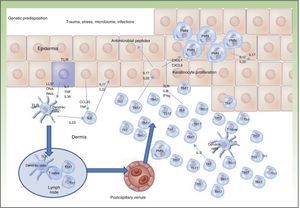
The pathogenesis of IBD can be explained by the interaction between environmental factors and gastrointestinal flora in a genetically susceptible individual. In IBD, 163 susceptibility loci have been identified, the majority of which are common to CD and UC.1 Many of the polymorphisms and mutations identified play a potential pathogenetic role as the corresponding proteins are implicated in lymphocyte activation pathways, adaptive immunity, intestinal barrier function, intestinal epithelial barrier repair, and immune tolerance.1
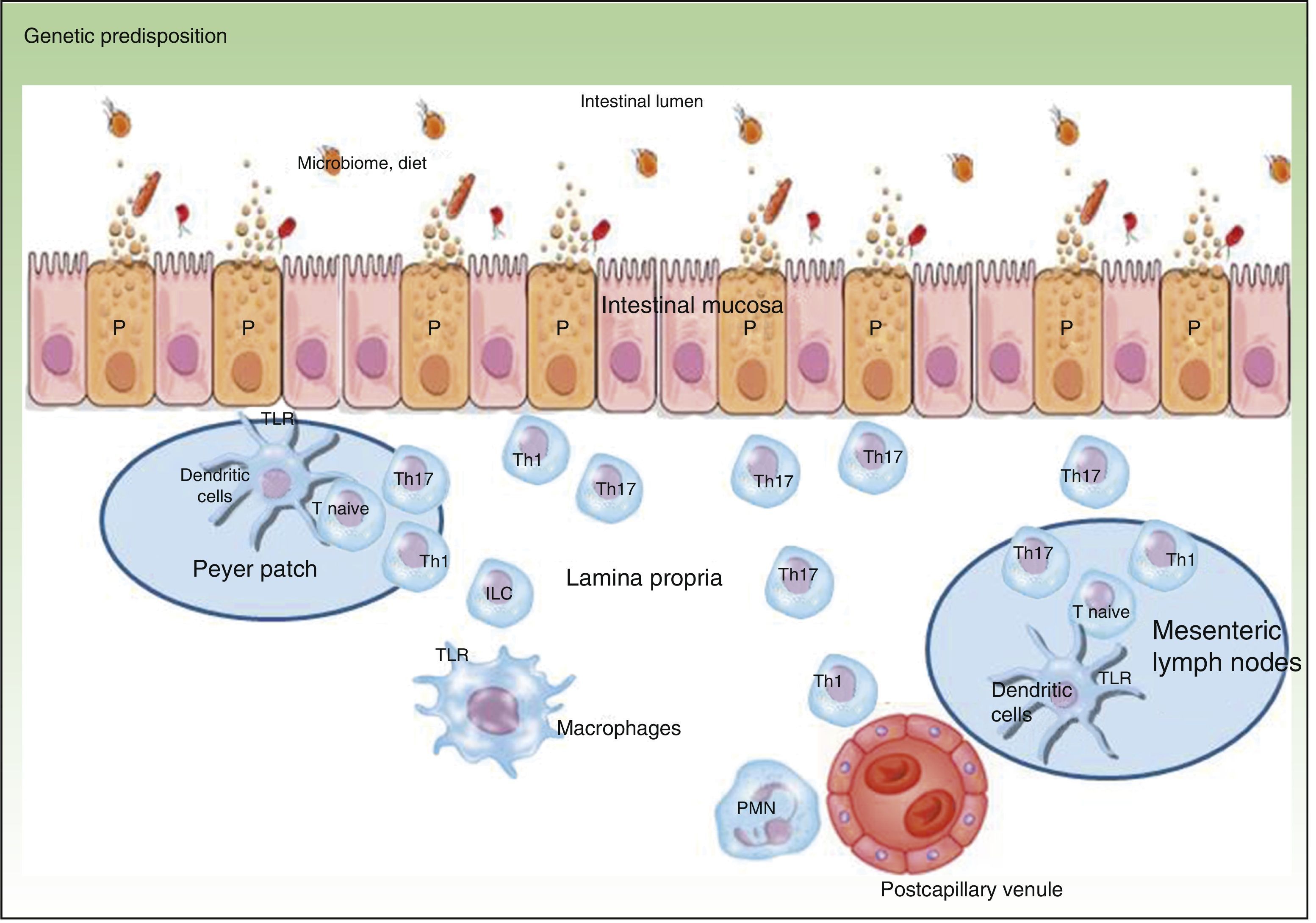
Traditionally, CD is considered to be characterized by T-helper (Th) 1 polarization while CU is characterized by Th2 polarization associated with decreased lymphocyte apoptosis.2,3 Currently, innate immunity is thought to predominate initially with disturbance in intestinal mucosa, secretion of antimicrobial peptides such as defensins, and activation of bacterial antigen recognition in the intestinal lumen. Bacterial antigens are captured by presenting cells, which migrate to the secondary lymphoid organs (Peyer patches) for presentation to naive T lymphocytes. Depending on the phenotype and the cytokines present in the medium, these lymphocytes differentiate to Th1, Th2, Th17, and regulatory T lymphocytes, which modulate response to these antigens.3 When certain cytokines such as TNF, interleukin (IL) 12, or IL2 are present, clonal expansion of the T lymphocytes occurs with recruitment of circulating T lymphocytes towards the lamina propria, leading to perpetuation of inflammation. The trigger has not yet, however, been identified. Studies have shown that Th1 response