The induction of antinuclear antibodies (ANA) and the onset of autoimmune diseases have been reported after treatment with tumor necrosis factor (TNF) inhibitors, though controversy persists.
ObjectivesTo determine the frequency of onset of autoimmune diseases and of the appearance of autoantibodies in psoriasis patients administered TNF inhibitors (adalimumab and etanercept) subcutaneously and to correlate this with the effectiveness of treatment, adverse effects, and the order of use of TNF inhibitors. We also tried to identify any factors that might predict the appearance of ANA and autimmune diseases.
MethodsWe performed a retrospective study of a cohort of 121 patients monitored over an 11-year period. ANA were measured at baseline and at 3, 6, and 12 months; positive results were followed up by study of antibodies to double-stranded DNA. Extractable nuclear antigen (ENA) antibodies were also studied at baseline and at 3, 6, and 12 months. Patients with a baseline assay of ANA and ENA at least one more assay during the first year were included in the study, and these antibodies were measured annually thereafter. Psoriasis area severity index was calculated and adverse effects were recorded at each visit.
ResultsA significant increase in ANA positivity was observed during treatment of moderate-to-severe psoriasis with adalimumab and etanercept, but this was not associated with the onset of autoimmune diseases. No correlation was observed with treatment efficacy, the order of use of TNF inhibitors, or the appearance of adverse effects. No predictive factors for the appearance of ANA were identified, except for the body mass index.
ConclusionsWe recommend ANA measurement and screening for autoimmune diseases prior to treatment with TNF inhibitors, but not routine serial measurements of ANA during follow-up except in patients with signs or symptoms suggestive of autoimmune disease.
Se ha descrito la inducción de anticuerpos antinucleares (ANA) y el desarrollo de enfermedades autoinmunes tras el tratamiento con fármacos anti-TNFα, aunque existe controversia sobre su significado.
ObjetivosDeterminar la aparición de enfermedades autoinmunes y de autoanticuerpos en pacientes psoriásicos tratados con fármacos anti-TNFα subcutáneos (adalimumab y etanercept). Relacionar su desarrollo con la efectividad del tratamiento, posibles efectos adversos y con el orden de administración del fármaco anti-TNFα. Evaluar los factores predictores de aparición de ANA y de enfermedades autoinmunes.
MétodosEstudio retrospectivo sobre una cohorte de 121 pacientes seguidos en un período de 11 años. Se determinaron los ANA (si fueran positivos), también se investigaron los anticuerpos anti-ADN de doble cadena y los anticuerpos extraíbles del núcleo basales a los 3, 6, 12 meses (admitiendo en el estudio a aquellos pacientes con una determinación basal y otra durante el primer año) y posteriormente cada año. En cada consulta se calculó el Psoriasis area and severity index y se recogieron los posibles efectos adversos.
ResultadosDurante el tratamiento de la psoriasis moderada-grave con adalimumab y etanercept se produce un aumento significativo en la positivización de los ANA, no acompañada del desarrollo de enfermedades autoinmunes. No se observa correlación con la efectividad del tratamiento, el orden cronológico de utilización de los fármacos anti-TNFα ni, aparentemente, con la aparición de efectos adversos. No se demuestran factores predictores del desarrollo de ANA excepto el índice de masa corporal.
ConclusiónRecomendamos la determinación de ANA y el despistaje de enfermedades autoinumnes previos al tratamiento con fármacos anti-TNFα, pero no una determinación seriada y rutinaria durante el seguimiento, excepto en aquellos casos en los que existan signos o síntomas de sospecha de enfermedad autoinmune.
Tumor necrosis factor (TNF) α inhibitors have a good safety and efficacy profile in the treatment of psoriasis. However, induction of antinuclear antibodies (ANAs) and onset of autoimmune diseases during treatment have been reported, mainly in rheumatology journals and, to a lesser extent, in dermatology journals.1–10 Studies are generally based on a low number of patients and have short follow-up periods. The significance of these phenomena remains unclear and is open to debate. Adverse drug effects could play a role, although the possibility of a latent underlying disease when the drugs are administered cannot be ruled out.
We decided to perform a study with the primary objective of evaluating the emergence of autoimmune disease, ANAs, extractable nuclear antigen antibodies (ENAs), and double-stranded DNA (dsDNA) antibodies in patients with psoriasis treated with subcutaneous TNFα inhibitors (adalimumab [ADA] and etanercept [ETN]). The secondary objectives of this study were as follows: (1) to verify whether there is an association between the emergence of antibodies or autoimmune disease and the effectiveness of treatment, possible adverse events, and order of administration of the TNFα inhibitor; and (2) to evaluate factors that predict emergence of ANAs and autoimmune diseases.
Material and MethodsWe performed a retrospective, single-center cohort study of patients seen at the Dermatology Department of Hospital Universitario de la Princesa, Madrid, Spain between February 2004 and March 2016. The study was approved by the local ethics committee (registration no. 27989).
Inclusion criteria- -
Patients had to be aged ≥18 years with moderate-to-severe psoriasis (defined based on the 2016 consensus document of the Spanish Psoriasis Group of the Spanish Academy of Dermatology and Venereology11).
- -
Patients had to have received continuous therapy with TNFα inhibitors (ADA and ETN) for at least 3 months. At the baseline visit, a washout period of ≥1 month was necessary for conventional systemic drugs, ETN, and ADA and ≥2 months for infliximab.
- -
Patients had to have undergone determination of ANAs at baseline and on at least 1 other occasion (3, 6, or 12 months after starting treatment).
The data collected from the clinical history were as follows:
- -
Demographic data, data on psoriasis, previous treatment for psoriasis, and presence of autoimmune disease.
- -
Screening before treatment with TNFα inhibitors (complete blood count, blood biochemistry, serology [hepatitis B and C, human immunodeficiency virus], tuberculin skin test and booster, chest x-ray).
- -
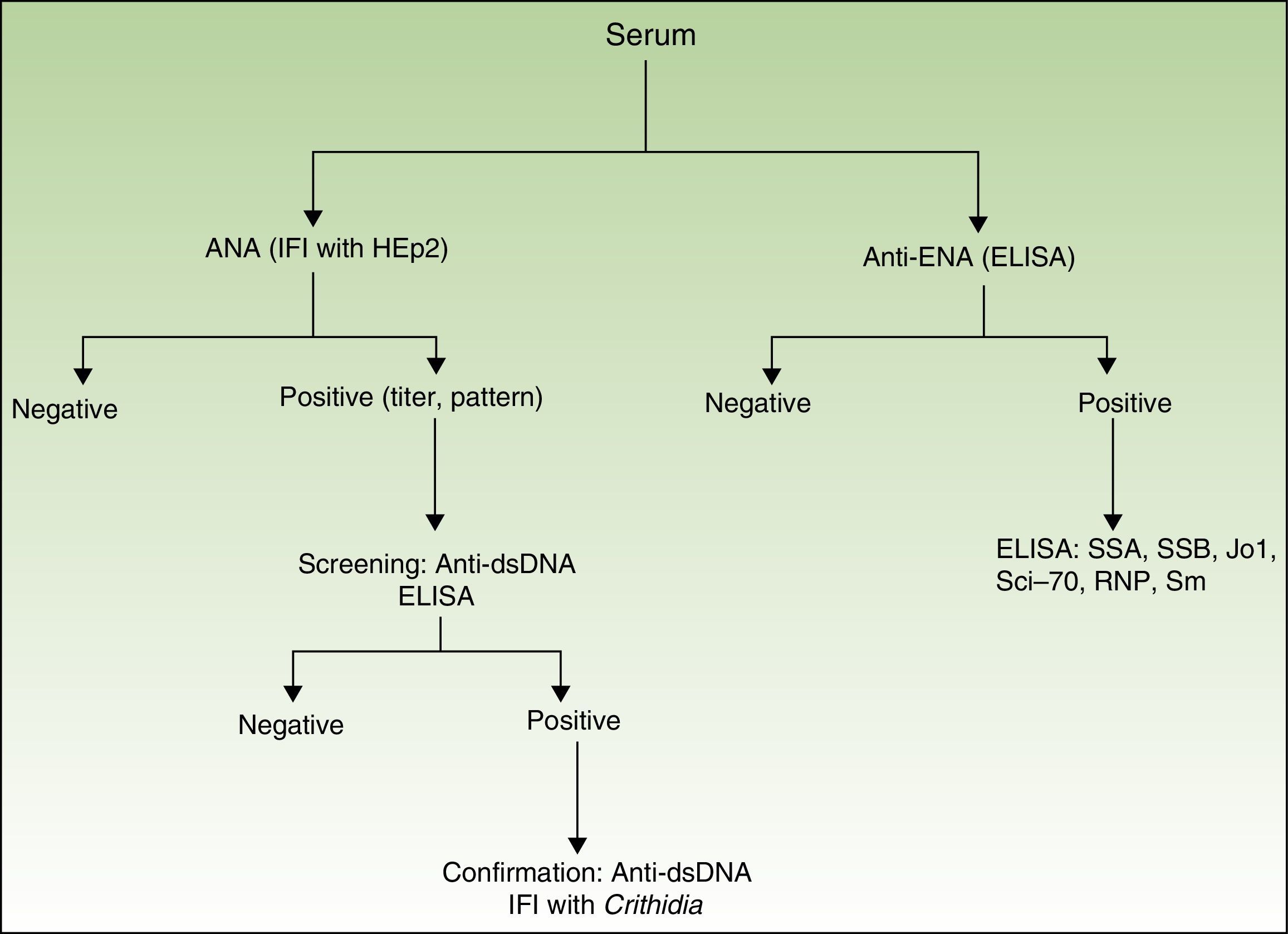
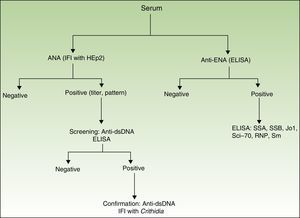
Presence, titer, and pattern of ANAs. The ANA analysis was performed using indirect immunofluorescence (based on human epithelial cells, HEp-2). If the result was positive (≥1/160), anti-dsDNA antibody was determined using enzyme-linked immunosorbent assay (ELISA [high sensitivity, lower specificity]). If these results were positive, they were confirmed using indirect immunofluorescence with Crithidia luciliae (high specificity, lower sensitivity)12 (Fig. 1).
