Corticosteroids are widely used in clinical practice. In dermatology, their application is generally topical. These substances may act as allergens and produce immediate and delayed hypersensitivity reactions. Allergic contact dermatitis is the most frequent presentation of corticosteroid allergy and it should be studied by patch testing in specific units. The corticosteroids included in the Spanish standard battery are good markers but not ideal. Therefore, if those makers are positive, it is useful to apply a specific battery of corticosteroids and the drugs provided by patients. Immediate reactions are relatively rare but potentially severe, and it is important to confirm the sensitization profile and to guide the use of alternative corticosteroids, because they are often necessary in several diseases. In this article we review the main concepts regarding these two types of hypersensitivity reactions in corticosteroid allergy, as well as their approach in the clinical practice.
Los corticoides son fármacos ampliamente utilizados en la práctica clínica, especialmente de forma tópica en dermatología. Estas sustancias pueden actuar como alérgenos y producir tanto reacciones de hipersensibilidad inmediata como retardada. La alergia en forma de dermatitis de contacto es la reacción más frecuente, y debe estudiarse mediante pruebas epicutáneas en unidades especializadas. Actualmente la batería estándar española tiene buenos marcadores para su detección, pero no ideales. Por ello, es rentable aplicar una batería específica de corticoides si dichos marcadores son positivos, así como los corticoides propios aportados por los pacientes. En cuanto a las reacciones de hipersensibilidad inmediata, son mucho menos habituales, pero potencialmente más graves. Debido a que estos fármacos son necesarios en múltiples enfermedades, es importante confirmar la sensibilización a estas sustancias, y orientar el uso de corticoides alternativos. En el presente artículo pretendemos revisar los principales conceptos respecto a estos 2 tipos de reacciones de hipersensibilidad en la alergia a corticoides, así como su abordaje en la práctica clínica.
Corticosteroids are widely used in dermatology, especially as topical drugs. In 1952, Sulzberger and Witten1 synthesized a topically active compound called substance F, which was later known as hydrocortisone or cortisol. Since their introduction, corticosteroids have proven highly efficacious as anti-inflammatory and immunomodulatory drugs, although, paradoxically, they can behave as allergens, leading to hypersensitivity reactions. This effect was first reported in the 1950s,2–5 and, since then, many more cases have been reported. Type IV (delayed) hypersensitivity reactions, which occur with symptoms of allergic contact dermatitis (ACD), are more common than type I (immediate) reactions, which are less well-known.6
Given that corticosteroids share a similar structure, cross-reactions between them are not uncommon. Consequently, it is difficult to study sensitization to these substances. Several authors have tried to classify the drugs into different groups. A current classification applied in ACD divides corticosteroids into 3 groups and patients into 2 groups, thus providing us with a more practical means of assessment. However, no ideal classification has been designed to date, and disagreement is common in daily clinical practice. Furthermore, this classification does not enable us to group the cross-reactions observed in immediate hypersensitivity reactions to corticosteroids.
Despite the fact that corticosteroids were named contact allergen of the year in 2005,7 corticosteroid allergy has received little attention and constitutes a challenge in clinical practice. The presentation and diagnostic test results are often difficult to interpret and subject to peculiarities that are worthy of analysis. In suspected allergic reactions to corticosteroids, it is important to identify both the culprit drug and drugs that can be used as alternatives, since corticosteroids may become almost indispensable for the control or treatment of specific diseases.
The present article reviews key concepts in both types of hypersensitivity reaction to corticosteroids and examines their management in clinical practice.
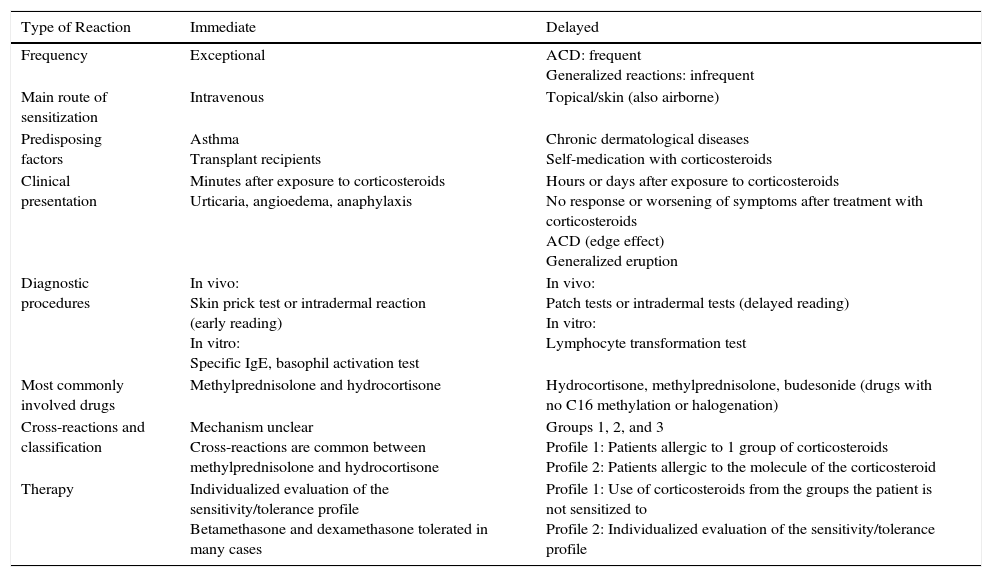
Types of AllergyCorticosteroids can give rise to type I hypersensitivity reactions (immunoglobulin [IgE]–mediated), which occur only minutes after exposure, and type IV hypersensitivity reactions (T cell–mediated), which occur hours or days after exposure. In the latter, the characteristics of T cells have been studied, and analysis of skin biopsy specimens has revealed an inflammatory infiltrate composed of CD3+ T cells with a predominantly type 2 helper T-cell cytokine profile.8Table 1 shows the main characteristics of both types of reaction.