predominates in the early phase of CD,4 with increased IL2 or IL12, whereas in advanced CD, an exaggerated Th17 response may predominate.5 These observations may have implications for the most appropriate therapeutic strategy according to the stage of disease. Mutation of the IL10 receptor has been reported in early CD.6 While this may have pathogenic implications, the therapeutic approach, which depends on phenotype and not genotype, is unaffected. In addition, only one third of patients with IBD have one of the polymorphisms described to date.6 This suggests that the environmental component is much more important than the genetic one and that development of CD is due to interaction between genetic load, the environment, the microbiome, and the genes themselves (Figure 1).
Psoriasis also has a genetic base, attributable in 30% to 50% to the susceptibility locus PSORS1, located on chromosome 6,7 although in some patient subgroups, the genetic load is even greater (100% in the case of guttate psoriasis).3 More than 40 susceptibility loci have been identified, and their gene products are related to synthesis of epidermal proteins, innate and acquired immunity, and intracellular transcription processes.8
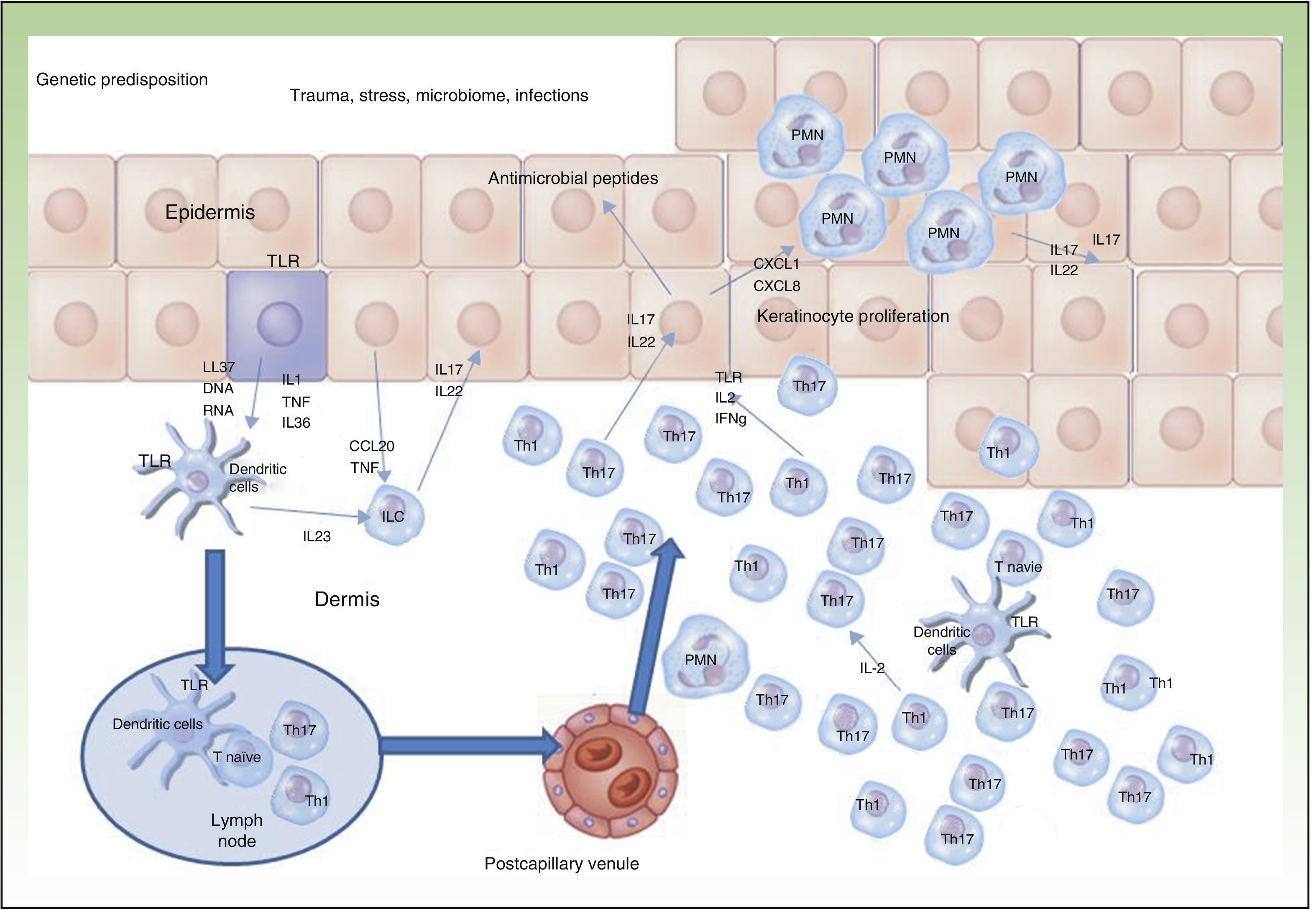
Currently, the main effector molecule with pathogenic importance in psoriasis is thought to be IL17.9,10 This molecule may be synthesized or released by different cell types implicated in innate immunity (polymorphonuclear cells, mast cells, resident γ-δ T lymphocytes) and acquired immunity (Th17 and CD8+ lymphocytes [Tc17, predominant in the epidermis of psoriasis lesions]).11–13 Our increased genetic knowledge of psoriasis has made a personalized