Lichen sclerosus (LiS) is a chronic scleroatrophic condition that usually affects the anogenital area and occasionally the extragenital sites. CD34-positive dermal dendritic cells (DDCs) contribute to the maintenance of the dermal microarchitecture and modulation of the immune response. p53 is a tumor suppressor gene important for the regulation of the cell cycle and apoptosis. Similar to morphea (a LiS-closely related scleroatrophic condition), dermal sclerosis, alterations of DDCs, and dermal microvasculature may be important underlying pathogenetic mechanisms in LiS.
ObjectivesTo examine the profile of CD34-positive DDCs, microvessel density (MVD), and p53 protein in LiS.
Materials and methodsThe immunohistological profiles of DDCs, MVD, and p53 were examined in 19 cases of LiS and their age- and sex-matched normal skin (10 specimens), using antibodies against CD34 and p53.
ResultsThere was a markedly decreased counts (1.7 ± 0.5/mm2) or complete loss of CD34-positive DDCs in LiS against their abundance in the normal skin (23.4 ± 2.1/mm2, p = 0.000). MVD was markedly increased in LiS lesions (20 ± 0.47) as compared to normal skin (5.50 ± 0.20, p = 0.000). Discontinuous single-cell p53 weakly positive nuclear staining was seen in the epidermal basal cell keratinocytes in normal skin and LiS lesions.
ConclusionsTo the best of this author's knowledge, this is the first study analyzing DDCs, MVD, and p53 profiles together in LiS. The findings suggest that alterations of DDCs and MVD have roles in the pathogenesis of LiS.
El liquen escleroso (LiE) es una enfermedad crónica escleroatrófica que afectará generalmente el área anogenital y ocasionalmente a localizaciones extragenitales. Las células dendríticas dérmicas CD34 positivas (DDC) contribuyen al mantenimiento de la microarquitectura dérmica y a la modulación de la respuesta inmunitaria. El p53 es un gen supresor de tumores importante para la regulación del ciclo celular y de la apoptosis. De manera similar a lo que ocurre en la morfea (una condición escleroatrófica estrechamente relacionada con el LiE), la esclerosis dérmica, las alteraciones de las DDC y de la microvasculatura dérmica pueden ser mecanismos patogénicos subyacentes importantes en el LiE.
ObjetivosExaminar el perfil de las DDC positivas para el CD34, la densidad de microvasos (MVD) y la proteína p53 en el LiE.
Materiales y métodosSe evaluaron los perfiles inmunohistológicos de las DDC, de la MVD y del p53 en 19 casos de LiE y en la piel normal de pacientes emparejados por edad y sexo (10 muestras), utilizando los anticuerpos contra el CD34 y el p53.
ResultadosHubo una marcada disminución de los recuentos (1,7 ± 0,5/mm2) o pérdida completa de DDC CD34 + en el LiE en comparación con su elevada expresión en la piel normal (23,4 ± 2,1/mm2, p = 0,000). La MVD estaba notablemente aumentada en las lesiones de LiE (20 ± 0,47) en comparación con la de la piel normal (5,50 ± 0,20, p = 0,000). Se observó una tinción nuclear discontinua, de células aisladas, débilmente positiva para el p53, localizada en los queratinocitos de las capas basales epidérmicas de la piel sana y de la piel afectada por el LiE.
ConclusionesHasta donde tenemos conocimiento, este es el primer estudio que analiza los perfiles de las DDC, de la MVD y del p53 de manera simultánea en el LiE. Los hallazgos sugirieron que las alteraciones de las DDC y de la MVD tienen papeles en la patogénesis del LiE.
Lichen sclerosus (LiS) is an insidious, chronic scleroatrophic inflammatory disease of unknown etiology. It affects the anogenital area such as the vulva (vulvar LiS), the male genitalia (LiS of the male genitalia or balanitis xerotica obliterans), and occasionally some extragenital sites such as buttocks, inner thigh, and under the breasts (extragenital LiS). LiS of the male genitalia usually affects the glans penis, foreskin (prepuce), the anterior urethra, and meatus, usually of uncircumcised males, resulting in phimosis and meatal stenosis1. Squamous cell carcinoma is the most common malignancy of the vulva. Its precursor lesions include the usual and the differentiated types of vulvar intraepithelial neoplasia (VIN). The differentiated VIN is associated with chronic dermatoses such as LiS and can progress to invasive squamous cell carcinoma. Human papillomavirus (HPV) DNA is always absent in these lesions. The usual type VIN affects is associated with high-risk HPV infection2.
In 1984, CD34 antigen was first discovered on hematopoietic stem and progenitor cells. It is a transmembrane phosphoglycoprotein that consists of a heavily sialylated, O-linked glycosylated extracellular domain, a single transmembrane helix, and a cytoplasmic tail. Dendritic cells (DCs) are professional antigen-presenting cells. In the skin, there are CD34-positive dendritic cells (DDCs) in the dermis. CD34 is also expressed by vascular endothelial progenitors, and clusters of newly formed endothelial cells (microvessels), and therefore CD34 expression is an indicator of microvessel density (MVD) and tissue vascularization3,4.