Allergy to Corticosteroids: Immediate and Delayed Hypersensitivity Reactions.
| Type of Reaction | Immediate | Delayed |
|---|---|---|
| Frequency | Exceptional | ACD: frequent Generalized reactions: infrequent |
| Main route of sensitization | Intravenous | Topical/skin (also airborne) |
| Predisposing factors | Asthma Transplant recipients | Chronic dermatological diseases Self-medication with corticosteroids |
| Clinical presentation | Minutes after exposure to corticosteroids Urticaria, angioedema, anaphylaxis | Hours or days after exposure to corticosteroids No response or worsening of symptoms after treatment with corticosteroids ACD (edge effect) Generalized eruption |
| Diagnostic procedures | In vivo: Skin prick test or intradermal reaction (early reading) In vitro: Specific IgE, basophil activation test | In vivo: Patch tests or intradermal tests (delayed reading) In vitro: Lymphocyte transformation test |
| Most commonly involved drugs | Methylprednisolone and hydrocortisone | Hydrocortisone, methylprednisolone, budesonide (drugs with no C16 methylation or halogenation) |
| Cross-reactions and classification | Mechanism unclear Cross-reactions are common between methylprednisolone and hydrocortisone | Groups 1, 2, and 3 Profile 1: Patients allergic to 1 group of corticosteroids Profile 2: Patients allergic to the molecule of the corticosteroid |
| Therapy | Individualized evaluation of the sensitivity/tolerance profile Betamethasone and dexamethasone tolerated in many cases | Profile 1: Use of corticosteroids from the groups the patient is not sensitized to Profile 2: Individualized evaluation of the sensitivity/tolerance profile |
Abbreviations: ACD, allergic contact dermatitis; Ig, immunoglobulin.
The frequency of ACD to corticosteroids reported in the literature ranges from 0.2% to 5%.9 In Spain, the prevalence of sensitization to corticosteroids is 1.1% in patients analyzed using patch tests,10 which is lower than those reported in Europe (2.6%)11 and the United States (4.6%).12 The main allergen in Spanish series is budesonide,13,14 whereas in the United States it is tixocortol.15 The sensitization rates for budesonide, tixocortol, and hydrocortisone 17-butyrate seem to be similar in Europe.16
Variations in the prevalence of ACD to corticosteroids resulting from delayed hypersensitivity reactions could be due to a series of factors, such as the frequency of corticosteroid use in each country, prescription habits, knowledge of allergy to corticosteroids, and the diagnostic tests used.17 In this sense, the concentrations of corticosteroids used in patch tests in Europe are lower than in the United States.
Delayed hypersensitivity to corticosteroids is more common in women. The patients who are most predisposed to ACD to corticosteroids are those with chronic skin diseases (eg, chronic eczema, atopic dermatitis, stasis dermatitis, chronic ulcers), owing mainly to alteration of the skin barrier and the proinflammatory nature of the drugs. Corticosteroid-based self-medication without medical supervision is another predisposing factor. Genetic predisposition has also been postulated.18