approach to treatment possible (genetic stratification) by identifying prognostic factors for response to treatment such as, for example, the HLACw6 haplotype, whose carriers are more likely to respond to ustekinumab than those who are not carriers (Figure 2).14
In the case of environmental factors, smoking and use of drugs such as nonsteroid antiinflammatory agents and oral contraceptives have been reported as risk factors for IBD, although smoking is the only one with pathogenic potential and a differential risk in CD compared to UC. Thus, in CD, active smoking increases the susceptibility to the disease, and the effect is even seen in exsmokers. In contrast, in UC, active smokers are less likely to have the disease and exsmokers have an increased risk even with respect to individuals who have never smoked.15 Other factors recently associated with IBD and with CD in particular are administration of antibiotics in the first years of life,16 environmental contamination,17,18 and vitamin D deficiency.19–21
One of the pathogenic factors currently generating most interest in the field of IBD is the microbiome or intestinal microbiota. Each Individual has his or her own distinctive intestinal flora, which can be influenced by genetic load, food intake, intestinal pathogens, and drugs. Disturbances characteristic of patients with IBD such as increased presence of enteroinvasive Escherichia coli22–24 accompanied by Faecalibacterium prausnitzii deficiency have been identified.25–27 These bacteria therefore seem to have pathogenic impact on the course of the disease.27
The role of the skin microbiome in the pathogenesis of psoriasis28 and psoriatic arthritis29 is also under debate.