- -
Presence of anti-ENA antibodies determined using ELISA. If the result was positive, a second ELISA was performed for the following antigens (SSA, SSB, Jo1, Scl-70, RNP, and Sm) (Fig. 1).
The data collected at each visit were as follows:
- -
Efficacy of treatment based on the Psoriasis Area and Severity Index (PASI)
- -
Type of treatment administered, dose, administration interval.
- -
Safety. Adverse effects during treatment, with particular attention to the development of autoimmune diseases.
- -
At visits where additional tests were performed, results were recorded for complete blood count, blood biochemistry, ANAs, anti-dsDNA antibodies, and anti-ENA antibodies, which were determined using the same techniques as at the baseline visit. In the case of positive antibody titers, a specific history was taken to rule out connective tissue disease with clinical symptoms.
Results for quantitative variables are presented as mean (SD), minimum, and maximum; results for qualitative variables are presented as absolute frequencies and percentages. Groups were compared using the t test for independent samples or the Mann-Whitney test, depending on whether or not the distribution was normal in the case of quantitative variables, and with the Fisher exact test using contingency tables or the chi-square test using larger tables in the case of qualitative variables.
We constructed a multivariate logistic regression model with ANAs (negative/positive) as the dependent variable. The independent variables were age, sex, body mass index (BMI), time since onset of the disease, psoriatic arthritis (yes/no), previous treatment (naïve, 1-2 previous TNFα inhibitors), baseline PASI, and current TNFα inhibitor (ADA/ETN). The approach adopted included forced entry of variables and backward stepwise selection.
The Mantel-Haenszel test was used to test the conditional independence of the induction of ANAs against the current TNFα inhibitor, while controlling for the effect of previous treatment.
Statistical significance was set at P<.05. The statistical analysis was performed using IBM SPSS 21.0 (IBM Corp).
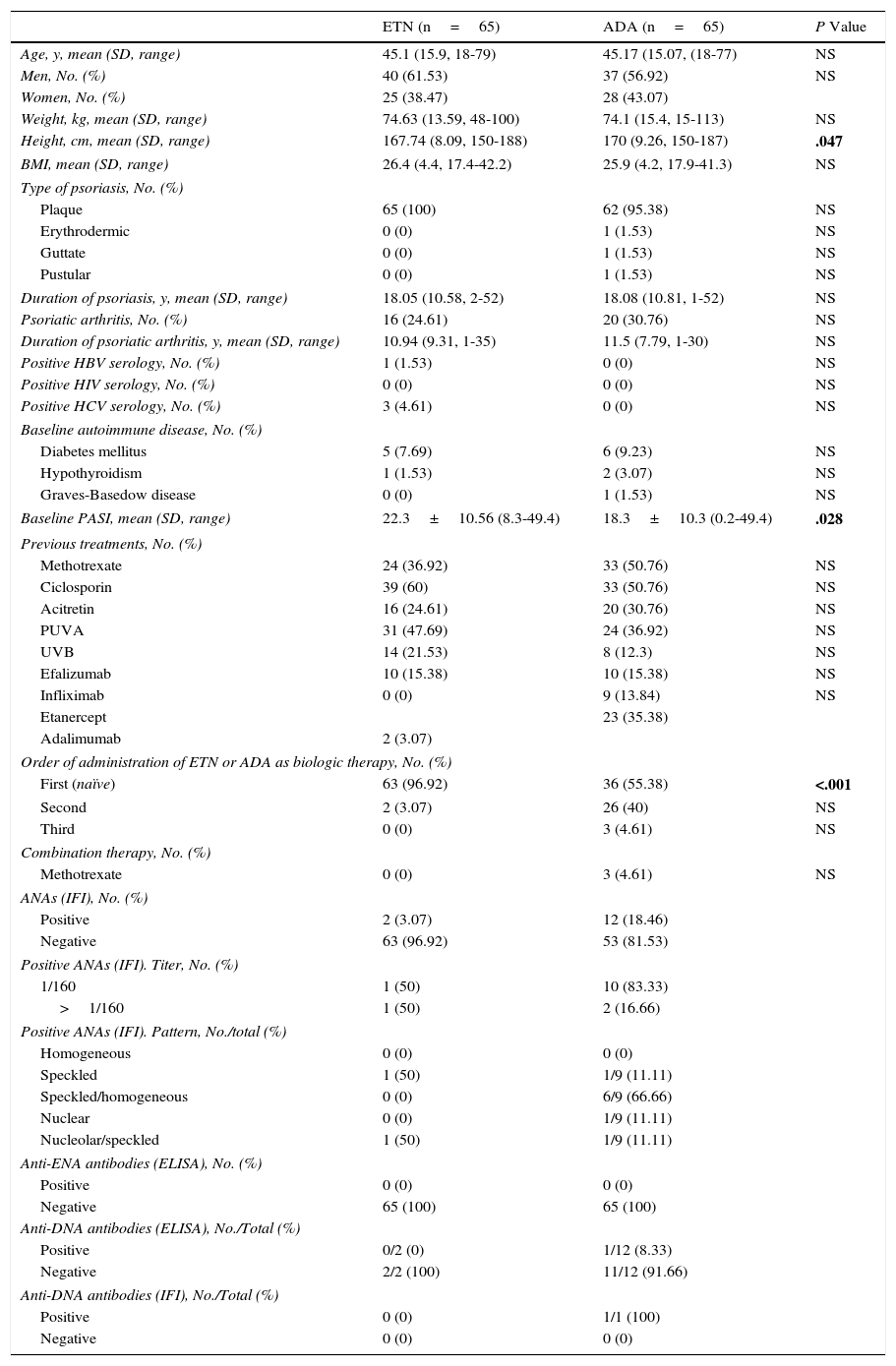
ResultsThe study population comprised 121 patients. Nine patients were treated with ETN and ADA, 56 were treated with ETN alone, and 56 with ADA alone. Therefore, ADA was used in 65 patients and ETN in 65 (77 men and 53 women). Table 1 summarizes the main baseline characteristics. Patients in the ADA group were taller and had a significantly lower baseline PASI than patients taking ETN. The number of naïve patients was clearly higher in the ETN group, thus explaining the differences in baseline PASI between the groups.