Etiology and PathogenesisRoutes of Sensitization/Sources of ExposureThe most common route of sensitization to corticosteroids is direct contact with the skin, although there have also been reports of indirect contact via airborne mechanisms, for example, in the case of patients with relatives who use a budesonide inhaler.19,20 Other routes of sensitization include the conjunctival mucosa, nasal mucosa, respiratory tract, and gastrointestinal tract, although these are less common.21–23
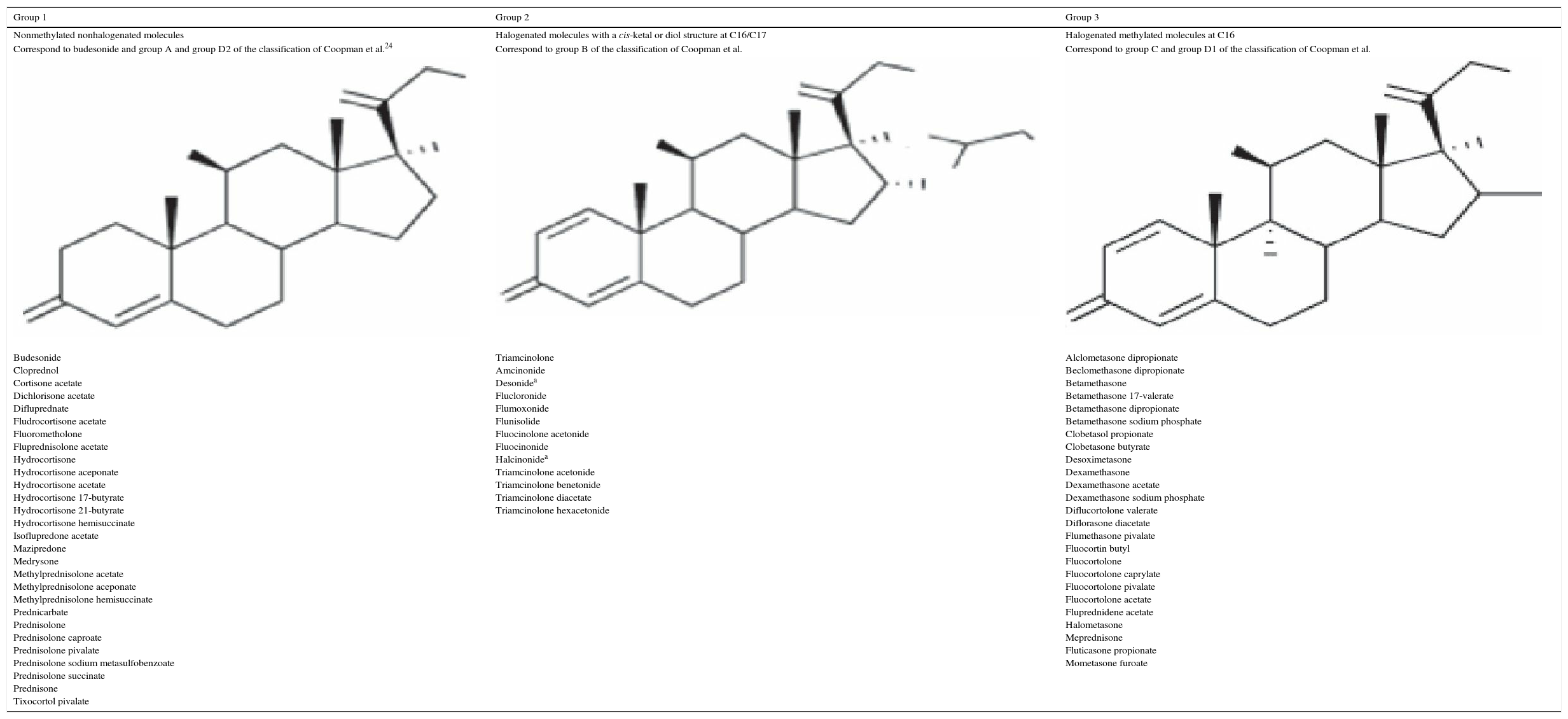
Cross-Reactions and ClassificationThe basic structure of corticosteroids is the cyclopentanoperhydrophenanthrene ring. Modification of this ring through halogenation and/or esterification improves the drug's therapeutic properties, thus enabling it to better penetrate the skin and increasing its effectiveness and potency. However, this chemical similarity leads to frequent cross-reactions, which are difficult to interpret. Several authors have tried to classify corticosteroids into groups in order to better understand sensitization to them.
In 1989, Coopman et al.24 classified corticosteroids into 4 groups, namely, A, B, C, and D, depending on chemical structure and cross-reactivity patterns. In 2000, Matura and Goossens25 divided group D into 2 subgroups, D1 and D2. It was later observed that cross-reactions were occasionally not as predicted or expected according to the classification, which was then modified. In 2009, Baeck et al.9 studied molecular models of corticosteroids and the skin test results of 315 corticosteroid-sensitive patients. In 2011, they proposed a new, simpler classification that divided the drugs into 3 groups based on cross-reactions and according to molecular structure (Table 2).26,27
| Group 1 | Group 2 | Group 3 |
|---|---|---|
| Nonmethylated nonhalogenated molecules | Halogenated molecules with a cis-ketal or diol structure at C16/C17 | Halogenated methylated molecules at C16 |
| Correspond to budesonide and group A and group D2 of the classification of Coopman et al.24 | Correspond to group B of the classification of Coopman et al. | Correspond to group C and group D1 of the classification of Coopman et al. |
| Budesonide Cloprednol Cortisone acetate Dichlorisone acetate Difluprednate Fludrocortisone acetate Fluorometholone Fluprednisolone acetate Hydrocortisone Hydrocortisone aceponate Hydrocortisone acetate Hydrocortisone 17-butyrate Hydrocortisone 21-butyrate Hydrocortisone hemisuccinate Isoflupredone acetate Mazipredone Medrysone Methylprednisolone acetate Methylprednisolone aceponate Methylprednisolone hemisuccinate Prednicarbate Prednisolone Prednisolone caproate Prednisolone pivalate Prednisolone sodium metasulfobenzoate Prednisolone succinate Prednisone Tixocortol pivalate | Triamcinolone Amcinonide Desonidea Flucloronide Flumoxonide Flunisolide Fluocinolone acetonide Fluocinonide Halcinonidea Triamcinolone acetonide Triamcinolone benetonide Triamcinolone diacetate Triamcinolone hexacetonide | Alclometasone dipropionate Beclomethasone dipropionate Betamethasone Betamethasone 17-valerate Betamethasone dipropionate Betamethasone sodium phosphate Clobetasol propionate Clobetasone butyrate Desoximetasone Dexamethasone Dexamethasone acetate Dexamethasone sodium phosphate Diflucortolone valerate Diflorasone diacetate Flumethasone pivalate Fluocortin butyl Fluocortolone Fluocortolone caprylate Fluocortolone pivalate Fluocortolone acetate Fluprednidene acetate Halometasone Meprednisone Fluticasone propionate Mometasone furoate |
aNonhalogenated molecules.