Another important aspect in IMIDs is the presence of comorbidities. In IBD, common diseases include those usually associated with smoking (lung cancer), immunosuppression, chronic intestinal inflammation (secondary carcinogenesis), and other IMIDs, with psoriasis being the most common of these.30 The proinflammatory state in IBD may have systemic repercussions and treating an episode of CD has been shown to reduce serum C reactive protein, although it is generally accepted that inflammation in IBD is more local then systemic.31
In the case of psoriasis, the most extensively studied comorbidities are those associated with cardiovascular disease and traditional risk factors of cardiovascular disease such as different components of metabolic syndrome, above all in the case of psoriatic arthritis. Abdominal obesity plays a key role because adipocytes function as an endocrine organ that acts by releasing proinflammatory cytokines and adipokines with a synergistic effect, thereby promoting a persistent inflammatory state, peripheral insulin resistance, atherosclerosis, and the onset of liver steatosis.32 In psoriasis, there is also an increased percentage of smokers, and smoking has been associated with stimulation of innate immunity and expression of genes related to psoriasis susceptibilility.33
Diagnosis of Inflammatory Bowel Disease in Patients with PsoriasisWe should suspect IBD on recurrent appearance of any of the following signs or symptoms in patients with psoriasis: abdominal pain, weight loss, episodes of diarrhea with or without bloody stools, tenesmus or urgent defecation, and presence of anogenital lesions and certain extraintestinal (skin, joint, or ocular) manifestations. Of note is that any extraintestinal manifestation may occur years before diagnosis of IBD; in other cases, these manifestations occur in the context of an inflammatory episode or may follow an independent course.34
Diagnosis of IBD is established according to the Lennard-Jones definition, which includes 4 groups of diagnostic criteria: clinical, radiological, endoscopic, and anatomic and clinical pathologic. The presence of at least 4 criteria is required for diagnosis, with the anatomic and clinical pathologic criterion being the definitive one.34
For this reason, all patients with clinical suspicion of IBD are referred for endoscopy (colonoscopy+ileoscopy) with biopsy sampling. In cases in which the first test cannot establish diagnosis, examination with an endoscopic capsule is indicated in order to detect lesions in proximal segments. Once the capsule detects lesions, enteroscopy is indicated to take biopsies and thus establish diagnosis of IBD.
Imaging tests (abdominal magnetic resonance imaging and computed tomography) are considered useful for the diagnosis of transmural complications of CD but not of IBD itself.34
Furthermore, some of these symptoms that are grounds for clinical suspicion are highly prevalent in the general population. Measurement of calprotectin in feces can be very sensitive for differential diagnosis between organic and functional disease, thus reducing the number of endoscopic procedures required.35
In patients with diagnosis of IBD, fecal calprotectin is useful for monitoring, given its good correlation with endoscopic findings.
Paradoxical Reactions and New Treatments in Crohn DiseaseA paradoxical reaction is an effect of medical treatment, generally with a drug, that is opposite to what would normally be expected. Examples include the appearance of new-onset psoriasis induced by treatment indicated for psoriasis, worsening of pre-existing psoriasis, or appearance of granuloma annulare, sarcoidosis, and pyoderma gangrenosum. It may present in patients with IMID after treatment with different biological agents, and in particular with anti-TNF agents.