Analysis of the Main Baseline Characteristics of the Study Patients.
| ETN (n=65) | ADA (n=65) | P Value | |
|---|---|---|---|
| Age, y, mean (SD, range) | 45.1 (15.9, 18-79) | 45.17 (15.07, (18-77) | NS |
| Men, No. (%) | 40 (61.53) | 37 (56.92) | NS |
| Women, No. (%) | 25 (38.47) | 28 (43.07) | |
| Weight, kg, mean (SD, range) | 74.63 (13.59, 48-100) | 74.1 (15.4, 15-113) | NS |
| Height, cm, mean (SD, range) | 167.74 (8.09, 150-188) | 170 (9.26, 150-187) | .047 |
| BMI, mean (SD, range) | 26.4 (4.4, 17.4-42.2) | 25.9 (4.2, 17.9-41.3) | NS |
| Type of psoriasis, No. (%) | |||
| Plaque | 65 (100) | 62 (95.38) | NS |
| Erythrodermic | 0 (0) | 1 (1.53) | NS |
| Guttate | 0 (0) | 1 (1.53) | NS |
| Pustular | 0 (0) | 1 (1.53) | NS |
| Duration of psoriasis, y, mean (SD, range) | 18.05 (10.58, 2-52) | 18.08 (10.81, 1-52) | NS |
| Psoriatic arthritis, No. (%) | 16 (24.61) | 20 (30.76) | NS |
| Duration of psoriatic arthritis, y, mean (SD, range) | 10.94 (9.31, 1-35) | 11.5 (7.79, 1-30) | NS |
| Positive HBV serology, No. (%) | 1 (1.53) | 0 (0) | NS |
| Positive HIV serology, No. (%) | 0 (0) | 0 (0) | NS |
| Positive HCV serology, No. (%) | 3 (4.61) | 0 (0) | NS |
| Baseline autoimmune disease, No. (%) | |||
| Diabetes mellitus | 5 (7.69) | 6 (9.23) | NS |
| Hypothyroidism | 1 (1.53) | 2 (3.07) | NS |
| Graves-Basedow disease | 0 (0) | 1 (1.53) | NS |
| Baseline PASI, mean (SD, range) | 22.3±10.56 (8.3-49.4) | 18.3±10.3 (0.2-49.4) | .028 |
| Previous treatments, No. (%) | |||
| Methotrexate | 24 (36.92) | 33 (50.76) | NS |
| Ciclosporin | 39 (60) | 33 (50.76) | NS |
| Acitretin | 16 (24.61) | 20 (30.76) | NS |
| PUVA | 31 (47.69) | 24 (36.92) | NS |
| UVB | 14 (21.53) | 8 (12.3) | NS |
| Efalizumab | 10 (15.38) | 10 (15.38) | NS |
| Infliximab | 0 (0) | 9 (13.84) | NS |
| Etanercept | 23 (35.38) | ||
| Adalimumab | 2 (3.07) | ||
| Order of administration of ETN or ADA as biologic therapy, No. (%) | |||
| First (naïve) | 63 (96.92) | 36 (55.38) | <.001 |
| Second | 2 (3.07) | 26 (40) | NS |
| Third | 0 (0) | 3 (4.61) | NS |
| Combination therapy, No. (%) | |||
| Methotrexate | 0 (0) | 3 (4.61) | NS |
| ANAs (IFI), No. (%) | |||
| Positive | 2 (3.07) | 12 (18.46) | |
| Negative | 63 (96.92) | 53 (81.53) | |
| Positive ANAs (IFI). Titer, No. (%) | |||
| 1/160 | 1 (50) | 10 (83.33) | |
| >1/160 | 1 (50) | 2 (16.66) | |
| Positive ANAs (IFI). Pattern, No./total (%) | |||
| Homogeneous | 0 (0) | 0 (0) | |
| Speckled | 1 (50) | 1/9 (11.11) | |
| Speckled/homogeneous | 0 (0) | 6/9 (66.66) | |
| Nuclear | 0 (0) | 1/9 (11.11) | |
| Nucleolar/speckled | 1 (50) | 1/9 (11.11) | |
| Anti-ENA antibodies (ELISA), No. (%) | |||
| Positive | 0 (0) | 0 (0) | |
| Negative | 65 (100) | 65 (100) | |
| Anti-DNA antibodies (ELISA), No./Total (%) | |||
| Positive | 0/2 (0) | 1/12 (8.33) | |
| Negative | 2/2 (100) | 11/12 (91.66) | |
| Anti-DNA antibodies (IFI), No./Total (%) | |||
| Positive | 0 (0) | 1/1 (100) | |
| Negative | 0 (0) | 0 (0) | |
Abbreviations: ANA, antinuclear antibody; BMI, body mass index; DNA, deoxyribonucleic acid; ENA, extractable nuclear antigen; HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus; IFI, indirect immunofluorescence; NS, not significant; PASI, Psoriasis Area and Severity Index; PUVA, psoralen UV-A.
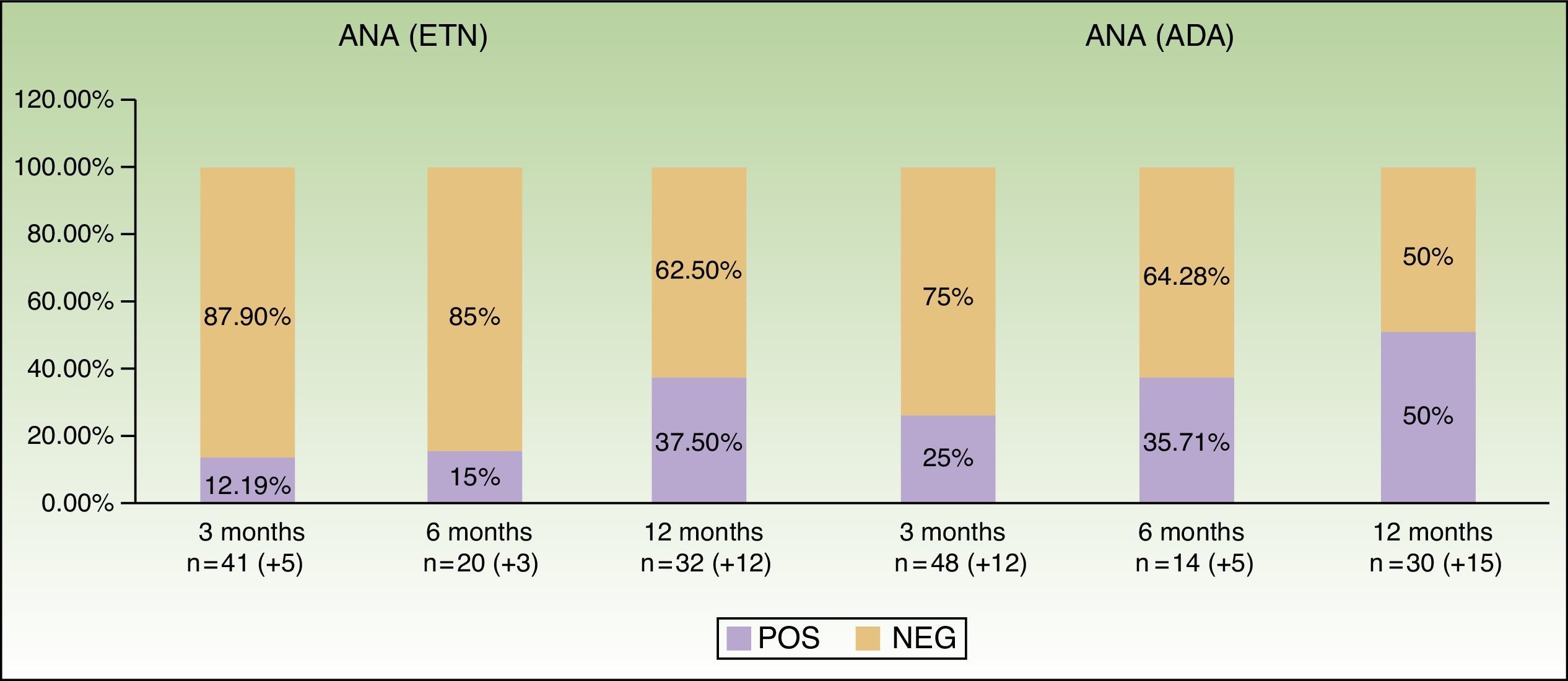
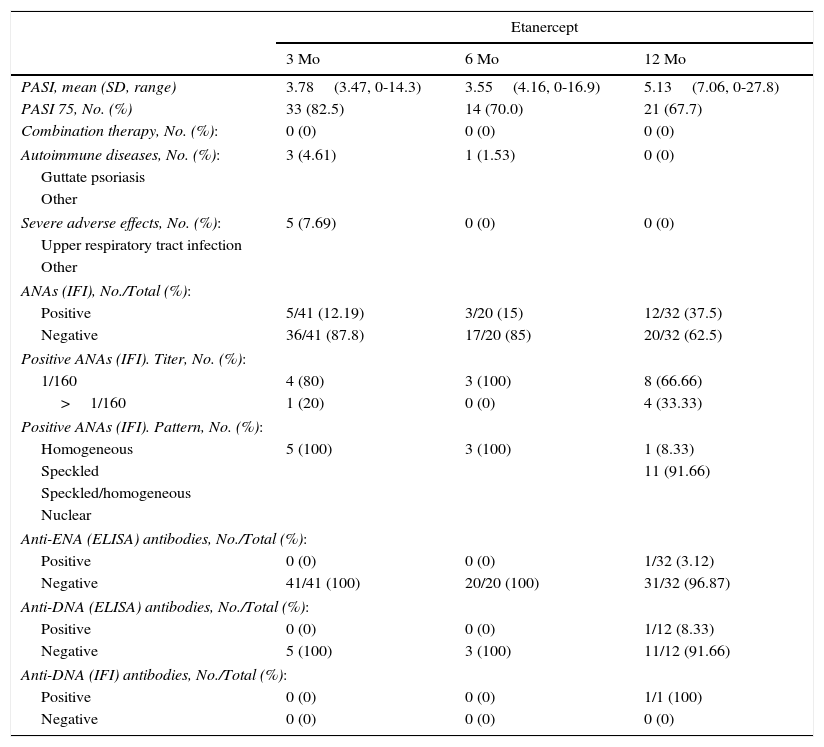
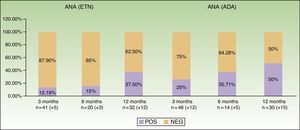