Group 1 corticosteroids produced allergic reactions more frequently, whereas group 3 drugs had the lowest sensitizing capacity and produced the fewest cross-reactions,28 probably because the metabolites resulting from the degradation process are determinants in cross-reactions. The formation of degradation products (steroid-glyoxal) has been observed in vitro on the C17 side chain. These products cause alterations on C21. The resulting complex, which can bind to most amino acids, binds irreversibly to arginine guanidine groups in serum proteins to become an antigen.29 Thus, the most allergenic corticosteroids are those whose degradation products bind more easily to arginine (eg, budesonide).30,31 Halogenation of the molecule seems to diminish this ability to bind to arginine, thus providing a potential explanation for the fact that betamethasone-type halogenated corticosteroids are less allergenic.
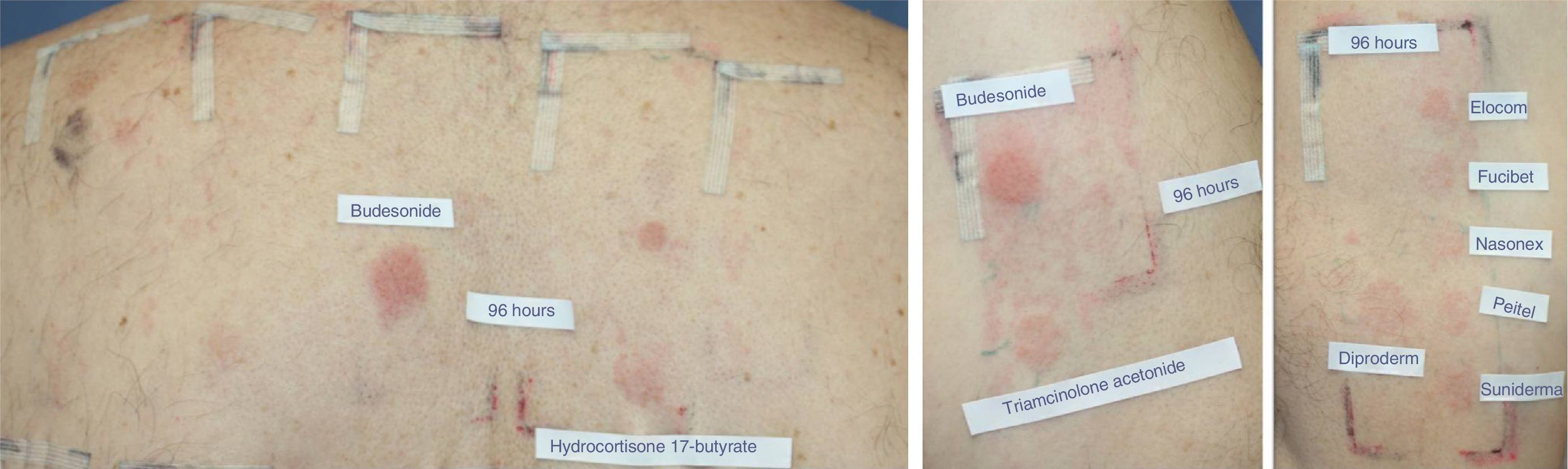
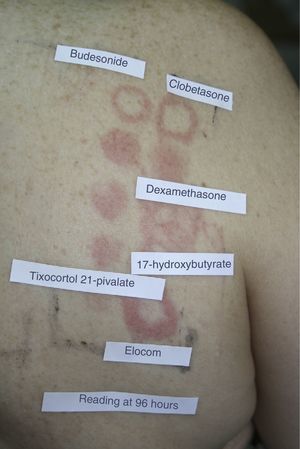
In addition to this new classification into 3 groups, the authors proposed dividing patients into 2 profiles depending on whether they were sensitized to 1 or more groups, so that profile 1 patients would react only to a single group and profile 2 patients would react to the whole spectrum of corticosteroids by recognizing the skeleton of the substances involved (Fig. 1). The latter profile includes patients who seem to be at greater risk of generalized skin reactions after administration of systemic corticosteroids.
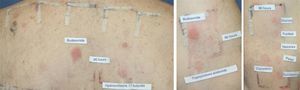
Example of a profile 2 patient who reacted to the complete spectrum of corticosteroids. The patient was diagnosed with inverse psoriasis and was found to be sensitized to all 3 groups of corticosteroids. Reading at 96hours with the modified standard series revealed positive results for budesonide (++) and hydrocortisone 17-butyrate (+) (group 1). Testing with the corticosteroid series revealed positive results to triamcinolone acetonide (+) (group 2). Testing with the patient's own corticosteroids revealed allergy to group 3 drugs, with positive results for the commercially available creams Diproderm (+), Elocom (+), Fucibet (+), Nasonex (+), Peitel (+), and Suniderma (+).