LiS is closely related to other scleroatrophic conditions such as morphea and scleroderma. Some authorities consider LiS as a superficial or subepidermal morphea, or as scleroderma of the papillary dermis5,6. These viewpoints are supported by several observations including the coexistence of morphea and LiS in some patients, the overlapping clinicopathological features, and their common possible underlying etiologies (Borrelia infection and autoimmunity)5,7 in both of them. Histologically, both morphea and LiS are characterized by the attenuation of the epidermis, which is reasoned to the aftermath of the dermal stromal changes including dermal hyalinization, sclerosis with decreased vascularization, and impaired tissue perfusion5–7.
Although to date the exact role and origin of DDCs are largely unknown, some investigations have suggested that DDCs are important for maintaining the dermal microarchitecture and the modulation of the immune response, and as such, they contribute to the development of scleroatrophic conditions such as LiS, morphea, and scleroderma8–11. In morphea and scleroderma, previous studies have shown a decreased quantity of CD34-positive DDCs in the dermis8–12. Aiba et al. examined the skin biopsies from lesions of 27 cases with scleroderma and also biopsies from normal skin of 17 individuals using antibodies against CD34. CD34-positive DDCs were either few or completely absent in the lesional skin of scleroderma. The authors suggested that CD34-positive cells may represent primary target cells in the autoreactive phenomenon in scleroderma8. Similarly, Gilmour et al. examined the number of CD34-positive DDCs in 33 skin biopsies of morphea and found a progressive decline in the numbers of these cells with the progression from the inflammatory to the sclerotic stages of morphea11. Moreover, there was an increased MVD both in both morphea and scleroderma13,14. Taken together, CD34-positive DDCs seem to contribute to the development of scleroderma and morphea. To date, similar studies in LiS are limited13,14.
The tumor suppressor p53 gene encodes for a 53 KD nuclear protein that plays important roles in the regulation of cell cycle, and apoptosis15. In most benign cells, including the keratinocytes of the penile foreskin and vulvar skin16,17, the expression of p53 protein is either absent or weak. Overexpression of p53 protein is frequent in the dysplastic and malignant squamous epithelial cells18. In LiS, p53 protein expression is weak to moderate nuclear, discontinuous, and localized to the basal cell keratinocytes (Pattern 0)17,19.
To date, we have limited knowledge about the profiles of CD34-positive DDCs, MVD, and p53 protein expression in LiS. This study used immunohistochemical techniques to address these issues.
Materials and methodsThis is a retrospective case series in which the archival formalin-fixed paraffin-embedded tissues of 19 cases of LiS (sclerotic stage), from the Pathology Department, Assuit University Hospitals, and consultation files of this author, were used. All the materials (paraffin-embedded tissue blocks, slides, and pathology reports) were coded. The information obtained was analyzed and reported in such a way that the identities of the participants cannot be ascertained. The study did not include any interaction or intervention with human subjects or included any access to any identifiable private information. Therefore the study did not require institutional review board review20. The study was performed in accordance with the principles of the Declaration of Helsinki. The materials represented vulvar (11 cases), extragenital LiS (two cases), and LiS of the male genitalia (6 cases). The control group (normal skin) included ten healthy age and sex-matched individuals who underwent skin excisions for benign skin lesions. Paraffin-embedded tissues were sectioned for routine histological (Hematoxylin and Eosin stains) and special stains (elastic fibers, Verhoeff-van Gieson stain, VVG). The histologic sections were examined for the features of the usual and differentiated types of VIN. The tissue sections were prepared for immunostaining following other groups3,18. Heat-induced epitope antigen retrieval technique was used (Dako Target Retrieval Solution, S1699; Dako, Carpinteria, California, USA) for 30 min at 95 °C followed by a 20-minute cooldown. The endogenous peroxidase activity was blocked with 10% hydrogen peroxide. Anti-CD34 primary antibody (DakoCytomation (Glostrup, Denmark; anti-human CD34 antibody), diluted in phosphate-buffered saline (PBS) was applied to each section. Antibodies against p53 (at a dilution of 1:200 dilution, Thermo scientific, Lab Vision Corporation, Fermont, Clone SP5, RM-9105-S1, USA) were applied to each section. Sections were incubated with primary antibodies at 4 °C overnight. Then sections were treated using the labeled polymer peroxidase method (DAKO EnVision™ + Peroxidase Kit, Dako, Carpinteria, CA) for 60 min following the manufacturer's instructions. Proteins were visualized using a liquid diaminobenzidine substrate kit (Zymed Laboratories, San Francisco, CA). Sections were counterstained in Mayer's Hematoxylin. Sections from hemangioma and squamous cell carcinoma were used as positive controls for the anti-CD34 and anti-p53 antibodies, respectively. Sections with PBS replacing the primary antibody were used as negative controls3. All slides were coded and evaluated by two observers blinded for clinical details and identity of the patients21.
