Incontinentia pigmenti (Bloch-Sulzberger syndrome) is a rare neuroectodermal dysplasia. It is an X-linked dominant disorder caused by mutations in the IKBKG/NEMO gene on Xq28. Approximately 80% of patients have a deletion of exons 4 to 10. Incontinentia pigmenti has an estimated incidence of 0.7 cases per 100 000 births. In hemizygous males, it is usually lethal, while in females, it has a wide spectrum of clinical manifestations. Incontinentia pigmenti is a multisystemic disease that invariably features skin changes. These changes are the main diagnostic criteria and they evolve in 4 stages, in association with other abnormalities affecting the central nervous system, eyes, teeth, mammary glands, hair, nails, skin, and other parts of the body. The aim of this brief review is to highlight the clinical features of this genodermatosis and underline the importance of case-by-case interdisciplinary management, including genetic counseling.
La incontinencia pigmenti (síndrome de Bloch-Sulzberger) es una displasia neuroectodérmica infrecuente, con patrón de herencia ligado al X dominante, causada por mutaciones en el gen IKBKG/NEMO y se encuentra localizado en Xq28. La deleción del exón 4 al 10 corresponde con la principal causa en aproximadamente el 80% de los casos. La incidencia estimada es de 0,7 en 100.000 nacimientos, usualmente letal en hombres hemicigotos y en el sexo femenino puede exhibir hallazgos clínicos variables. Es una entidad multisistémica, que incluye defectos en la piel, siempre presente y principal criterio diagnóstico, la cual evoluciona en cuatro etapas, asociada a alteraciones en el sistema nervioso central, globo ocular, dientes, glándula mamaria, pelo, uñas, entre otros. El objeto de esta breve revisión es resaltar los hallazgos clínicos de esta genodermatosis, con la finalidad de brindar el seguimiento médico individualizado e interdisciplinario que incluya un adecuado asesoramiento genético familiar.
Incontinentia pigmenti (OMIM 308300), a rare neuroectodermal dysplasia,1,2 is an X-linked dominant disorder caused by mutations in the IKBKG/NEMO gene (GenBankNM_003639.3, OMIM 300248), formerly known as NEMO. The gene is located on Xq28 and encodes a kappa light polypeptide gene enhancer in B cells, kinase gamma, which has a key role in the modulation of nuclear transcription factor kappa B (NF-κB).1,3–7
Incontinentia pigmenti was described by Bloch in 1926 and Sulzberger in 19284 and is hence also known as Bloch–Sulzberger syndrome.6,8 The causative gene has high penetrance and variable expressivity.4 The 11.7-kb deletion spanning exons 4 to 10 is the main cause of incontinentia pigmenti and is found in approximately 80% of cases.3,6,9,10 It is caused by recombination between two MER67B repeats in introns 3 and 10 of the gene, resulting in loss of protein function. Small insertion-deletion and nonsense mutations have also been described. Some hypomorphic IKBKG/NEMO mutations cause a distinct entity: hypohidrotic ectodermal dysplasia with immunodeficiency (OMIM 300291).6,11
EpidemiologyIncontinentia pigmenti has an estimated incidence of 0.7 cases per 100 000 births10 and 27.6 new cases annually. Between 65% and 75% of cases are due to sporadic mutations and the remaining cases are familial.2 The condition is usually lethal in hemizygous male embryos.2–4,12,13 Female patients can survive due to functional mosaicism resulting from X chromosome inactivation.2,4,9 The clinical findings in such cases are highly variable.3 Incontinentia pigmenti is therefore predominant in females, with a female to male ratio of 37:1. Male patients with clinical features of incontinentia pigmenti may also have Klinefelter syndrome (47, XXY). Survival in such cases is possible due to the second chromosome X or somatic mosaicism for the above-mentioned common deletion.4,5,8
Clinical Features and ManagementIncontinentia is a multisystemic disease4,5,8 that affects both ectodermal and mesodermal tissues. The skin is always involved and is the main diagnostic criterion.9 Additional alterations affect the central nervous system, eyes, teeth,3,4,10,12 mammary glands, hair, nails, and skeleton. Cardiopulmonary alterations are also observed, albeit less frequently.12
Landy and Donnai14 established the first diagnostic criteria for incontinentia pigmenti in 1993, before discovery of the causative gene.10 In 2014, Minić et al.15 proposed a series of updated criteria, and in 2018, with the permission of the authors, Rosser3 modified these criteria to improve the phenotypic characterization of the disease and further update the diagnostic criteria. The major criteria proposed were the typical dermatologic findings observed in incontinentia pigmenti that are used to establish a clinical diagnosis,3,5 while the minor criteria corresponded to other possible alterations. Additional diagnostic criteria include a positive family history (in a first-degree relative) and a mutation in the IKBKG/NEMO gene. These are described in Table 1,2–20 together with the other criteria described by Rosser3 and the respective frequencies as reported in the literature.
