To evaluate health-related quality of life (HRQOL), patient satisfaction, and adherence to treatment in patients with moderate or severe atopic dermatitis on maintenance therapy.
Material and methodsWe performed a national, multicenter, cross-sectional, epidemiological study in adults and children with moderate or severe atopic dermatitis of at least 16 months’ duration who were receiving maintenance therapy. We used the Dermatology Life Quality Index (DLQI), the children's version of this scale (cDLQI), and the Morisky medication adherence scale. Visual analog scales were used to measure treatment satisfaction. We used the Mann-Whitney U test to compare HRQOL between patients with moderate and severe disease and the Wilcoxon test to compare the frequency and duration of flares before and after the start of maintenance therapy.
ResultsWe studied 141 children and 141 adults; the prevalence of moderate AD in these groups was 85.8% and 79.4%, respectively. The impact of AD on HRQOL was mild to moderate. Maintenance therapy led to a significant decrease in the frequency and duration of flares (P < .001). While treatment satisfaction was high in both groups, adherence was poor (18.4%-42.6% in children and 14.9%-27.0% in adults).
ConclusionsPatients with moderate and severe AD receiving maintenance therapy experience a reduction in the number and duration of flares and an improvement in HRQOL. While treatment satisfaction is high, adherence rates could be improved.
Evaluar la calidad de vida relacionada con la salud (CVRS), la satisfacción y cumplimiento en pacientes con dermatitis atópica (DA) moderada-grave en tratamiento farmacológico de mantenimiento.
Material y métodosEstudio epidemiológico, multicéntrico, nacional, transversal con pacientes adultos y pediátricos diagnosticados de DA moderada o grave de al menos 16 meses de evolución y en tratamiento de mantenimiento. Se aplicó el Índice de Calidad de Vida en Dermatología (DLQI), el Cuestionario Dermatológico de Calidad de Vida Infantil (CDLQI), la versión para menores de 4 años (IDQOL), la Escala de Afectación de la Dermatitis Atópica (EADA), el test de Morisky-Green y escalas visuales analógicas de satisfacción. Se comparó la CVRS entre pacientes con afectación moderada y grave (U de Mann-Whitney) y la duración y número de brotes antes y después de la terapia de mantenimiento (prueba de Wilcoxon).
ResultadosParticiparon 141 pacientes pediátricos y 141 adultos con DA moderada en el 85,8% y 79,4% de los casos, respectivamente. El impacto en CVRS fue leve-moderado. La duración y número de los brotes disminuyeron desde la aplicación del tratamiento de mantenimiento (p<0.001). Aunque la satisfacción fue alta en ambos grupos, el cumplimiento fue muy bajo (entre el 18,4%-42,6% en pediátricos y entre el 14,9%-27,0% en adultos).
ConclusionesLos pacientes con DA moderada o grave que siguen tratamiento farmacológico de mantenimiento presentan una reducción en la duración y número de los brotes y menor afectación de su CVRS. Además, los pacientes están satisfechos con el tratamiento aunque su cumplimiento es mejorable.
Atopic dermatitis (AD) is a chronic inflammatory skin disease that appears in early childhood. It is characterized by intense pruritus, dry skin, and skin lesions that appear in flares and have a considerable impact on patients’ lives.1,2 In recent years, the prevalence of AD has increased to between 7% and 20% in children and between 2% and 7% in adults.2,3 The etiology of AD has been linked to genetic, biological, immunological, and environmental factors.4 As a consequence of certain pathophysiological features of AD, such as the persistence of subclinical inflammation and skin barrier dysfunction, there has been a paradigm shift in the approach to treating the disease. It is now possible, after the resolution of a flare, to recommend a proactive treatment strategy aimed at preventing new flares and achieving longer flare-free intervals.5,6
AD can cause various difficulties. Numerous studies have highlighted the physical, psychological, and social impact of the disease on patients’ lives, especially during acute episodes.1,7–9 AD flares can interfere with sleep and rest, disrupt personal performance in school, at work, and in social relationships, and lead to considerable emotional distress associated with symptom severity. We believed it would be interesting to assess how patients with AD on maintenance therapy perceive their health-related quality of life (HRQOL), as there is little evidence on this group of patients in Spain. Previous studies have examined patients’ attitudes toward medical recommendations and treatment for AD flares.10,11 Nevertheless, research on patient satisfaction with maintenance strategies and on adherence to maintenance therapy is needed because these factors have important clinical implications.12–14 The primary objective of this study was to evaluate HRQOL, patient satisfaction, and treatment adherence in patients receiving maintenance therapy to control AD. The secondary objective was to confirm the basic psychometric properties of the Atopic Dermatitis Impact Scale (ADIS).