Immunohistochemical evaluation of the MVD, CD34-positive DDCs, and p53 protein expression: The immunostained sections were examined. In normal and lesional skins, CD34 immunostaining was absent from the epidermis. CD34-positive dermal dendritic cells in the dermis were counted in three randomly chosen square fields (1 mm2) following other groups21. Evaluation of MVD was performed following other groups. The sections were examined at low power magnification (×40) to identify the hot spots of microvessels in the dermis. Single endothelial cells or a cluster of endothelial cells reactive (membrane/cytoplasm staining) for CD34 in the membrane/cytoplasm was regarded as a distinct single countable microvessel. The number of microvessels was counted at higher magnification (×400). Vessel lumen and red cells, though often present, were not required to define a microvessel3,22. p53 protein expression values were scored and reported following other groups18. Only nuclear staining was considered positive. Initially, the distribution of p53 positive cells was categorized into basal versus suprabasal. The staining intensity was scored as follows: weak 1+, moderate 2+, or strong 3+. The percentage of positive cells was calculated as the number of positive cells per 100 consecutive cells (0–25, 26–50, 51–75%, or >75%)17,18. p53 staining profiles included three patterns. Pattern 0 indicates the presence of a nuclear, discontinuous; weak to moderate p53 staining that is localized to the basal cell keratinocytes (no suprabasal extension). Pattern 1 indicates the presence of a significantly increased p53 moderate to strong nuclear staining in the basal cell layer but without prominent suprabasal extension. Pattern 2 indicates the presence of a significantly increased p53moderate to strong nuclear reactivity in the basal cell layer, which is associated with prominent suprabasal extension23.
Statistical analysis: Differences between the groups (normal skin versus LiS) were determined using the non-parametric Wilcoxon-Mann-Whitney following other groups12,24. p values of less than 0.05 were treated as statistically significant. The results were presented as the Mean and standard error of the mean (SEM). Statistical tests were performed using SPSS version 23 (IBM Corp, New York, U.S.A.).