Paradoxical reactions observed in patients with IBD treated with anti-TNF agents include psoriasis, palmoplantar pustulosis, skin fold or scalp lesions, nail disorders, and psoriatic dermatitis, with this latter condition being the most common. These lesions have a varied histology in which we can observe psoriasiform reaction, subcorneal pustule±eosinophils, eczematous and/or lichenoid reaction. Palmoplantar pustular lesions may be keratoderma-like and disabling, and respond poorly to topical and systemic treatment. The pathogenesis of these reactions is not known, but the main hypothesis points to imbalance between TNF and interferon α. This phenomenon is considered a class effect, and so may occur with all anti-TNF agents, with a mean latency of 10.5 months (range, 2 weeks to 80 months) after the start of treatment. Paradoxical reactions in IBD affect men and women equally and there is no personal or family history in 70% of cases. They appear to occur more frequently in patients treated with infliximab, adalimumab, and certolizumab, but the incidence is proportional to the number of patients and the years of treatment.36 These patients have a 50% chance of developing a repeat paradoxical reaction if an anti-TNF drug is reintroduced, even if it is a different agent to the causal one.37 Recently, IL12/23 and the Th17 pathway have been implicated in the pathogenesis of IBD. This would explain the good response to ustekinumab, and this agent can be considered a possible treatment in these cases of paradoxical reactions to anti-TNF agents.36
Among the risk factors for the development of paradoxical reactions in patients with IBD are CD and factors such as smoking, obesity, and treatment with infliximab, although in the case of infliximab the risk detected may be driven by bias arising from being the first anti-TNF agent used.38
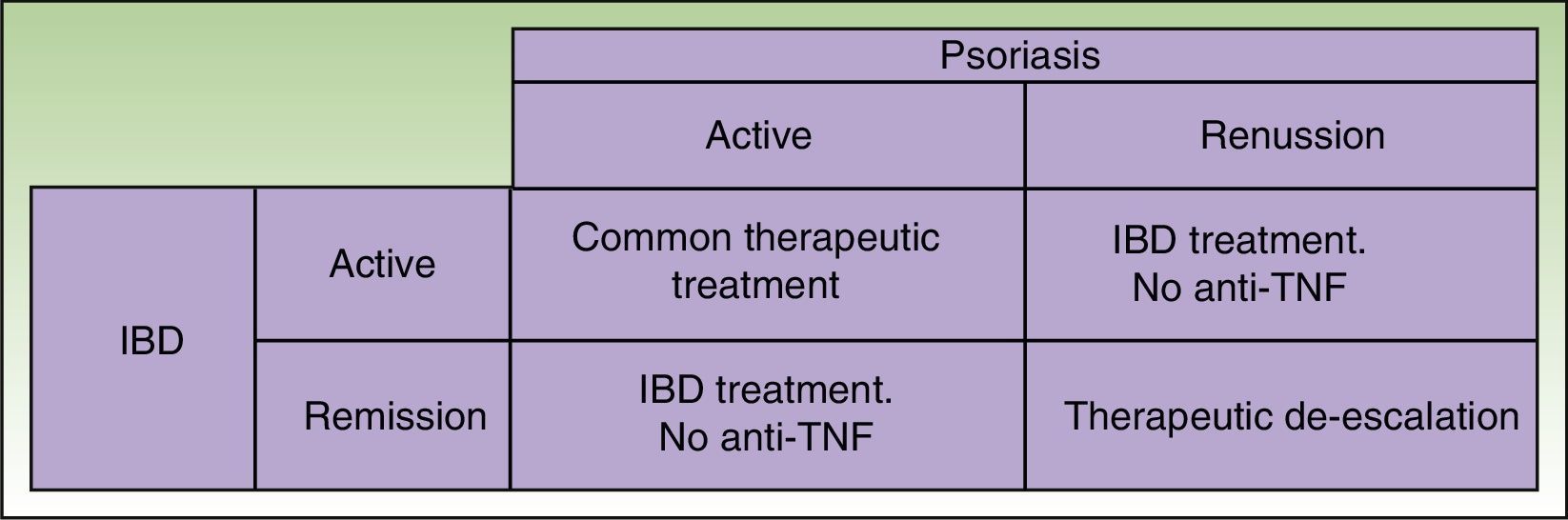
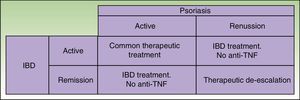
Given that some patients may develop a new episode of IBD on withdrawing the anti-TNF agent and that replacement with another anti-TNF agent may often lead to recurrence or worsening, the anti-TNF agent should be maintained if the paradoxical reaction is not very intense and topical and/or systemic treatments, generally immunosuppressants, are administered39 in agreement with the dermatologist. But in some cases, this curative approach is not sufficient, and it is necessary to change the therapeutic class. Ideally, the new drug should be as effective for skin manifestations of the paradoxical reaction as for IBD control, and so ustekinumab currently appears the best alternative (Figure 3).38
Other Skin Manifestations in IBD PatientsApproximately 30% of patients with IBD develop skin lesions. These may be specific to IBD, reactive such as erythema nodosum and pyoderma gangrenosum in those patient with known genetic susceptibility at TRAF31P2 in which humoral immunity and response to IL17 are implicated, or secondary to treatments, such as nonmelanoma skin cancer.40
Associated Spondyloarthropathy and Therapeutic ApproachJoint involvement in patients with IBD and psoriasis is common, and often it is necessary to collaborate with expert rheumatologists. An exhaustive structured medical history is essential to offer the best treatment in each case. Axial and/or peripheral inflammatory involvement is a defining feature when deciding on the therapeutic approach. In IBD, there are 3 forms of athritis.41 Type i is a form with essentially peripheral involvement associated with an episode of intestinal disease. It is acute, asymmetric, and affects fewer than 6 joints. Type ii is a polyarticular, migratory form that tends to be symmetric. In this form, symptoms do not bear any relationship with IBD activity. Both the type i and type ii forms present as nonerosive arthritis. Finally, type iii or the spondylitic form follows a similar course to inflammatory back pain with morning stiffness, although sacroiliitis may be asymptomatic in 4% to 18% of patients. In the event of clinical suspicion with inconclusive radiography of sacroiliac joints, magnetic resonance imaging is considered the technique of choice for detection in the early phases.42,43