Patients and MethodsA total of 156 dermatologists throughout Spain participated in this multicenter, cross-sectional, epidemiological study. The enrollment period lasted from November 2009 to June 2010. During this period, each dermatologist consecutively interviewed 2 patients—1 adult (aged > 16 years) and 1 child (aged 2-15 years)—who had been clinically diagnosed with moderate or severe AD in accordance with the diagnostic criteria described by Hanifin and Rajka.15 Patients enrolled in the study had a disease duration of at least 16 months and had been receiving topical pharmacologic maintenance therapy for at least 4 months as a proactive strategy to prevent flares and control the disease. Topical pharmacologic maintenance therapy was defined as the prescription of topical calcineurin inhibitors, topical corticosteroids, or alternatives, with or without emollients, once the dermatologist had determined that the AD flare for which the patient had previously been treated was under control (stable or inactive AD lesions in the dermatologist's judgment). This topical therapy was prescribed according to the dermatologist's criteria, following the routine clinical practice of each hospital. All patients (or their parents, in the case of children) gave their written informed consent to participate in the study, which was conducted in accordance with Good Clinical Practice guidelines and the Declaration of Helsinki.
Study Variables and Measurement ToolsThe dermatologists recorded the following sociodemographic and clinical variables for each participating patient: age, sex, duration of AD, clinical assessment of disease severity (moderate or severe) at the time of diagnosis, number of flares in the previous year, duration of flares before the start of maintenance therapy, topical maintenance therapy prescribed for flare-free intervals (topical calcineurin inhibitors, topical corticosteroids, or alternatives), and number and duration of flares after at least 4 months of maintenance therapy. They also indicated their clinical impression of each patient's adherence to maintenance therapy and the treatment of flares.
Specific HRQOL Questionnaires (Completed by Patients or Their Parents)Dermatology Life Quality Index (DLQI).16,17 This 10-item questionnaire was used to evaluate patients’ symptoms and feelings as well as problems related to daily activities, leisure, work and school, personal relationships (including sexual difficulties), and treatment.
Children's Dermatology Life Quality Index (cDLQI).18 This version of the DLQI scale was used for patients between the ages of 5 and 16 years.
Infants’ Dermatitis Quality of Life Index (IDQOL).19 This scale was used for patients under the age of 5.
On the basis of these 3 specific questionnaires, patients were given a total aggregate score of between 0 (minimum impact, better HRQOL) and 30 (maximum impact, worse HRQOL). In addition, on the basis of their overall scores on the DLQI or cDLQI, patients were classified in 5 categories according to the impact of AD on HRQOL: no effect (0-1 points), small effect (2-5 points), moderate effect (6-10 points), very large effect (11-20 points), and extremely large effect (21-30 points). In addition, the IDQOL questionnaire included an item that evaluated the severity of the eczema on a scale of 1 (extremely severe) to 5 (fairly good or none).
Atopic Dermatitis Impact Scale (ADIS).11 This questionnaire has 9 items in the adult version and 8 items in the children's version. Respondents chose from 4 possible answers on a Likert-type scale and their overall scores were linearly transformed into a scale of 0 (minimum impact on HRQOL) to 10 (maximum impact).
Adherence and SatisfactionMorisky Medication Adherence Scale.20 This indirect measure was included in order to take into account self-reported adherence to the prescribed treatment regimen, thereby complementing our assessment from another relevant perspective. In the case of pediatric patients, the test was taken by the patients themselves if they were able to understand it and by their parents otherwise. Patients were considered to have adhered to treatment if they answered “no” to questions 1, 3, and 4 and “yes” to question 2.
Satisfaction. Patients used a visual analog scale of 0 (minimum satisfaction) to 10 (maximum satisfaction) to report their satisfaction with several aspects: the maintenance therapy overall, prevention of new flares, length of flare-free intervals, frequency of application, disease control, and information provided by the dermatologist about the maintenance therapy and about treating new flares.
Statistical AnalysisFirst, we carried out a descriptive analysis of the clinical and sociodemographic characteristics of the pediatric and adult patients. Second, we used the Mann-Whitney U test to compare the HRQOL of patients considered by the dermatologists as having moderate disease with that of patients considered as having severe disease. Third, we used the Wilcoxon test to compare the frequency and duration of AD flares before and after the start of maintenance therapy. Fourth, we used a forward conditional logistic regression model (entry criterion, P≤.05; exit criterion, P≥.1) to study which factors (sex, age, severity of AD, HRQOL, and satisfaction) were associated with treatment adherence and we calculated the odds ratios (ORs). Fifth, we analyzed the relationship between ADIS scores and DLQI, cDLQI, and IDQOL scores (Spearman correlation coefficient). Finally, we used exploratory factor analysis to determine whether the unidimensionality of the 2 versions of the ADIS was replicated and we calculated the Cronbach α to confirm adequate internal consistency. In all statistical tests with outcome variables, a P value of .05 was considered statistically significant. The statistical analyses described in this section were carried out using the Stata 10 (StataCorp) and SPSS (version 15.0) statistical packages.