Table 2 and Figure 2 show data from the first year of follow-up, especially with respect to induction of antibodies and development of autoimmune diseases. We can observe that induction of ANAs becomes more frequent as the time on treatment increases: from 12.19% at baseline to 37.5% at 12 months with ETN and from 25% to 50% with ADA. In the case of ADA, the patterns revealed by indirect immunofluorescence varied widely. No autoimmune connective tissue diseases were observed during follow-up.
Changes in ANAs During the First Year of Follow-up.
| Etanercept | |||
|---|---|---|---|
| 3 Mo | 6 Mo | 12 Mo | |
| PASI, mean (SD, range) | 3.78(3.47, 0-14.3) | 3.55(4.16, 0-16.9) | 5.13(7.06, 0-27.8) |
| PASI 75, No. (%) | 33 (82.5) | 14 (70.0) | 21 (67.7) |
| Combination therapy, No. (%): | 0 (0) | 0 (0) | 0 (0) |
| Autoimmune diseases, No. (%): | 3 (4.61) | 1 (1.53) | 0 (0) |
| Guttate psoriasis | |||
| Other | |||
| Severe adverse effects, No. (%): | 5 (7.69) | 0 (0) | 0 (0) |
| Upper respiratory tract infection | |||
| Other | |||
| ANAs (IFI), No./Total (%): | |||
| Positive | 5/41 (12.19) | 3/20 (15) | 12/32 (37.5) |
| Negative | 36/41 (87.8) | 17/20 (85) | 20/32 (62.5) |
| Positive ANAs (IFI). Titer, No. (%): | |||
| 1/160 | 4 (80) | 3 (100) | 8 (66.66) |
| >1/160 | 1 (20) | 0 (0) | 4 (33.33) |
| Positive ANAs (IFI). Pattern, No. (%): | |||
| Homogeneous | 5 (100) | 3 (100) | 1 (8.33) |
| Speckled | 11 (91.66) | ||
| Speckled/homogeneous | |||
| Nuclear | |||
| Anti-ENA (ELISA) antibodies, No./Total (%): | |||
| Positive | 0 (0) | 0 (0) | 1/32 (3.12) |
| Negative | 41/41 (100) | 20/20 (100) | 31/32 (96.87) |
| Anti-DNA (ELISA) antibodies, No./Total (%): | |||
| Positive | 0 (0) | 0 (0) | 1/12 (8.33) |
| Negative | 5 (100) | 3 (100) | 11/12 (91.66) |
| Anti-DNA (IFI) antibodies, No./Total (%): | |||
| Positive | 0 (0) | 0 (0) | 1/1 (100) |
| Negative | 0 (0) | 0 (0) | 0 (0) |
| Adalimumab | |||
|---|---|---|---|
| 3 Mo | 6 Mo | 12 Mo | |
| PASI, mean (SD, range) | 2.6(4, 0-19) | 1.9(4.1, 0-26.6) | 1.6(3.4, 0-15) |
| PASI 75, No. (%) | 28 (75.7) | 9 (81.8) | 17 (94.4) |
| Combination therapy, No. (%): | 3 (4.61) | 3 (4.61) | 1 (1.53) |
| Methotrexate | ACI: 1 (1.53) | NBUVB: 1 (1.53) | |
| Other: acitretin, NBUVB | ACI: 1 (1.53) | ||
| Autoimmune diseases, No. (%): | 0 (0) | ||
| Guttate psoriasis | 2 (3.07) | 1 (1.53) | |
| Alopecia areata | 1 (1.53) | ||
| Severe adverse effects, No. (%): | 0 (0) | 0 (0) | |
| Upper respiratory tract infection | 3 (4.61) | ||
| Urinary tract infection | 1 (1.53) | ||
| Double bypass | 1 (1.53) | ||
| ANAs (IFI), No./Total (%): | |||
| Positive | 12/48 (25) | 5/14 (35.71) | 15/30 (50) |
| Negative | 36/48 (75) | 9/14 (64.28) | 15/30 (50) |
| Positive ANAs (IFI). Titer, No. (%): | |||
| 1/160 | 9/12 (75) | 1/5 (20) | 7/15 (46.66) |
| >1/160 | 3/12 (25) | 4/5 (80) | 8/15 (53.33) |
| Positive ANAs (IFI), Pattern, No. (%): | |||
| Homogeneous | 1 (8.33) | 1/4 (25) | 4 (26.66) |
| Speckled | 7 (58.33) | 3/4 (75) | 10 (66.66) |
| Speckled/homogeneous | 1 (8.33) | ||
| Nuclear | 2 (16.66) | 1 (6.66) | |
| Homogeneous/nuclear | 1 (8.33) | ||
| Anti-ENA antibodies (ELISA), No./Total (%): | |||
| Positive | 1 (Scl 70) -> 2.08% | ||
| Negative | 47/48 (97.91) | 14/14 (100) | 30 (100) |
| Anti-DNA antibodies (ELISA), No./Total (%): | |||
| Positive | 0 (0) | 0 (0) | 1 (6.66) |
| Negative | 12/12 (100) | 5/5 (100) | 14 (93.33) |
| Anti-DNA (IFI), No./Total (%): | |||
| Positive | 0 (0) | 0 (0) | 0 (0) |
| Negative | 0 (0) | 0 (0) | 1 (100) |
Abbreviations: ACI, acitretin; ANA, antinuclear antibody; ENA, extractable nuclear antibody; IFI, indirect immunofluorescence; PASI, Psoriasis Area and Severity Index; NBUVB, narrowband UV-B.