Proposed Diagnostic Criteria for Incontinentia Pigmenti and Prevalence of Some of the Corresponding Conditions as Reported in the Literature.a
| Criteria | Frequency, % |
|---|---|
| Major | |
| Typical skin lesions following the lines of Blaschko | 100 |
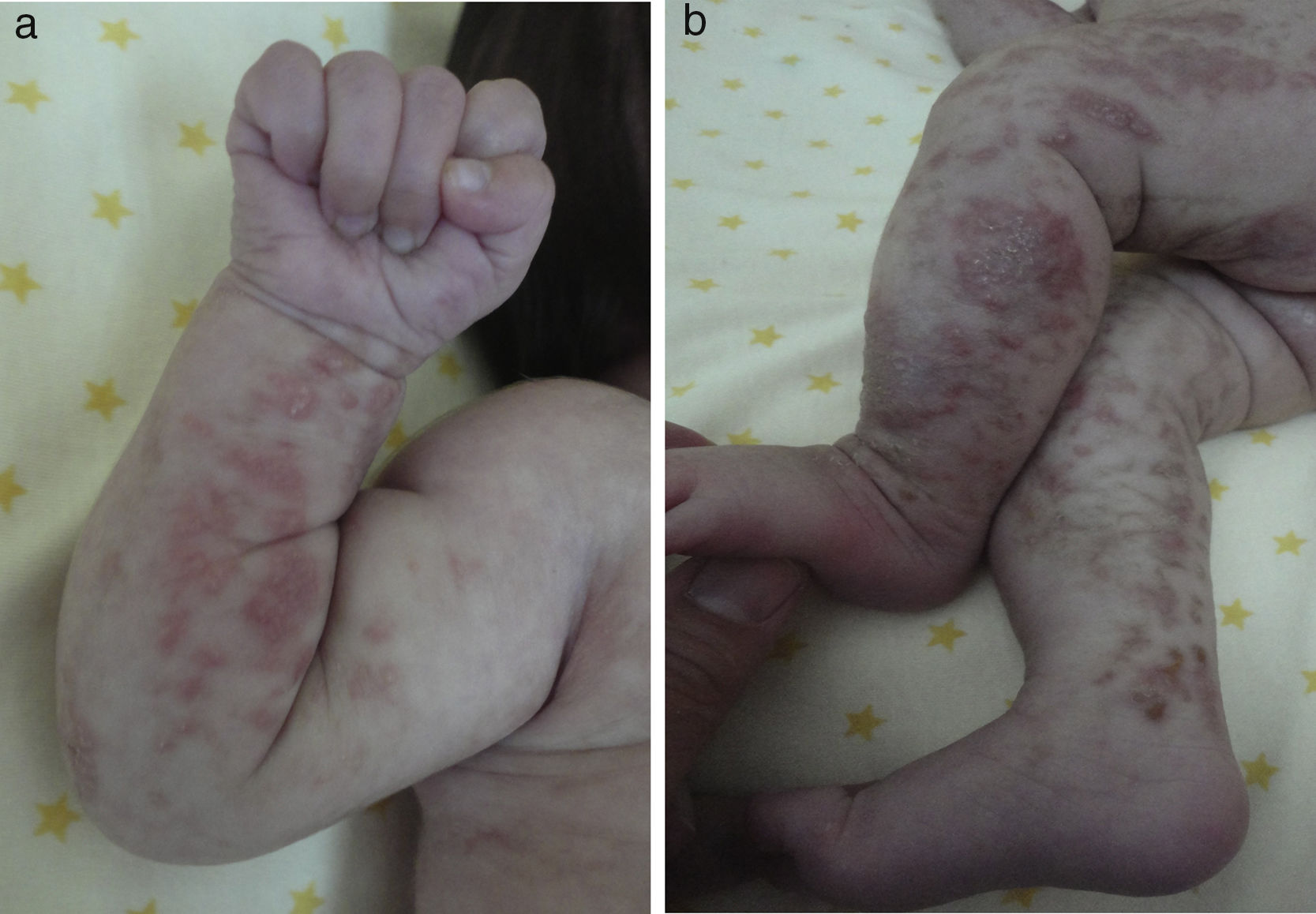
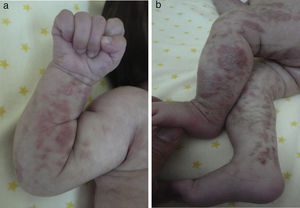
| Stage I — vesiculobullous stage: erythematous or inflammatory lesions characterized by vesicles, bullae, or pustules; more common on extremities and scalp | |
| Present from birth to second week of life | |
| Stage II — verrucous/hyperkeratotic stage: hyperpigmented pustules or crusts | |
| Occurs from week 2 to week 6 | |
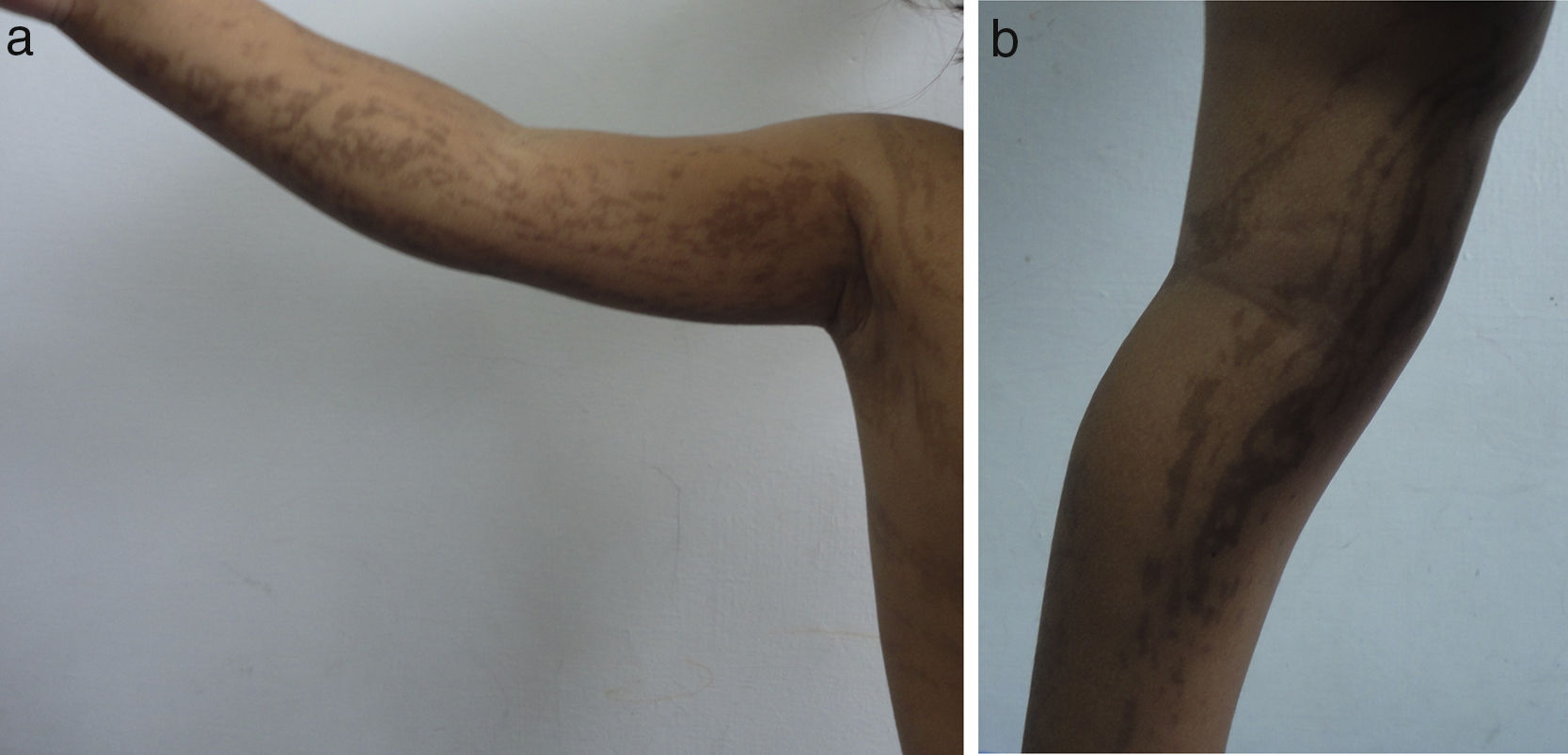
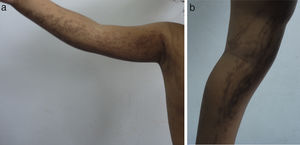
| Stage III — hyperpigmentation stage: whorled hyperchromic macules; predominant in intertriginous areas and on trunk | |
| These appear between week 12 and 26 and can improve in adolescence or persist into adulthood | |
| Stage IV — atrophy/hypopigmentation stage: hypochromic macules and possibly alopecia | |
| This stage is not always present | |
| The above stages may overlap and not necessarily follow the given order | |
| Minor | |
| Central nervous system/neurologic impairments | 30 |
| Seizures | 40.2 |
| Spastic paralysis | 16.5 |
| Psychomotor delay | 26 |
| Mental retardation | 29.9 |
| Microcephaly | 11.3 |
| Cerebral/cerebellar atrophy | 13.4a |
| Microgyria/polymicrogyria | |
| Hypoplasia of the corpus callosum | |
| Basal ganglia alterations | |
| Periventricular leukomalacia | |
| Hydrocephaly | 5.1 |
| Porencephaly | |
| Ischemic stroke | |
| Diffuse hemorrhagic necrosis | |
| Encephalomyelitis | |
| Eye alterations | |
| Visual defects | 17 |
| Retinopathy | 15.9 |
| Retinal detachment | 8.5 |
| Retinal vascular alteration | 5.3 |
| Pigmentation disorders (hyper/hypopigmentation) | |
| Optic atrophy | |
| Foveal hypoplasia | |
| Retrolental fibroplasia | |
| Cataracts | |
| Microphthalmia | 6.4 |
| Strabismus | |
| Nystagmus | |
| Dental alterations | 17-34 |
| Delayed primary tooth eruption | 34.3 |
| Anodontia/hypodontia | |
| Microdontia | |
| Dental dystrophy | 17.2 |
| Abnormal (conical) shape | 22.3 |
| Impacted teeth | 17.2 |
| Diastema | |