Most delayed hypersensitivity reactions to corticosteroids present as chronic eczema. These reactions are often difficult to recognize because the onset of symptoms is slow and the chronology atypical; therefore, the information provided by the patient is essential. We should suspect ACD to corticosteroids when eczema occurs at the site where the drug was applied or when chronic skin complaints do not improve after application of the drug.32 Also noteworthy are a more intense reaction at the edges of the lesion than in the center of the area treated owing to the anti-inflammatory effect and the presence of signs resulting from prolonged use of corticosteroids (eg, atrophy, rosacea, and perioral dermatitis) due to diagnostic delay. However, presentation of the reaction in the form of hand eczema is less frequent than in the remaining patients studied using patch tests.9 Reactions should also be taken into account in people who work in hospitals and in the pharmaceutical industry, although the literature contains few cases of occupational dermatitis (Table 3).33
Criteria for Suspicion of Corticosteroid-Induced Allergic Contact Dermatitis.
| Persistent chronic eczematous dermatitis |
| Eruption more intense at the edges |
| No improvement in symptoms despite appropriate treatment with corticosteroids |
| Worsening of skin disease after treatment with corticosteroids |
| Occupational exposure to corticosteroids (persons working in hospitals and the pharmaceutical industry) |
Patients sensitized to corticosteroid eyedrops can experience periocular eczema and/or edema, conjunctivitis, itching, burning pain, and/or tearing.21 Therapy with inhaled corticosteroids can lead to periorificial eczematous eruptions (perioral, perinasal) that could extend to the mucosa and cause nasal congestion, rhinitis, stomatitis, and even bronchospasm.22,34
In patients who are sensitized to topical corticosteroids, generalized eruptions have been reported after oral, inhaled, parenteral, and transmucosal administration of corticosteroids.35–38 These delayed systemic reactions can present as maculopapular or eczematous eruptions, or even as blisters and purpura. Eruptions have also been reported in corticosteroid-allergic patients after administration of steroid hormones.39–41
Furthermore, there have been isolated reports of the reaction presenting as delayed angioedema/urticaria,42 fixed drug eruption,43 erythema multiforme,44,45 and acute generalized exanthematous pustulosis.46
DiagnosisThe most commonly used methods for the diagnosis of hypersensitivity reactions are in vivo tests such as patch tests and delayed reading of intradermal tests. Patch tests are the approach of choice, whereas delayed reading of intradermal tests is not carried out routinely owing to the associated risk of cutaneous atrophy.47 In vitro testing of delayed hypersensitivity to corticosteroids is based on the lymphocyte transformation test, which tends to be restricted to specialized centers.48–51
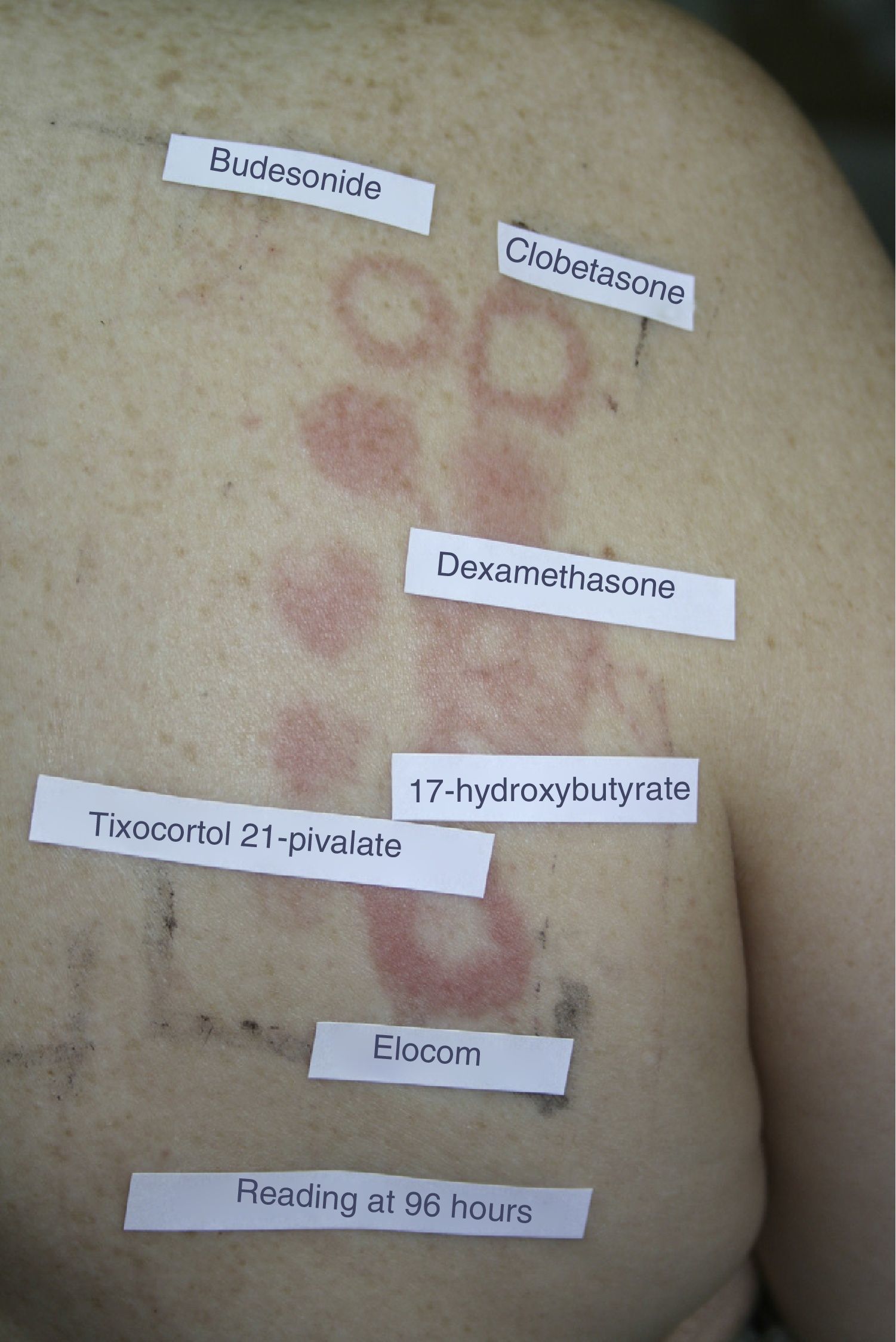