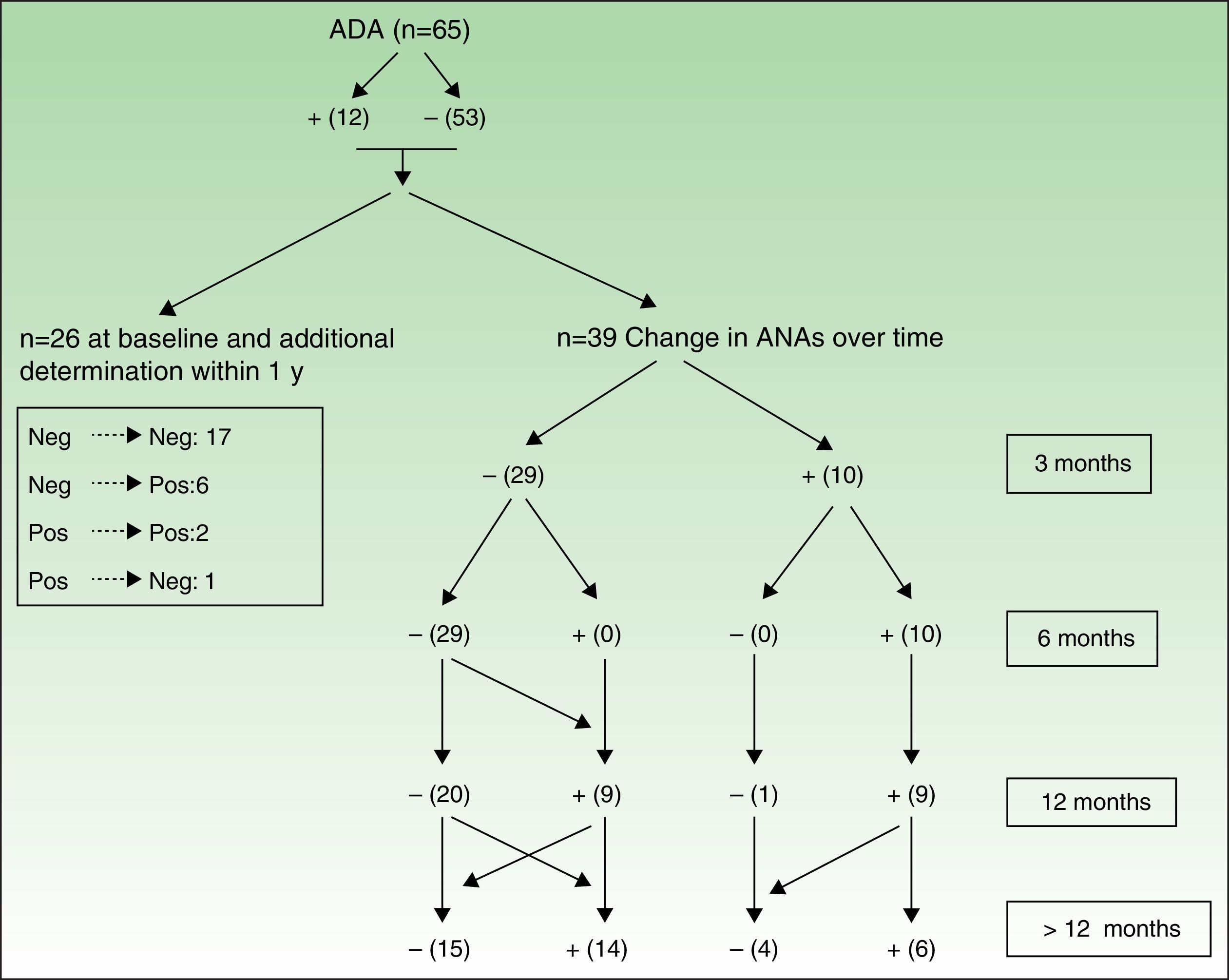
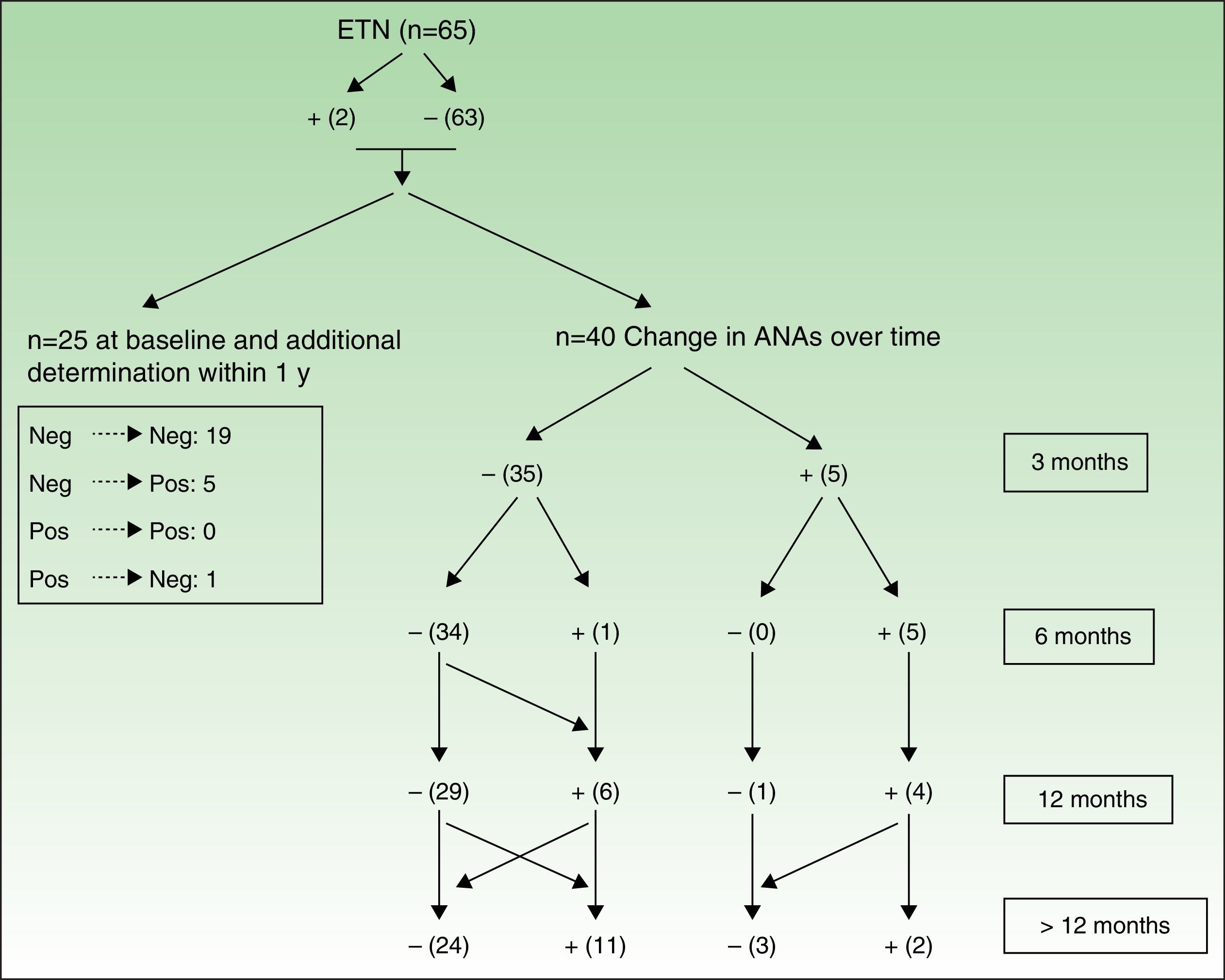
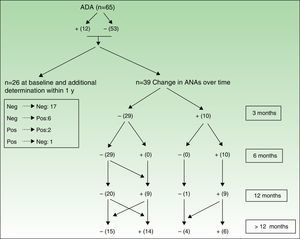
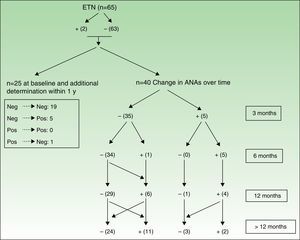
Figures 3 and 4 show how ANAs changed during the evaluation period.
Changes in ANAs after treatment with adalimumab. The values in the box on the left are from those patients who only had 1 baseline determination and at least 1 other determination in under 1 year, thus enabling them to enter the study but not to be followed up over time. On the right (Changes over time), we can see determination of ANA in patients who were followed up. All patients whose antibodies changed were considered to have undergone determination of ANA at all follow-up points in order to construct the table.
Changes in ANAs after treatment with ETN. See Figure 3 for explanation.