| Malocclusion | |
| Palate disorders | |
| High-arched palate | |
| Mammary gland alterations | |
| Supernumerary nipple | |
| Hair abnormalities (hair, eyebrows, eyelashes) | 28-38 |
| Alopecia | 9.7 |
| Hypertrichosis | 3.6 |
| Nail disorders | |
| Dystrophy | 64 |
| Yellowing | |
| Vertical or horizontal splitting | |
| Male miscarriage | |
| Typical histopathologic findings in skin | |
| Additional conditions for confirming diagnosis | |
| No history of incontinentia pigmenti in a first-degree female relative | |
| If molecular diagnosis is not available, at least 2 major criteria or 1 major criterion plus 1 minor criterion are needed to confirm diagnosis | |
| An IKBKG mutation combined with any major or minor criterion confirms diagnosis | |
| History of incontinentia pigmenti in a first-degree female relative | |
| One major criterion or 2 minor criteria | |
| In all cases, diagnosis is supported by eosinophilia and skewed X-chromosome inactivation |
aAdapted from references 2–20
Skin lesions are the presenting manifestation. They appear at birth or in the first 2 weeks of life, progress over a period of years, and are distributed along the Blaschko lines. There are 4 classic stages: a vesicobullous stage (Fig. 1), a verrucous/hyperkeratotic stage, a hyperpigmented stage (Fig. 2), and an atrophic/hypopigmented stage.19,20 Not all patients experience the 4 stages,19 and the first 3 can appear simultaneously.20 These lesions are described in more detail in Table 1.2–20 The term incontinentia pigmenti is linked to the histologic characteristics of the lesions in stage 3 of the disease (hyperpigmentation) and specifically to melanin incontinence from melanocytes in the basal epidermal layer and deposits in the superficial dermis.4 These manifestations generally do not require specific intervention3 or treatment. Treatment with topical corticosteroids or tacrolimus can delay progression of the vesicobullous stage, although the lesions resolve spontaneously.18