ResultsClinicopathologic findings: The study included 19 cases with LiS with a mean age of 60.6 ± 4.5 years. The study included 11 cases of vulvar LiS (mean ± standard error of the mean of age: 64.3 ± 3.2 years). The sites of the vulvar lesions were labia minor (9 cases), perineum, and the posterior fourchette (one case, each). The study also included 6 cases of LiS of male genitalia involving the foreskin (mean ± standard error of the mean of age: 53.7 ± 13.1 years), and two cases of extragenital LiS (right breast and paraumbilical region, one case each, mean ± standard error of the mean of age: 60.0 ± 1.0 years). The histological sections were examined to obtain the diagnosis of LiS and to rule out squamous neoplasia. Features diagnostic of early differentiated type VIN/differentiated squamous cell carcinoma was seen in one case of LiS. Histologically, sections from both genital and extragenital LiS showed attenuation of the epidermis with the loss of the regular rete ridge pattern. A granular layer was present. There was no basal nuclear atypia. There was no acanthosis. There was papillary dermal edema and homogenization of the dermal collagen, fragmentation, and haphazard arrangement (horizontal, vertical, and random orientation) of the dermal elastic fibers (VVG stain). There were condensation and curling of the elastic fibers in the surrounding dermis. No fungal elements were detected (periodic acid–Schiff with and without diastase). Few, scattered lymphocytes were seen in the dermis. No evidence of squamous neoplasia could be identified in 18 cases. A summary of these findings was shown in Figs. 1–4.
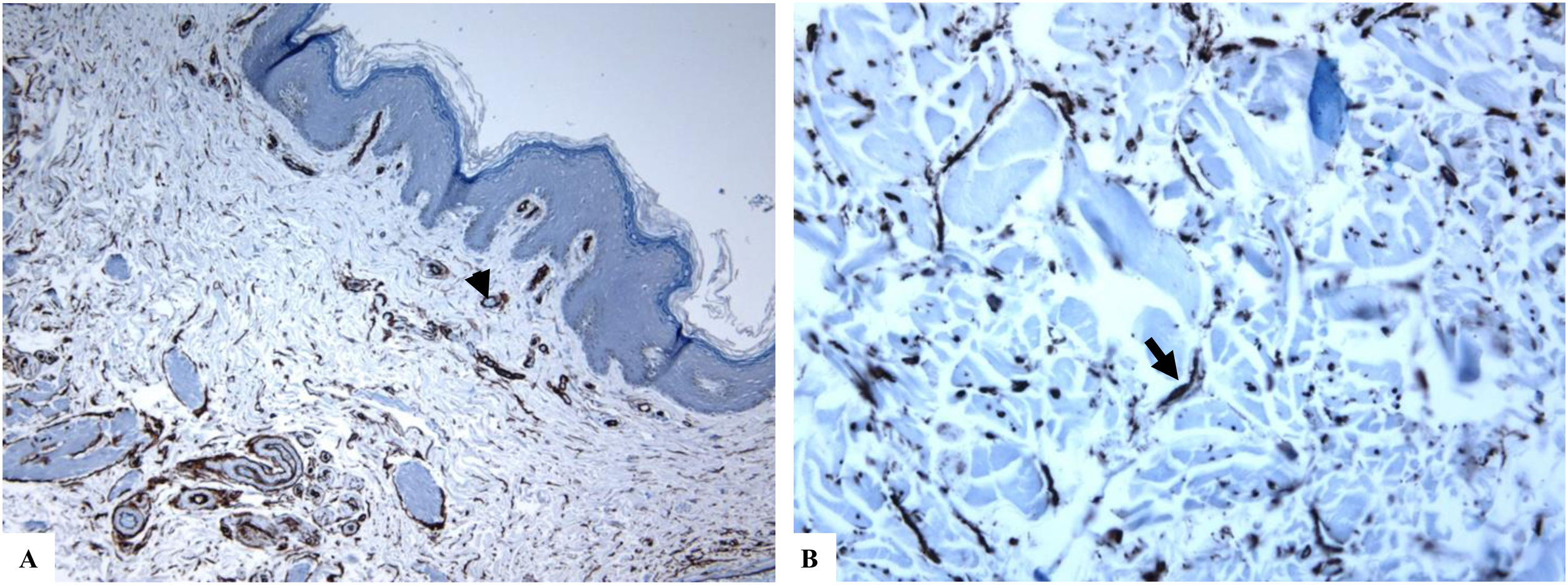
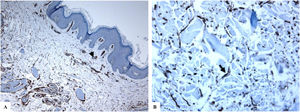
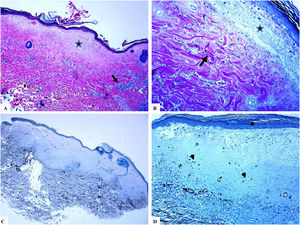
Genital (vulvar) lichen sclerosus. A–B: The features of LiS include the markedly attenuated epidermis with a transition from the edematous papillary dermis (star) to the hyalinized homogenized dermal collagen (arrow), and subtle inflammatory cell infiltrate (arrowhead). C-D: CD34 staining shows loss of CD34-labeled dermal dendritic cells and an increased microvessel density (arrowheads). (Original magnifications. A: ×40, B: ×100, C: ×40, and D: ×100).
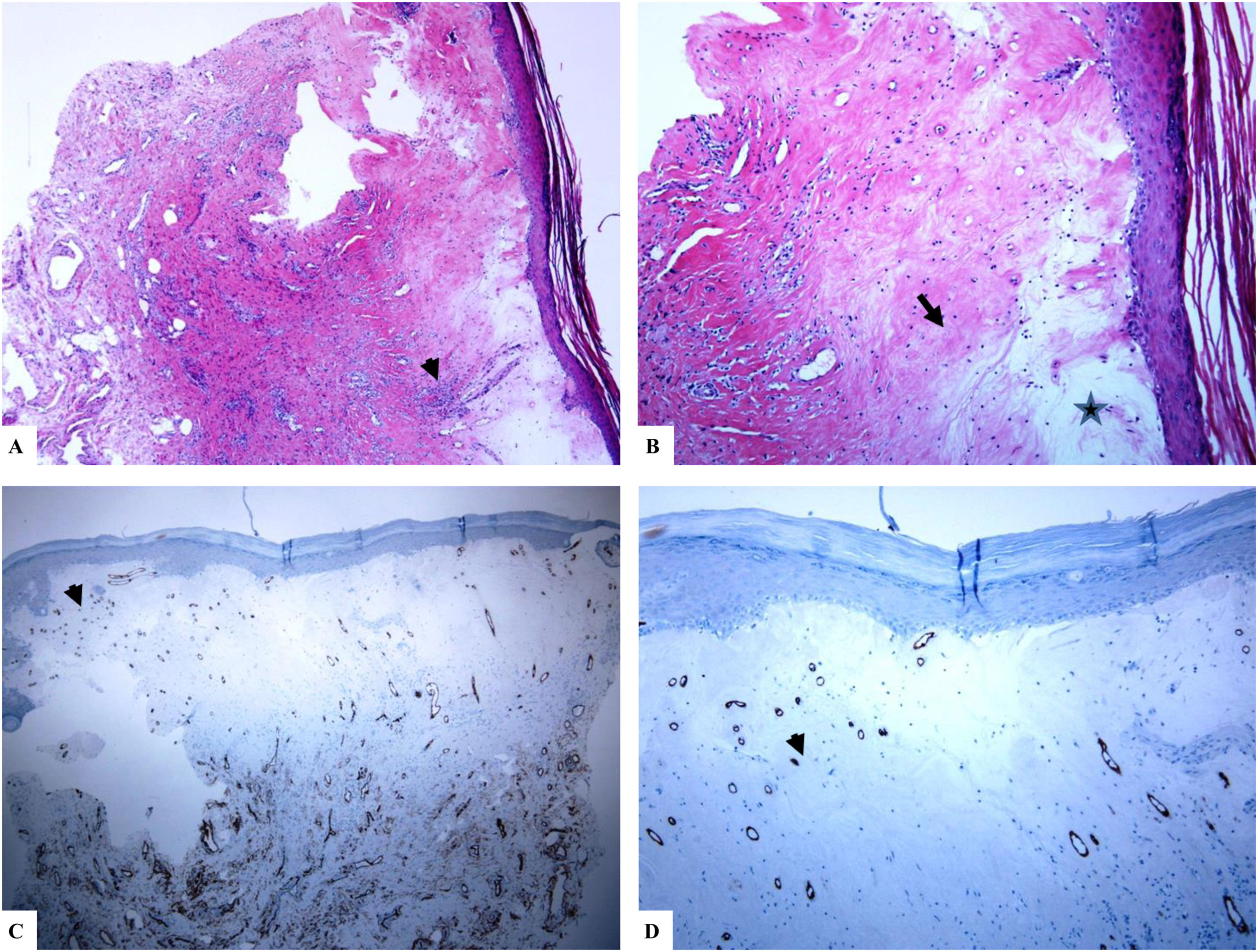
Genital (penile) lichen sclerosus (Balanitis xerotica obliterans of the foreskin, prepuce).