In treatment of IBD-associated arthritis, nonsteroidal antiinflammatory agents may be of use, although they should be used with caution as they can aggravate IBD (UC). Celecoxib is the only nonsteroidal antiinflammatory agent for which no IBD flares have been reported.44,45 Low-dose corticosteroids can also control peripheral joint involvement, but immunosuppressants such as sulfasalazine and methotrexate are the most widely used therapeutic options, particularly when peripheral involvement is present.46 However, methotrexate has not been shown to be effective with axial symptoms and so is not recommended in these cases.47,48 In the event of refractory peripheral or axial involvement, TNF antagonists such as infliximab are recommended.49–51
Similarities and Differences in the Treatment of IMIDs in Dermatology and Digestive PathologyIn both specialties, topical treatments, systemic immunomodulators such as methotrexate, and biologic agents are all available, but certain therapeutic characteristics need to be taken into account. Azathioprine is the first systemic agent of choice in IBD and when faced with ineffectiveness of etanercept. It is worth mentioning that the range of options is more limited in the case of IBD as only a few approved effective anti-TNF agents are available (infliximab and adalimumab; golimumab only in UC), although in the near future the approval of molecules other than anti-TNF agents is expected (ustekinumab, tofacitinib, vedolizumab). These agents have shown promising results in different trials.52,53 Treatment of CD traditionally followed a stage-wise approach, starting with corticosteroids, adding immunomodulators, and, finally, adding anti-TNF agents if remission was not achieved. Currently, however, treatment is considered according to site, pattern, and severity of the disease, opting for a stage-wise approach, accelerated stage-wise approach, or direct intensive approach.52,53 With this strategy, currently, more patients are receiving treatment with anti-TNF agents for control of flares. Both in IBD and in psoriatic arthritis, it is very important to start treatment in the so-called window of opportunity, when therapeutic intervention can modify the disease course and slow the development of complications.54,55
In IBD, the use of combined treatments of azathioprine and/or 6-mercaptopurine with anti-TNF agents is common. In psoriasis, in contrast, monotherapy is usually applied, whether with biologic agents or traditional agents although combination with phototherapy or methotrexate increases the efficacy of treatment and may impede the development immunogenicity in the case of anti-TNF agents.56
ConclusionIMIDs have pathogenic similarities that suggest an overlap in the therapeutic approach with anti-TNF agents, but differences are also present related to the characteristics of the target organs themselves. The presence of microbial antigens, destruction of mucosa, and the resulting sequelae in cases of IBD define a window of opportunity. The skin and joint manifestations are common in patients with IBD and require a combined approach with key dermatologists and rheumatologists, who are experienced in systemic treatment of psoriasis and spondyloarthropathies, and have particular experience in the use of biologic agents. It is therefore of utmost importance that there is cross-training between specialties and that rapid referral pathways and access to consultants are set up. This will enable integrated care for the patient, management of paradoxical reactions, and safety of biological treatment, given the limited effective therapeutic alternatives in patients with IBD.