Incontinence pigmenti largely becomes serious when there are neurological alterations,9,10 as these can cause considerable morbidity and mortality.19 Neurologic impairments are varied in nature, but the most common are seizures,9,21 which are generally tonic22 or focal clonic, last for a short time, and cause loss of consciousness. They are more common in the neonatal period,9 are resistant to treatment, and have a poor prognosis. Other disorders include microcephaly, ischemic stroke, cerebellar ataxia, and delayed psychomotor development. Acute signs of encephalitis mainly appear in the first 4 days of life and include food intolerance, lethargy, and seizure-like movements. Brain magnetic resonance imaging with angiography is therefore essential in neonates with characteristic cutaneous incontinentia pigmenti lesions. Follow-up studies are also necessary to prevent sequelae.3,9,22
Eye impairment is observed in between 50% and 77% of patients. The most serious alterations involve the retina,16 and retinal problems such as detachment and avascular retina are the most feared of complications. These can be treated with laser photocoagulation targeting retinal neovascularization. Cryotherapy can also be used and promising results have been observed with vascular endothelial growth factor inhibitors.18 Optical coherence tomography images show internal and external retinal layer thinning. Eye abnormalities similar to retinoblastoma have been described in some cases of incontinentia pigmenti and include leukocoria, strabismus, intraocular calcification, and, as already mentioned, retinal detachment. The differential diagnosis is therefore important.16 Patients should have an eye examination every month for the first 4 months, every 3 months up to 1 year of age, every 6 months up to 3 years of age, and every 12 months thereafter.18
Etiology and PathogenesisNF-κB is activated by the protein product of IKBKG/NEMO, which plays a key modulatory role in multiple physiological functions, such as immune response and stress, inflammatory reactions, ectodermal development, cell adhesion, and protection of cells against tumor necrosis factor–induced apoptosis.3,4,6,22
The IKBKG/NEMO mutation leads to decreased NF-κB activity, thereby increasing susceptibility to apoptosis.4 Extensive apoptosis in males is responsible for early fetal death10 and liver disorders.2