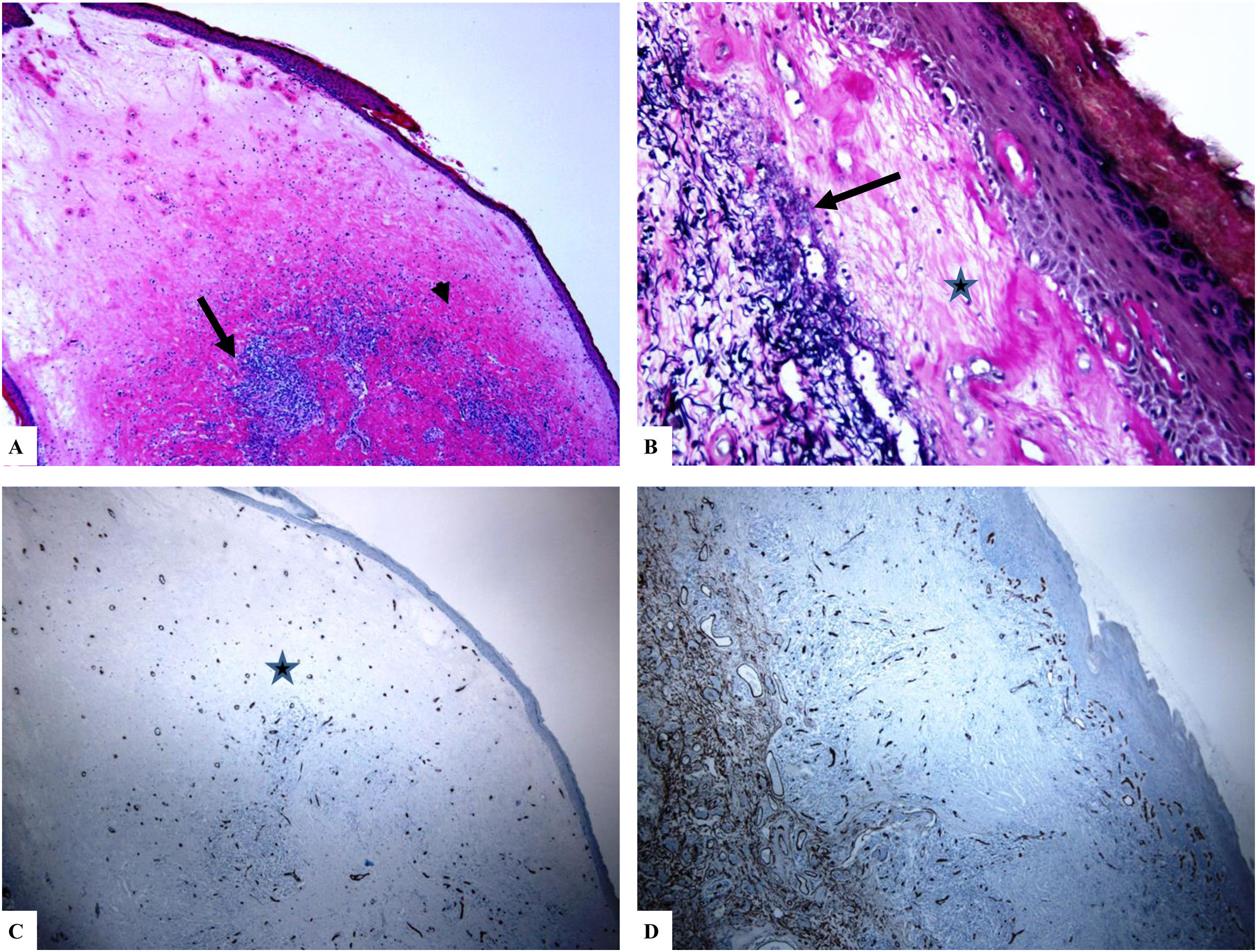
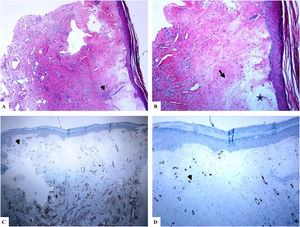
A: The features of the penile LiS include the thinning of the epidermis with loss of the normal rete ridge pattern, broad condensation of the homogenously, and massively hyalinized papillary dermal collagen (arrowhead), and the dense patchy dermal lymphocytic infiltrate (arrow). B: Edema, condensation, and curling of the elastic fibers (Verhoeff-van Gieson stain for elastic fibers, arrow) at the base of the homogenized collagen (star). C-D: CD34 staining shows loss of CD34 dermal dendritic cells and an increased microvessel density (star). (Original magnifications. A: ×20, B: ×200, C:×20, and D: ×100).