A series of points should be taken into account when reading and interpreting the results of patch tests with corticosteroids. In early readings, corticosteroids can produce a doughnut-type reaction or edge effect, which consists of a reaction at the edges of the patch and not in the middle area, probably because of the anti-inflammatory effect in the central area, where the concentration is higher (Fig. 2). Whitening of the skin has also been reported at the site of application of the patch after 48hours owing to a vasoconstriction effect. Furthermore, it is important to draw attention to delayed erythema, since corticosteroids can suppress or delay a positive response as a result of their anti-inflammatory and immunomodulatory effect.52 Consequently, the current guidelines of the International Contact Dermatitis Research Group include the possibility of a third reading 7 days after the patches have been applied in order to avoid potential false negatives.
Example of the edge effect (“doughnut-type” reaction) in a reading of corticosteroid patches at 96hours. The reaction appears at the edge of the patch and not in the middle area, probably because of the predominant anti-inflammatory effect in the central area, where the concentration is higher.
Patch tests are based on markers such as budesonide, tixocortol-pivalate, and hydrocortisone 17-butyrate. Budesonide and tixocortol-pivalate are included in the European and Spanish standard series,47 and hydrocortisone 17-butyrate is included in the TRUE test and the standard series of the North American Contact Dermatitis Group.
In Spain, the standard series of the Spanish Contact Dermatitis and Skin Allergy Research Group includes budesonide 0.01% in petrolatum (pet) and tixocortol 21-pivalate 0.1% pet. Although petrolatum is a good vehicle for the study of corticosteroids, some authors have postulated that the best method for detecting delayed hypersensitivity to corticosteroids is by using the active ingredients diluted in ethanol.53 Both markers can identify sensitization in most patients, since both belong to group 1 of the classification; however, sensitization may go undetected in patients who are allergic to groups 2 and/or 3. Therefore, it is important to assess patients using specific series and their own products. It could also prove useful to add a corticosteroid from groups 2 and/or 3 to the standard series, at least temporarily, to better determine the prevalence of sensitization to these groups.
In patients with positive results to corticosteroid markers from the standard series and in patients with a high clinical suspicion of allergy to corticosteroids but no positive results to markers of corticosteroid allergy, the study should be extended by applying a specific corticosteroid series and the patient's own products.54 One example of a corticosteroid-specific series is that supplied by Chemotechnique Diagnostics AB, which, in addition to corticosteroids from the standard series, includes triamcinolone acetonide (1.0% pet), dexamethasone 21-phosphate (1.0% pet), clobetasol 17-propionate (1.0% pet), betamethasone 17-valerate (1.0% pet), and alclometasone 17-21-dipropionate (1.0% pet).