Inflammatory reactions and epidermal recruitment of eosinophils, which occur in the first stage of disease, tend to have an important pathogenic role.4 This is probably related to eosinophil-selective chemokine (eotaxin),4,16 which is produced by specific leukocytes, such as eosinophils, macrophages, and T cells, and structural cells such as endothelial cells, fibroblasts, and epithelial cells.4
Differential DiagnosisThe entities that should be contemplated in the differential diagnosis vary with disease stage. During the first stage, incontinentia pigmenti may be confused with multiple infections of the newborn, such as congenital herpes simplex, varicella, epidermolysis bullosa, and bullous pemphigoid.18,23 As neonatal herpes and incontinentia pigmenti can coexist, treatment with acyclovir must be started as soon as a viral infection is confirmed. The differential diagnosis in the second stage is limited and should include linear epidermal nevus.23 The third stage is the hallmark stage of incontinentia pigmenti and must be distinguished from linear and whorled nevoid hypermelanosis. The fourth stage, if present, can be mistaken for hypomelanosis of Ito or vitiligo. These conditions, however, lack an initial inflammatory phase and do not generally affect the appendages.18
Conclusions and Genetic CounselingThe diagnosis of incontinentia pigmenti is based on clinical findings.18 Nonetheless, in view of the enormous variability of clinical features and the broad spectrum of molecular alterations, it is difficult to establish a homogeneous group of patients and standardize treatment. Although progress has been made in our understanding of the causes of incontinentia pigmenti and in clinical care research, the scarcity of patients seen in diagnostic centers makes it difficult to obtain a global epidemiological picture. Integration of these data could make an important contribution to future scientific endeavours.10
When dealing with a multisystemic disorder, long-term, individualized, multidisciplinary follow-up is necessary, with evaluation by dermatologists, neurologists, ophthalmologists, and dentists, among others.3,24 Congenital hypothyroidism, myasthenia gravis, and Wilms tumor have been described in certain cases of incontinentia pigmenti,18 and one group of authors who recently observed liver disease in a patient with incontinentia pigmenti suggested including hepatic evaluation in this setting, even though they were unable to establish a link between the 2 conditions.1 This could mean that even broader care may be warranted in patients with incontinentia pigmenti.