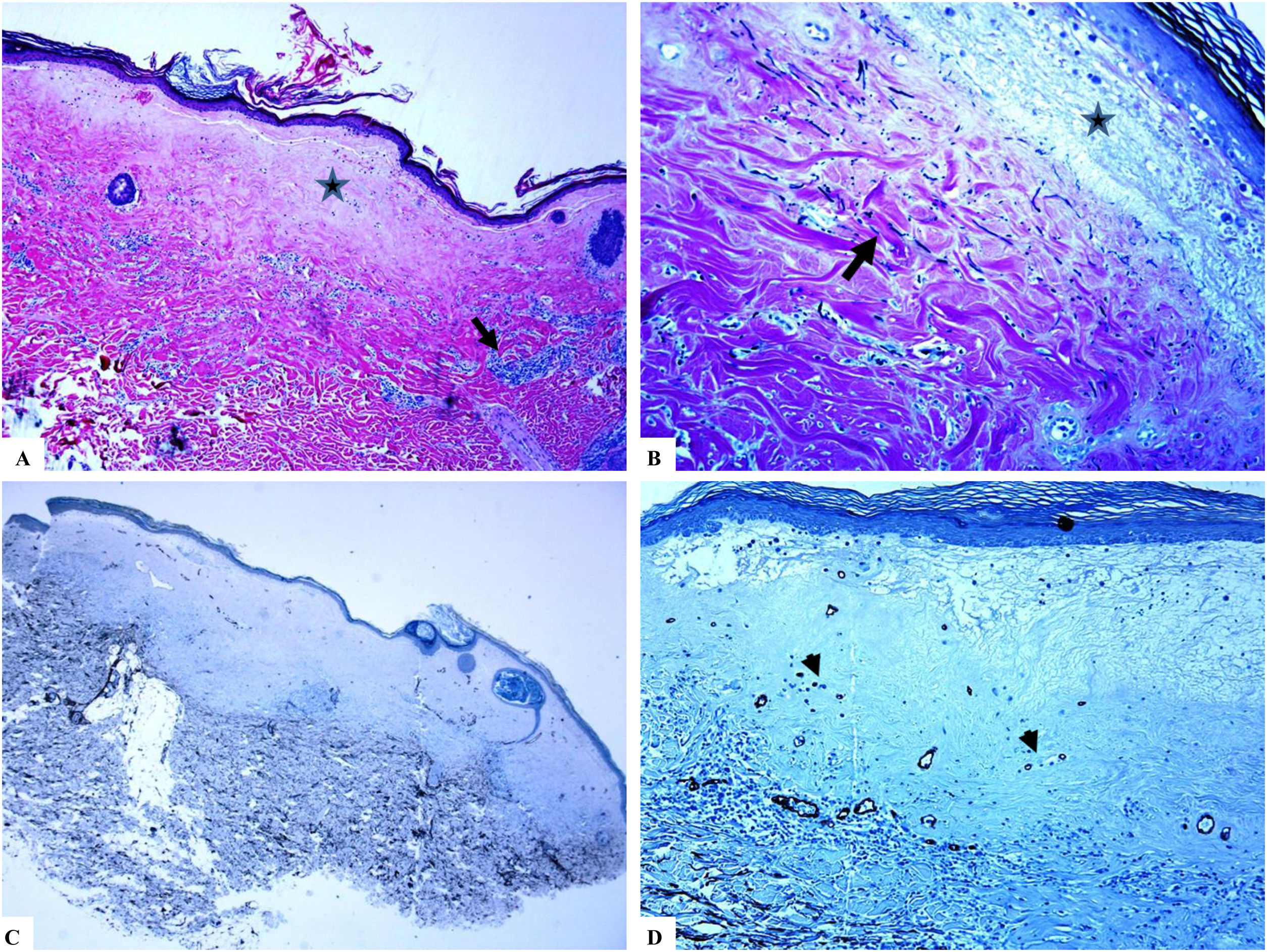
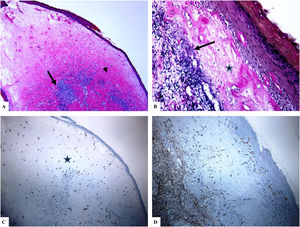
Extragenital lichen sclerosus (breast lesions). A: The features of LiS include the presence of focal hyperkeratosis, thinning of the epidermis, hyalinization of the papillary dermal collagen (star), and edema. B: Fragmentation and haphazard arrangement (horizontal, vertical, and random orientation) of the dermal elastic fibers (Verhoeff-van Gieson stain for elastic fibers, arrow). C–D: CD34 staining shows loss of CD34-dermal dendritic cells and an increased microvessel density (arrowheads). (Original magnifications. A: ×20, B: ×40, C: ×20, and D: ×40).