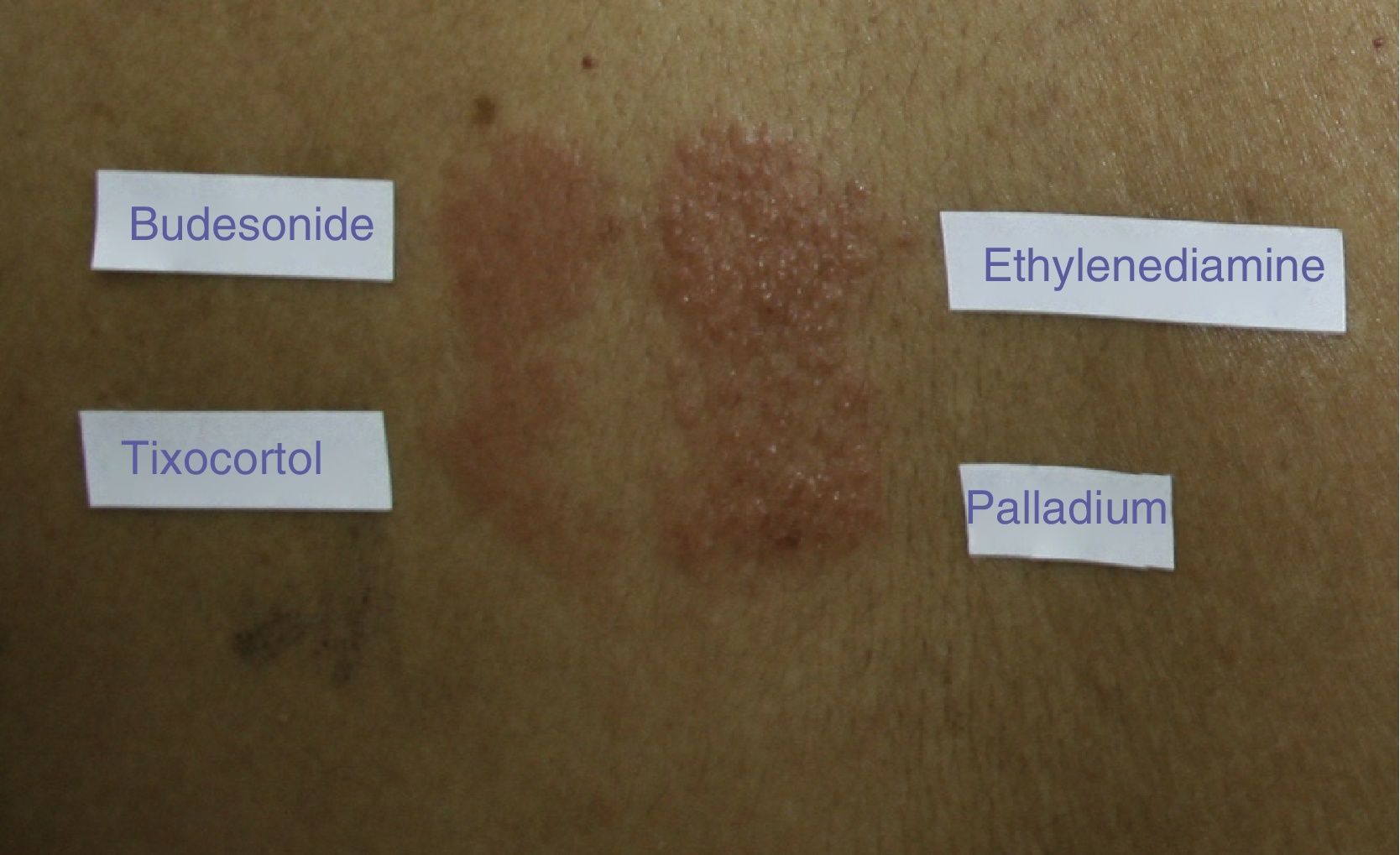
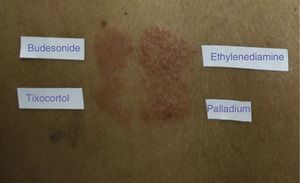
In our experience, it is common to encounter patients with a positive result to a marker who do not have positive results when tested with corticosteroids from the same group or with their own products containing corticosteroids from the same group. If we obtain a positive result to one of the patient's own products, we must rule out the possibility of allergy to an excipient, since this condition often manifests as ACD to corticosteroids. Allergenic excipients used in corticosteroid formulas include chlorocresol,55 benzyl alcohol, ethylenediamine hydrochloride, isopropyl palmitate, parabens, polysorbate 60, propylene glycol, stearyl alcohol, sodium dioctyl sulfosuccinate, sodium metabisulfite, and 1,2-hexanetriol.17,56–59
Cosensitization, or sensitization to multiple allergens, in corticosteroid-allergic patients is well documented.60 In their series of 315 patients, Baeck et al.9 found positive reactions to other allergens—mainly metals and fragrances—in up to 84% of cases. Other allergens that have traditionally been involved in cosensitization to corticosteroids are neomycin sulfate and ethylenediamine dihydrochloride (Fig. 3). Cosensitization was attributed to the presence of these allergens in the same pharmaceutical cream.
TherapyManagement of corticosteroid-allergic patients continues to be complicated. The recent classification of Baeck et al.26 and Baeck and Goosens27has made it possible to reclassify corticosteroids according to their chemical structure and patients according to their allergy to 1 or more groups. However, this classification is not ideal, since, during follow-up, some patients do not experience a reaction after application of topical corticosteroids from the group they were sensitized to. Similarly, it does not seem useful to recommend inhaled or systemic corticosteroids, since the cross-reaction pattern after inhaled or systemic administration is not well-known.
Although there is some doubt when recommending a specific drug, it is prudent to recommend that profile 1 patients avoid all topical corticosteroids from the group they are sensitized to according to the current classification, which serves as a guideline. In order to recommend a specific drug, it would be appropriate for the patient to undergo patch testing or a use test with the drug and thus rule out sensitization. The option that can be proposed to profile 2 patients is to recommend other measures, such as calcineurin inhibitors, and reserve corticosteroids only for emergency situations, when no alternative is available.