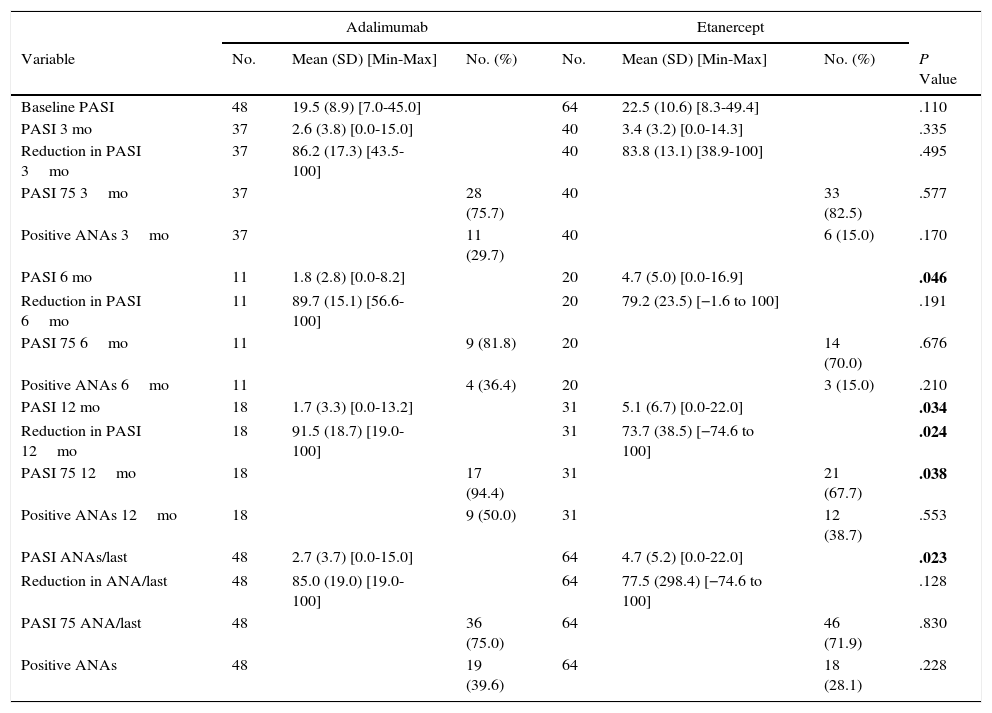
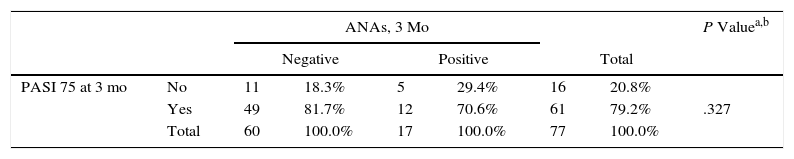
The effectiveness analysis revealed differences in favor of patients taking ADA compared with those taking ENT: the absolute mean PASI was lower at 6 and 12 months and overall (Table 3). However, this value is not reliable, since patients taking ADA started with statistically significantly lower baseline PASI values. At no point during the study was an association found between induction of ANAs and a 75% improvement in the PASI (PASI 75) (Table 4). Assessment of the effect of treatment using the Mantel-Haenszel test revealed that treatment did not significantly modify the association between PASI 75 and induction of ANAs at 3, 6, and 12 months or overall.
PASI and Time to PASI 75a,b
| Adalimumab | Etanercept | ||||||
|---|---|---|---|---|---|---|---|
| Variable | No. | Mean (SD) [Min-Max] | No. (%) | No. | Mean (SD) [Min-Max] | No. (%) | P Value |
| Baseline PASI | 48 | 19.5 (8.9) [7.0-45.0] | 64 | 22.5 (10.6) [8.3-49.4] | .110 | ||
| PASI 3 mo | 37 | 2.6 (3.8) [0.0-15.0] | 40 | 3.4 (3.2) [0.0-14.3] | .335 | ||
| Reduction in PASI 3mo | 37 | 86.2 (17.3) [43.5-100] | 40 | 83.8 (13.1) [38.9-100] | .495 | ||
| PASI 75 3mo | 37 | 28 (75.7) | 40 | 33 (82.5) | .577 | ||
| Positive ANAs 3mo | 37 | 11 (29.7) | 40 | 6 (15.0) | .170 | ||
| PASI 6 mo | 11 | 1.8 (2.8) [0.0-8.2] | 20 | 4.7 (5.0) [0.0-16.9] | .046 | ||
| Reduction in PASI 6mo | 11 | 89.7 (15.1) [56.6-100] | 20 | 79.2 (23.5) [−1.6 to 100] | .191 | ||
| PASI 75 6mo | 11 | 9 (81.8) | 20 | 14 (70.0) | .676 | ||
| Positive ANAs 6mo | 11 | 4 (36.4) | 20 | 3 (15.0) | .210 | ||
| PASI 12 mo | 18 | 1.7 (3.3) [0.0-13.2] | 31 | 5.1 (6.7) [0.0-22.0] | .034 | ||
| Reduction in PASI 12mo | 18 | 91.5 (18.7) [19.0-100] | 31 | 73.7 (38.5) [−74.6 to 100] | .024 | ||
| PASI 75 12mo | 18 | 17 (94.4) | 31 | 21 (67.7) | .038 | ||
| Positive ANAs 12mo | 18 | 9 (50.0) | 31 | 12 (38.7) | .553 | ||
| PASI ANAs/last | 48 | 2.7 (3.7) [0.0-15.0] | 64 | 4.7 (5.2) [0.0-22.0] | .023 | ||
| Reduction in ANA/last | 48 | 85.0 (19.0) [19.0-100] | 64 | 77.5 (298.4) [−74.6 to 100] | .128 | ||
| PASI 75 ANA/last | 48 | 36 (75.0) | 64 | 46 (71.9) | .830 | ||
| Positive ANAs | 48 | 19 (39.6) | 64 | 18 (28.1) | .228 | ||
Abbreviations. ANA, antinuclear antibody; PASI: Psoriasis Area and Severity Index.
Association Between Presence of ANAs and PASI 75.
| ANAs, 3 Mo | P Valuea,b | |||||||
|---|---|---|---|---|---|---|---|---|
| Negative | Positive | Total | ||||||
| PASI 75 at 3 mo | No | 11 | 18.3% | 5 | 29.4% | 16 | 20.8% | |
| Yes | 49 | 81.7% | 12 | 70.6% | 61 | 79.2% | .327 | |
| Total | 60 | 100.0% | 17 | 100.0% | 77 | 100.0% | ||
| ANAs, 6 Mo | ||||||||
|---|---|---|---|---|---|---|---|---|
| Negative | Positive | Total | ||||||
| PASI 75 at 6 mo | No | 7 | 29.2% | 1 | 14.3% | 8 | 25.8% | |
| Yes | 17 | 70.8% | 6 | 85.7% | 23 | 74.2% | .400 | |
| Total | 24 | 100.0% | 7 | 100.0% | 31 | 100.0% | ||
| ANAs, 12 Mo | ||||||||
|---|---|---|---|---|---|---|---|---|
| Negative | Positive | Total | ||||||
| PASI 75 at 12 mo | No | 6 | 21.4% | 5 | 23.8% | 11 | 22.4% | |
| Yes | 22 | 78.6% | 16 | 76.2% | 38 | 77.6% | 1.000 | |
| Total | 28 | 100.0% | 21 | 100.0% | 49 | 100.0% | ||
| ANAs Overall | ||||||||
|---|---|---|---|---|---|---|---|---|
| Negative | Positive | Total | ||||||
| PASI 75 last | No | 18 | 24.0% | 12 | 32.4% | 30 | 26.8% | |
| Yes | 57 | 76.0% | 25 | 67.6% | 82 | 73.2% | .370 | |
| Total | 75 | 100.0% | 37 | 100.0% | 112 | 100.0% | ||
Abbreviations. ANA, antinuclear antibody; PASI: Psoriasis Area and Severity Index.