A markedly decreased number or complete loss of CD34-positive DDCs in LiS against their presence in the normal skin: The CD34-positive DDCs show brownish cytoplasmic staining. The dermis from the lesional skin (LiS) showed either complete loss of CD34-positive DDCs or rare CD34-positive DDCs as compared to the normal skin group. The mean expression values of DDCs counts were 1.7 ± 0.5/mm2 versus 23.4 ± 2.1/mm2 for LiS (both genital and extragenital cases) versus normal skin, respectively. The mean values of CD34-positive DDCs counts were 2.0 ± 0.7/mm2 (vulvar LiS), 1.8 ± 0.7/mm2 (LiS of the male genitalia), and 0.0 ± 0.0/mm2 (extragenital LiS). The differences between the lesional (LiS) and normal skins were statistically significant (p < 0.000). A summary of these findings was shown in Table 1 and Figs. 1–4.
The clinical and immunohistochemical findings in patients with lichen sclerosus.
| Aspects | Patient group (19 cases) | Normal skin (10 cases) | p Value (LiS versus control groups) | |||
|---|---|---|---|---|---|---|
| Vulvar LiS | Penile LiS | ExtragenitalLiS | LiS (all cases) | (Control) | ||
| Age (years) (mean ± SEM) | 64.3 ± 5.0 | 53.7 ± 13.1 | 61.0 ± 1.0 | 60.6 ± 4.5 | 56.20 ± 2.66 | 0.111 |
| Sex | Females | Males | Females | Females and males | 3 males and 7 females | 0.000 |
| DDCs (mean ± SEM) | 2.0 ± 0.7 | 1.8 ± 0.7 | 0.0 ± 0.0 | 1.7 ± 0.5 | 23.4 ± 2.1 | 0.000 |
| MVD | 19.45 ± 0.82 | 23 ± 0.44 | 18.5 ± 0.5 | 20.47 ± 0.63 | 5.50 ± 0.16 | 0.000 |
| P53 staining values | ||||||
| SI (mean ± SEM) | 1.36 ± 0.15 | 1.66 ± 0.16 | 1.5 ± 0.5 | 1.31 ± 0.1 | 1.1 ± 0.10 | NS |
| PP% (mean ± SEM) | 4.36 ± 0.5 | 4.33 ± 0.55 | 4.5 ± 0.5 | 4.36 ± 0.33 | 3.5 ± 0.44 | NS |
| Pattern | 0 | 0 | 0 | 0 | 0 | NS |
LiS: lichen sclerosus, VLiS: vulvar lichen sclerosus, Penile LiS, CD34 + DDCs: CD34-positive dermal dendritic cells, and MVD: microvessel density. SI: staining intensity, PP%: percentage of positive cells, and SEM: standard error of mean. NS: not statistically significant.
A markedly increased MVD in the dermis of LiS as compared to the normal skin: CD34 marker reactivity in the microvessels appeared as brown cytoplasmic reactivity with or without membrane staining. Areas with vessels having caliber larger than eight red blood cells were excluded. Large and medium-sized blood vessels with thick muscular walls or vessels with a luminal diameter of greater than 50 mM were excluded from the final count. The MVD values in the lesional skin of both genital and extragenital LiS (20.47 ± 0.63, p = 0.000) were statistically significantly high (p < 0.000) as compared to the normal skin (5.50 ± 0.2). The mean values of MVD were 19.45 ± 0.82 (vulvar LiS), 23 ± 0.44 (LiS of the male genitalia), and 18.50 ± 0.50 (extragenital LiS). A summary of these findings is shown in Table 1 and Figs. 1–4.
p53 protein staining patterns in the normal skin, and LiS: The lesional and normal skins showed nearly similar p53 protein staining values and patterns. The reactivity was in the form of discontinuous individual single-cell p53+ nuclear staining (weak to moderate staining intensity) in the basal cell keratinocytes of the epidermis (in less than 10 % of basal keratinocytes). A suprabasal or continuous linear basal staining was not evident in all LiS cases. The expression values of p53 protein were summarized in Table 1 and Figs. 1–4. Ki67 staining was localized to the basal cell keratinocytes both in LiS and normal skin.
DiscussionTo date, our knowledge about the counts of CD34-positive DDCs, MVD, and expression patterns of p53 in LiS is limited. This study aims to get insights into these issues. I revealed the following observations: i) a markedly decreased number or complete loss of CD34-positive DDCs in LiS skin against their presence in normal skin, ii) a markedly increased MVD in the dermis of LiS as compared to normal skin and iii) lack of p53 protein accumulation in these lesions.