Immediate Hypersensitivity ReactionsEpidemiologyImmediate hypersensitivity reactions to corticosteroids are relatively uncommon, although they should be recognized, since they carry a greater risk than delayed reactions and are more difficult to manage, irrespective of the department they occur in. Their prevalence is estimated to be 0.1%-0.3%.61,62
Etiology and PathogenesisRoutes of Sensitization/Sources of ExposureSensitization in immediate hypersensitivity reactions is mainly through the intravenous route, although sensitization can also occur via the oral, intramuscular, intra-articular, and subcutaneous routes. The incidence of sensitization via these routes seems to be higher in patients with asthma63 and in patients receiving regular treatment with corticosteroids, for example, transplant recipients. However, in such cases, it is difficult to determine whether the increase in incidence is due to greater susceptibility or extensive exposure to corticosteroids.
Cross-reactions and ClassificationThe drugs most commonly involved in immediate hypersensitivity reactions are hydrocortisone and methylprednisolone. Although cross-reactions between them have been identified, we do not have sufficient data to demonstrate cross-reaction patterns and draw up classifications, as is the case with delayed hypersensitivity reactions. In fact, there have been reports of allergy to hydrocortisone/methylprednisolone in which the patient subsequently tolerated systemic administration of other group 1 corticosteroids, such as prednisone, and corticosteroids from other groups, such as betamethasone and dexamethasone.63–65
Furthermore, there are reports of hypersensitivity due not to corticosteroids themselves, but to the succinates added to the drugs to make them more soluble for intravenous administration.66–68
SymptomsThe various clinical pictures described for immediate hypersensitivity reactions to corticosteroids include angioedema, urticaria,69–71 and anaphylaxis.61 Although uncommon, contact urticaria can also be triggered by corticosteroids.
DiagnosisDiagnosis can be made using in vivo tests, which include mainly the skin prick test and early reading of intradermal tests. Challenge tests can be used when the results are negative, although the risk-benefit ratio should always be taken into consideration. In vitro tests include specific IgE determination69,70 and the basophil activation test.72
When interpreting test results, it is important to rule out the possibility that the reaction is caused by preservatives or additives used in medications, such as carboxymethylcellulose or macrogol.73,74
TherapySystemic corticosteroids are necessary for the treatment of many diseases. The finding that immediate reaction to a specific corticosteroid does not necessarily imply a reaction to others indicates the need for an allergy workup to confirm the patient's sensitization/intolerance. As when studying any drug, it is important to take a detailed clinical history in order to determine the reason the corticosteroid was indicated, the type and kinetics of the reaction, and possible concomitant factors. Excipients should also be taken into account. Most authors use skin tests and controlled exposure tests to demonstrate the involvement of the drug. In addition, controlled exposure tests must be performed with succinate-free corticosteroids from a group other than the one causing the reaction in order to offer the patient alternative treatment. In emergency cases, betamethasone and deflazacort are recommended when the patient is allergic to other corticosteroids.63
ConclusionsAllergy to corticosteroids, especially ACD, is increasingly common. Prevalence ranges from 0.2% to 5%, and the most common allergen varies according to the country. In Spain, the most common allergen is budesonide, followed by tixocortol, with a prevalence of 0.95% and 0.63%, respectively.75
Corticosteroid-induced hypersensitivity reactions are a major restriction for the patient and a therapeutic challenge for the dermatologist in daily clinical practice. Given that the presentation of corticosteroid allergy and diagnostic test results are often difficult to interpret, a thorough knowledge of this condition is essential.
The recent classification by Baeck et al.26,27 has made it possible to reclassify corticosteroids according to their chemical structure and patients according to whether they are allergic to corticosteroids from 1 or more groups, thus improving treatment options and recommendations on the corticosteroids that can be prescribed.
The markers available in the Spanish standard series for detection of allergy to corticosteroids belong to group 1; therefore, patients allergic to group 2 and 3 markers may go undiagnosed. Studies based on the specific corticosteroid series and the patient's own commercially available creams could help to identify the individual sensitization profile.
Nevertheless, discordance continues to be observed between the results of patch tests and the patient's tolerance to various commercial preparations. In order to provide alternative treatments, it is advisable to perform exposure tests with corticosteroids from a group other than the one the patient is sensitized to or to recommend other options such as calcineurin inhibitors.
Although immediate hypersensitivity reactions are rarer than delayed reactions, they can be potentially severe. These reactions are problematic for various types of specialist, since the drugs are often indispensable; therefore, an allergy workup should be performed to confirm sensitization. As when studying any type of drug, a detailed clinical history is necessary to ascertain why the corticosteroid was indicated. Furthermore, it is important to take into account not only the active ingredient, but also the excipients used. Once the culprit drug has been identified, the patient should be advised about alternative corticosteroids. Betamethasone and deflazacort are recommended in emergency cases.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Berbegal L, DeLeon FJ, Silvestre JF. Reacciones de hipersensibilidad a corticoides. Actas Dermosifiliogr. 2016;107:107–115.