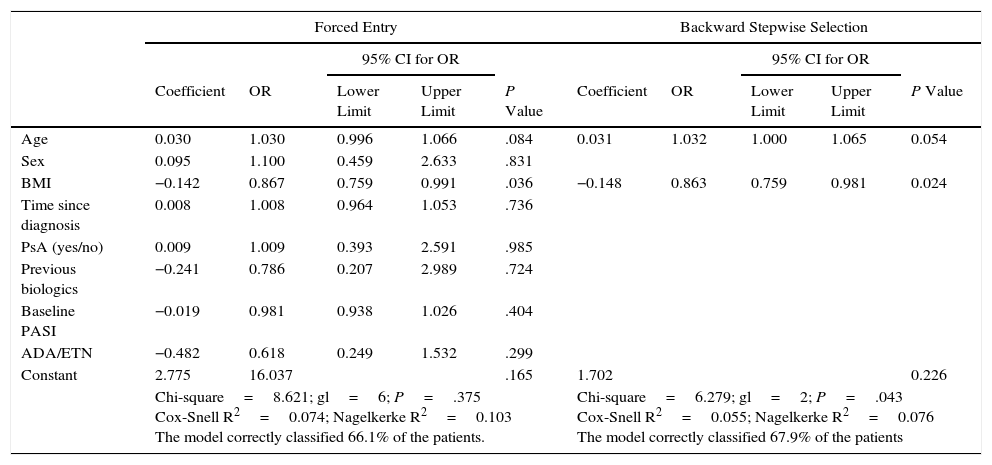
The only variable significantly associated with the induction of ANA was BMI (P=.024) (Table 5). The risk of induction of ANA fell as BMI increased, with an odds ratio (OR) of 0.867 (95% CI, 0.759-0.991), thus indicating that in the range 20-30 the risk decreases as BMI increases. The association with age did not reach statistical significance (P=.054). Although the overall adjustment was significant (P=.043), the ability of the independent variables to account for the dependent variable was very small (Cox-Snell R,2 0.055; Nagelkerke R,2 0.076). None of the variables studied correlated with the ability to reach PASI 75.
Analysis of Factors Associated With the Development of ANAs.
| Forced Entry | Backward Stepwise Selection | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 95% CI for OR | 95% CI for OR | |||||||||
| Coefficient | OR | Lower Limit | Upper Limit | P Value | Coefficient | OR | Lower Limit | Upper Limit | P Value | |
| Age | 0.030 | 1.030 | 0.996 | 1.066 | .084 | 0.031 | 1.032 | 1.000 | 1.065 | 0.054 |
| Sex | 0.095 | 1.100 | 0.459 | 2.633 | .831 | |||||
| BMI | −0.142 | 0.867 | 0.759 | 0.991 | .036 | −0.148 | 0.863 | 0.759 | 0.981 | 0.024 |
| Time since diagnosis | 0.008 | 1.008 | 0.964 | 1.053 | .736 | |||||
| PsA (yes/no) | 0.009 | 1.009 | 0.393 | 2.591 | .985 | |||||
| Previous biologics | −0.241 | 0.786 | 0.207 | 2.989 | .724 | |||||
| Baseline PASI | −0.019 | 0.981 | 0.938 | 1.026 | .404 | |||||
| ADA/ETN | −0.482 | 0.618 | 0.249 | 1.532 | .299 | |||||
| Constant | 2.775 | 16.037 | .165 | 1.702 | 0.226 | |||||
| Chi-square=8.621; gl=6; P=.375 Cox-Snell R2=0.074; Nagelkerke R2=0.103 The model correctly classified 66.1% of the patients. | Chi-square=6.279; gl=2; P=.043 Cox-Snell R2=0.055; Nagelkerke R2=0.076 The model correctly classified 67.9% of the patients | |||||||||
Abbreviations: ADA, adalimumab; ANA, antinuclear antibody; BMI, body mass index; ETN: etanercept; PsA, psoriatic arthritis; PASI, Psoriasis Area and Severity Index.
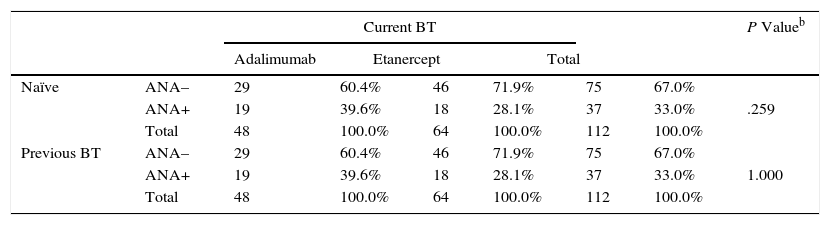
Given the possibility that administration of a TNFα inhibitor could lead to the induction of ANAs during treatment with a second TNFα inhibitor, we analyzed the order of use of ADA (high percentage of second treatments) and ETN (high percentage of patients who were biologic-naïve). However, no significant differences were observed between induction of ANA and previous therapy with biologics (naïve vs second or third TNFα inhibitor) (Table 6). The Mantel-Haenszel common OR was 0.544 (95% CI, 0.229-1.292), thus indicating that there was no reason to reject the independence between the variables analyzed.
Presence of ANAs and Previous Use of Antibiotic Treatmenta
| Current BT | P Valueb | |||||||
|---|---|---|---|---|---|---|---|---|
| Adalimumab | Etanercept | Total | ||||||
| Naïve | ANA– | 29 | 60.4% | 46 | 71.9% | 75 | 67.0% | |
| ANA+ | 19 | 39.6% | 18 | 28.1% | 37 | 33.0% | .259 | |
| Total | 48 | 100.0% | 64 | 100.0% | 112 | 100.0% | ||
| Previous BT | ANA– | 29 | 60.4% | 46 | 71.9% | 75 | 67.0% | |
| ANA+ | 19 | 39.6% | 18 | 28.1% | 37 | 33.0% | 1.000 | |
| Total | 48 | 100.0% | 64 | 100.0% | 112 | 100.0% | ||
Abbreviations: ANA, antinuclear antibody; BT, biologic therapy.
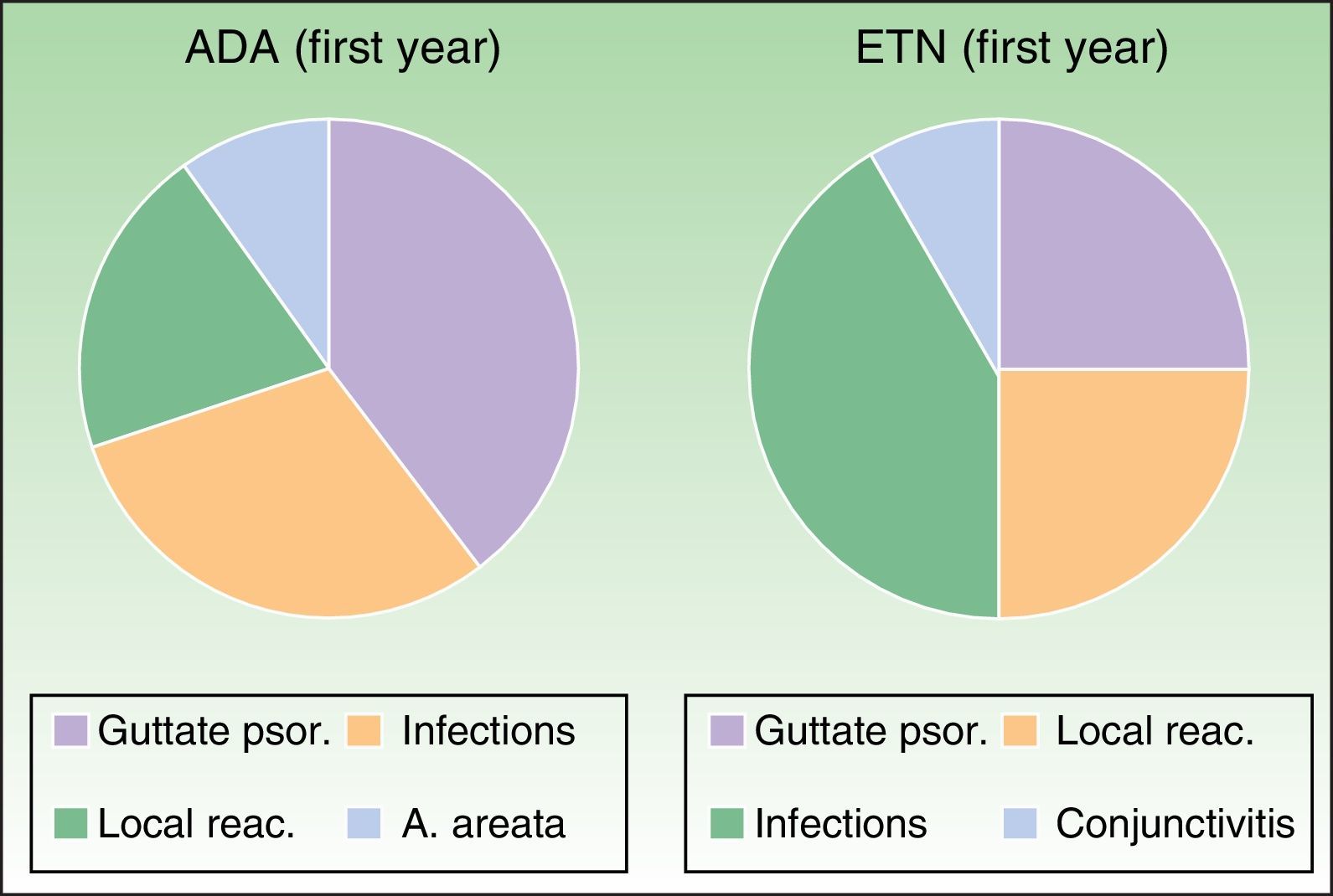
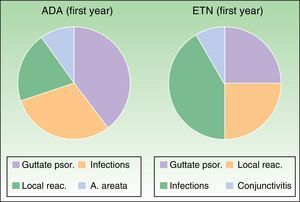
The adverse effects analysis showed that during the first year of follow-up in the ADA group, 4 cases were potentially caused by an autoimmune mechanism (1 paradoxical guttate psoriasiform reaction, 2local reactions at the injection site, and 1 case of alopecia areata, Fig. 5).