A markedly decreased number or complete loss of CD34-positive DDCs in LiS against their presence in normal skin: CD34-positive DDCs are present in the dermis of normal skin. They play roles in the development of the surrounding epithelial and mesenchymal cells and the modulation of the immune response21. In accordance with previous investigations in morphoeic skin8–12, and dermal scars21, the current study showed a markedly decreased number of CD34-positive DDCs or their complete loss or in the dermis of LiS lesions. Skobieranda et al. examined the expression of CD34-positive progenitor cells in 26 lesional skin biopsies from cases with morphea side by side with an additional 11 biopsies from normal skin. They found a decreased quantity of CD34-expressing cells in the lesional skin as compared to the normal skins. The authors proposed regulatory roles for CD34-positive DDCs in collagen synthesis and hence, the development of morphea9. Lee et al. examined the distribution of CD34-positive DDCs and smooth muscle actin (SMA-positive) myofibroblasts in punch skin biopsies from patients with morphea and healthy individuals (normal skin).CD34-positive DDCs were markedly reduced or absent in morphoeic skin as compared to the normal skin. SMA-positive myofibroblasts increased mostly in the dermal locations where CD34-positive DDCs declined or disappeared12. The authors proposed that the lack of CD34-positive DDCs in morphea was compatible with similar findings in the wound-healing process12,25, both are associated with phenotypic differentiation of CD34-positive DDCs into SMA-positive myofibroblasts. Taken together, these findings suggest that the DDCs contribute to the regulation of collagen production in the skin.
The myofibroblasts are smooth muscle actin positive cells that can play an important role in the pathogenesis of fibrosis. These cells can arise from the CD34-positive fibrocytes under the stimulation with fibrogenic cytokines26,27. Lee et al. used immunohistochemical staining methods to examine the expression profile of CD34, factor XIIIa, smooth muscle actin, CD31, and vascular cell adhesion molecule-1 in morphea. They found a reduced CD34 stromal stain in these lesions whereas factor XIIIa, smooth muscle actin, and vascular cell adhesion molecule-1 protein expression was high as compared to the normal skin. There was an inverse correlation between CD34 stromal stain and the spatial expression pattern with smooth muscle actin stain. The authors indicated that the mutually exclusive staining patterns of CD34 stromal and SMA stains suggest a phenotypic change of CD34+ DDCs into smooth muscle actin-positive myofibroblasts in morphea12. It is tempting for future studies to compare the expression pattern of these proteins in LiS.
A markedly increased MVD in the dermis of LiS as compared to normal skin: In this investigation, the findings of increased MVD in LiS support previous findings in morphoeic and sclerodermic skin13,14,28. Olejek et al. examined MVD (using CD34 antibodies) in punch skin biopsies from vulvarLiS in 28 women13 and 100 cases of anogenital LiS and they found a significant increase of MVD after photodynamic therapy14. In 2009, Dziankowska-Bartkowiak examined the vascular endothelial changes in scleroderma (systemic sclerosis and morphea). They analyzed the serum vascular endothelial growth factor (VEGF) and its soluble receptor 2 (sVEGFR2) using the ELISA method as well as the immunohistochemical expression of CD34 antigen in the lesional skin. There was a higher mean serum VEGF/sVEGFR2 ratio in systemic sclerosis patients as compared to the control group. Also, there were variations in the CD34 expression in the vascular endothelial cells28. Taken collectively, the increased MVD in LiS may be due to the release of angiogenic factors that enhance neoangiogenesis28.CD34-positive cells can also differentiate into progenitor cells that further incorporate into newly formed blood vessels29.
p53 protein expression in LiS: In agreement with other investigators, the current study reported that p53 staining in LiS was weak to moderate nuclear, discontinuous, and localized to the basal cell keratinocytes indicative of lack of p53 protein accumulaiton17,19. This pattern of p53 nuclear staining reflects a stress-related response secondary to dermal inflammation and ischemia rather than underlying p53 gene mutations19. Yang et al., examined the expression profile of p53 in 12 cases of differentiated VIN using immunohistochemical staining methods19. In differentiated VIN, p53 protein expression was seen in the basal cell keratinocytes with suprabasal extension. In contrast, suprabasilar p53 staining was not seen in LiS cases19. Hantschmann et al., examined p53 protein expression in patients with squamous cell carcinoma, VIN, LiS, and squamous hyperplasia. In LiS and the normal vulval skin, p53 protein expression was localized to the basal cell keratinocytes. The usual and differentiated types of VIN showed basal and suprabasal p53 nuclear reactivity. Most of the squamous cell carcinomas showed moderate to strong diffuse nuclear p53 staining17.
To conclude, to the best of this author's knowledge, this is the first study analyzing DDCs, MVD, and p53 profiles together in LiS. This study showed that the counts of CD34-positive DDCs were significantly lower in LiS than normal skin (controls), while MVD was significantly higher in patients than controls. These findings suggest that alterations of DDCs and MVD have possible roles in the pathogenesis of fibrosis in these lesions. Future investigations are needed to comprehend the mechanisms underlying these alterations. Moreover, the examination of the correlations among the expression of CD34, factor XIIIa, smooth muscle actin, CD31, and vascular cell adhesion molecule-1 in morphea is open for future investigations.
Please cite this article as: Hussein MRA. Análisis inmunohistológico de las células dendríticas dérmicas positivas para CD34 y de la densidad de microvasos en el liquen escleroso genital y extragenital. Actas Dermosifiliogr. 2021. https://doi.org/10.1016/j.ad.2021.02.009