FundingThe meeting received logistic support form Janssen-Cilag.
Conflicts of InterestJanssen-Cilag acted as facilitator for the meeting and provided technical and methodological support but no employees of the company participated in the development, drafting, and discussion of scientific content. Lluís Puig has received consulting honoraria from Merck, AbbVie, and Janssen.
Given that there is a limit to the number of authors, we would like to thank all meeting participants for their attendance and input:
Speakers: S. Calvet (Servicio de Patología Digestiva, Hospital Parc Taulí, Sabadell, Spain), J. M. Carrascosa (Servicio de Dermatología, Hospital Germans Trias i Pujol, Badalona, Spain), E. Domènech (Servicio de Patología Digestiva, Hospital Germans Trias i Pujol, Badalona, Spain), J. Guardiola (Servicio de Patología Digestiva, Hospital Universitari de Bellvitge, L’Hospitalet de Llobregat, Spain), S. Khorrami (Servicio de Patología Digestiva, Hospital Universitari de Son Espases, Palma de Mallorca, Spain), A. Laiz (Servicio de Reumatología, Hospital de la Santa Creu i Sant Pau, Barcelona, Spain), J. Luelmo (Servicio de Dermatología, Hospital Parc Taulí, Sabadell, Spain), J. Notario (Servicio de Dermatología, Hospital Universitari de Bellvitge, L’Hospitalet de Llobregat, Spain)
Participants: X. Aldeguer (Servicio de Patología Digestiva, Hospital Universitari de Girona Doctor Josep Trueta, Girona, Spain), M. Alsina (Servicio de Dermatología, Hospital Universitari de Girona Doctor Josep Trueta, Girona, Spain), M. Andreu (Servicio de Patología Digestiva, Hospital del Mar, Barcelona, Spain), G. Aparicio (Servicio de Dermatología, Hospital Universitari Vall d’Hebron, Barcelona, Spain), M. Ara (Servicio de Dermatología, Hospital Clínico Universitario Lozano Blesa, Zaragoza, Spain), M. T. Arroyo (Servicio de Patología Digestiva, Hospital Clínico Universitario Lozano Blesa, Zaragoza, Spain), N. Borruel (Servicio de Patología Digestiva, Hospital Universitari Vall d’Hebron, Barcelona, Spain), D. Busquets (Servicio de Patología Digestiva, Hospital Universitari de Girona Doctor Josep Trueta, Girona, Spain), M. Esquius (Servicio de Dermatología, Hospital Sant Joan de Déu, Manresa, Spain), F. Gallardo (Servicio de Dermatología, Hospital del Mar, Barcelona, Spain), J. Llao (Servicio de Patología Digestiva, Hospital Sant Joan de Déu, Manresa, Spain), L. Márquez (Servicio de Patología Digestiva, Hospital del Mar, Barcelona, Spain), M. Piqueras (Servicio de Patología Digestiva, Consorci Sanitari de Terrassa, Terrassa, Spain), A. Pol (Servicio de Dermatología, Consorci Sanitari de Terrassa, Terrassa, Spain), M. Ribera (Servicio de Dermatología, Hospital Parc Taulí, Sabadell, Spain), E. Ricart (Servicio de Patología Digestiva, Hospital Clínic, Barcelona, Spain), A. Sanso (Servicio de Patología Digestiva, Hospital de Manacor, Mallorca, Spain), A. Sapiña (Servicio de Patología Digestiva, Hospital de Manacor, Mallorca, Spain), D. Vila (Servicio de Dermatología, Hospital Sant Joan de Déu, Manresa, Spain).
Please cite this article as: Sánchez-Martínez MA, Garcia-Planella E, Laiz A, Puig L. Enfermedad inflamatoria intestinal: abordaje conjunto digestivo-dermatológico. Actas Dermosifiliogr. 2017;108:184–191.