Most common adverse events during the first year of treatment. Adalimumab group: Guttate psoriasis (1.53%), infections (6.15%), local reactions (3.07%), A. areata (1.53%). Etanercept group: Guttate psoriasis (3.07%), infections (9.23%, with 1 case of conjunctivitis), local reactions (4.61%). A. indicates alopecia; ADA, adalimumab; ETN, etanercept; psor., psoriasis; reac., reactions.
Subsequently, there were 2new cases of guttate psoriasiform reactions at 2 and 3 years and 1 case of urticaria at 5 years. The severe adverse effects recorded included 1 case of cervical and lumbar hernia and 1 fractured humerus, both of which required the patient to be hospitalized. Severe adverse effects also included adenocarcinoma of the head of the pancreas, type 1 papillary renal cell carcinoma, and 1 case of Alzheimer disease.
In the ETN group, the only adverse events during the first year that were considered to be autoimmune in origin comprised 4cases of paradoxical guttate psoriasis and 3 local reactions (Fig. 5). During subsequent follow-up, the only events described were mild respiratory tract infections, 1 abortion, 1 case of Alzheimer-type cognitive impairment, 1 case of chronic obstructive pulmonary disease, 2cataract operations, 2herniorraphies, and 1 case of lumbar-sciatic pain. There were no reports of lupus erythematosus, other connective tissue disease, or vasculitis. The abovementioned cases of potentially autoimmune origin were not associated with induction of ANAs in the study patients.
DiscussionTNFα inhibitors are of enormous benefit in patients with moderate-to-severe psoriasis whose previous treatment with other options has proven ineffective. However, induction of antibodies and subsequent adverse reactions mean that these agents have to be used with care in patients with autoimmune disease.5,7,9 Viana et al.13 believed that a homogeneous baseline ANA pattern and anti-dsDNA antibodies leave patients at greater risk of developing autoimmune diseases, such as lupus erythematosus.
A review of the literature reveals controversy over the role of previous therapy with a TNFα inhibitor in efficacy and its potential to predispose to induction of ANAs or autoimmune disease during treatment with a second or third TNFα inhibitor.6,14 We found no association whatsoever between these variables and previous therapy with biologics, thus indicating that they could be associated with genetic susceptibility. Given the possible transient increase in antibody titers after streptococcal infection resulting from the structural similarity between streptococcal M protein and keratin 12, some authors recommend verifying whether patients had an infection when their ANA titer was being determined.1
In addition to previous treatment, we analyzed other potential predictors of induction of ANAs, such as association with psoriatic arthritis, sex, and baseline PASI, although no significant associations arose. In the case of age, the association did not reach statistical significance, despite being close (P=.054). We found no references in the literature associating age with induction of ANA in patients treated with biologics. BMI was the only parameter associated with the development of ANA, albeit as a protective variable. However, we have no reasonable explanation for this observation and found no relevant data in the literature.
Recent studies have proposed that induction of antibodies could result from the reduction in concentrations of the cytokines that participate in clearance of nuclear material in apoptosis (as is the case with C-reactive protein), thus leading to a greater predisposition to formation of ANAs and development of autoimmunity. An association has also been reported with abnormalities of cell apoptosis, which could lead to the release of antigens in the nucleosome and formation of anti-dsDNA antibodies in predisposed individuals. Bardazzi et al.1 reported that whereas ADA and infliximab inhibit the transmembrane and soluble fraction of TNFα, ETN inhibits only the soluble fraction, thus potentially leading to less pronounced cell apoptosis and less pronounced induction of antibodies (although this would not explain the fact that induction of ANAs is less prevalent with ADA than with infliximab).1 Nevertheless, these hypotheses have not been confirmed and would depend to a large extent on individual susceptibility to the drug.3,7,10,15
Although induction of ANAs in patients taking biologics seems quite common (33.3%-77% depending on the series),1,4,13 development of autoimmune diseases is rather uncommon in psoriasis (<1%). This finding was reflected in our study: the only situations that potentially had an autoimmune origin during follow-up were 1 case of alopecia areata and various paradoxical psoriasiform reactions. This could be due to the isotypes of the anti-dsDNA antibodies induced by the drugs: while in other autoimmune diseases, it is IgG forms that participate in pathogenesis, therapy with TNFα inhibitors induces IgM and IgA anti-dsDNA.2–4
It is well known that some patients develop resistance to TNFα inhibitors, which is a cause of secondary failures of efficacy. Although the underlying mechanism is unknown, Pink et al.7 proposed that induction of ANAs during treatment could predispose to a failed response to the drug. In contrast, other studies have not shown any association between induction of antibodies and the loss of response to treatment.1,8 Lora et al.15 went so far as to suggest a trend towards a better response to the drug with more rapid development of ANA. In the present study, the Fisher exact test did not demonstrate a correlation between induction of ANAs and clinical response to the drug (PASI 75).
Our study is subject to a series of limitations. First, its design was retrospective. Consequently some data were lost, since not all data were collected at all the visits. Second, the number of patients was limited, there was a fair degree of variability between them, and the duration of follow-up varied. Third, the limitations of the techniques used to detect ANAs and other antibodies could have led to false positives and false negatives.
Conclusions- •
Induction of ANAs is significantly increased during treatment of moderate-to-severe psoriasis with ADA and ETN, although it is not accompanied by development of autoimmune disease. In contrast, there is no significant increase in induction of anti-dsDNA or anti-ENA antibodies.
- •
No correlation was observed between induction of ANAs and effectiveness of treatment, the order in which the drugs are administered, or the appearance of adverse effects. Similarly, it was not possible to demonstrate factors that predicted induction of ANAs, except for BMI (inversely proportional).
- •
We recommend determination of ANAs and screening for autoimmune diseases before starting therapy with TNFα inhibitors. However, serial, routine determination during follow-up is not necessary, except in those cases where there are signs or symptoms of suspected autoimmune disease.
The authors declare that no tests were carried out in humans or animals for the purposes of this study.
Confidentiality of dataThe authors declare that they have followed their institutional protocols on publication of patient data.
Right to privacy and informed consentThe authors declare that no private patient data appear in this article.
Conflicts of InterestThe authors declare that they have no conflicts of interest
We are grateful to Cristina Carazo for her help with data collection and her work on the database. We are also grateful to Jesús Garrido for the statistical analysis.
Please cite this article as: Oter-López B, Llamas-Velasco M, Sánchez-Pérez J, Dauden E. Inducción de anticuerpos y enfermedades autoinmunes en pacientes con psoriasis tratados con fármacos anti-TNFα. Actas Dermosifiliogr. 2017;108:445–456.