Moreover, the updated diagnostic criteria proposed by Rosser3 do not include skeletal alterations, (e.g., low stature, hemivertebra, kyphosis, scoliosis, supernumerary ribs, hip dysplasia, hemiatrophy, club foot, 4 or toe syndactyly4,10) or cardiopulmonary disorders (e.g., interatrial communication,4 left ventricular endomyocardial fibrosis, tricuspid insufficiency, tetralogy of Fallot,4,17 or pulmonary hypertension, even in the absence of cardiovascular alterations).2,4 Risk of recurrent infection is also not currently contemplated.10 Future updates will probably incorporate many of these conditions to cover the main clinical manifestations of this disease.
Genetic studies are essential for advancing our understanding of incontinentia pigmenti and confirming diagnoses through molecular diagnostic techniques, particularly in doubtful cases.18 Prognosis is generally good, but, as already mentioned, regular follow-up by a multidisciplinary team is important. Genetic family counseling is also important in patients with an X-linked dominant disorder,18,23 which is usually lethal in male patients. Female patients in this case have a 50% chance of having a daughter with incontinentia pigmenti. Miscarriage occurs in approximately 50% of male fetuses. Males that do survive should be referred for a cytogenetic study.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
We would like to thank the Incontinentia Pigmenti Genetic Biobank (www. igb. cnr. it/ ipgb), which forms part of the Biobanking and BioMolecular Resources Research Infrastructure-European Research Infrastructure Consortium (BBRMI-ERIC), and the CNR-DSB Flagship Project InterOmics.We also thank Rosalía Gumina F, Managing Director of the Library at the Instituto Autónomo Hospital Universitario de Los Andes, Universidad de Los Andes.
Please cite this article as: Cammarata-Scalisi F, Fusco F, Ursini MV. Incontinencia pigmenti. Actas Dermosifiliogr. 2019;110:273–278.