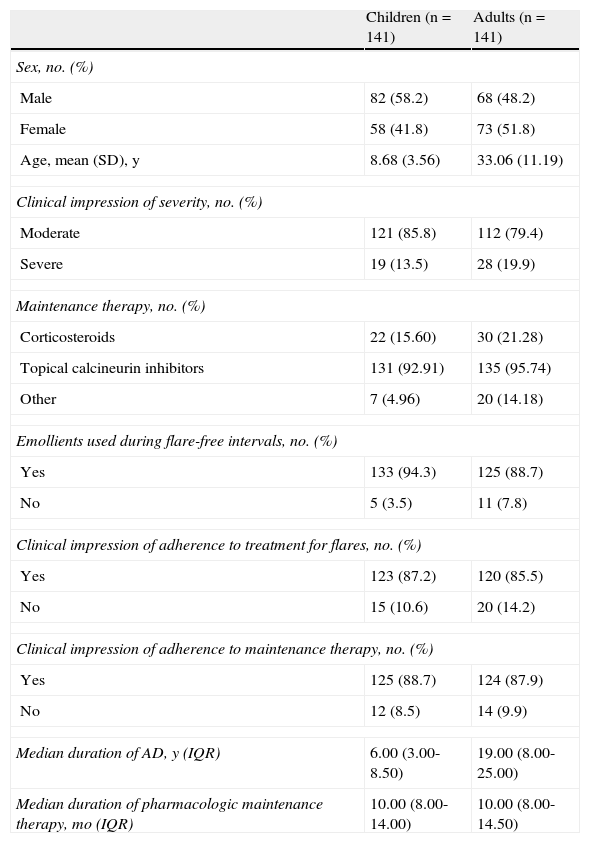
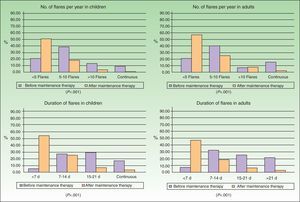
ResultsData were recorded for 309 patients with AD (155 adults and 154 children). Twenty-seven patients (14 adults and 13 children) were excluded from the study because they did not meet the inclusion criteria described in the previous section. The final sample for analysis comprised 282 patients (141 adults and 141 children). The sociodemographic and clinical characteristics of the sample are summarized in Table 1 and Fig. 1. According to the dermatologists’ clinical impression, AD severity was moderate in 85.8% of the children and 79.4% of the adults. The median time on maintenance therapy was 10 months (Table 1).
Description of Children and Adults With Atopic Dermatitis (AD).
| Children (n=141) | Adults (n=141) | |
| Sex, no. (%) | ||
| Male | 82 (58.2) | 68 (48.2) |
| Female | 58 (41.8) | 73 (51.8) |
| Age, mean (SD), y | 8.68 (3.56) | 33.06 (11.19) |
| Clinical impression of severity, no. (%) | ||
| Moderate | 121 (85.8) | 112 (79.4) |
| Severe | 19 (13.5) | 28 (19.9) |
| Maintenance therapy, no. (%) | ||
| Corticosteroids | 22 (15.60) | 30 (21.28) |
| Topical calcineurin inhibitors | 131 (92.91) | 135 (95.74) |
| Other | 7 (4.96) | 20 (14.18) |
| Emollients used during flare-free intervals, no. (%) | ||
| Yes | 133 (94.3) | 125 (88.7) |
| No | 5 (3.5) | 11 (7.8) |
| Clinical impression of adherence to treatment for flares, no. (%) | ||
| Yes | 123 (87.2) | 120 (85.5) |
| No | 15 (10.6) | 20 (14.2) |
| Clinical impression of adherence to maintenance therapy, no. (%) | ||
| Yes | 125 (88.7) | 124 (87.9) |
| No | 12 (8.5) | 14 (9.9) |
| Median duration of AD, y (IQR) | 6.00 (3.00-8.50) | 19.00 (8.00-25.00) |
| Median duration of pharmacologic maintenance therapy, mo (IQR) | 10.00 (8.00-14.00) | 10.00 (8.00-14.50) |
Abbreviation: IQR, interquartile range.
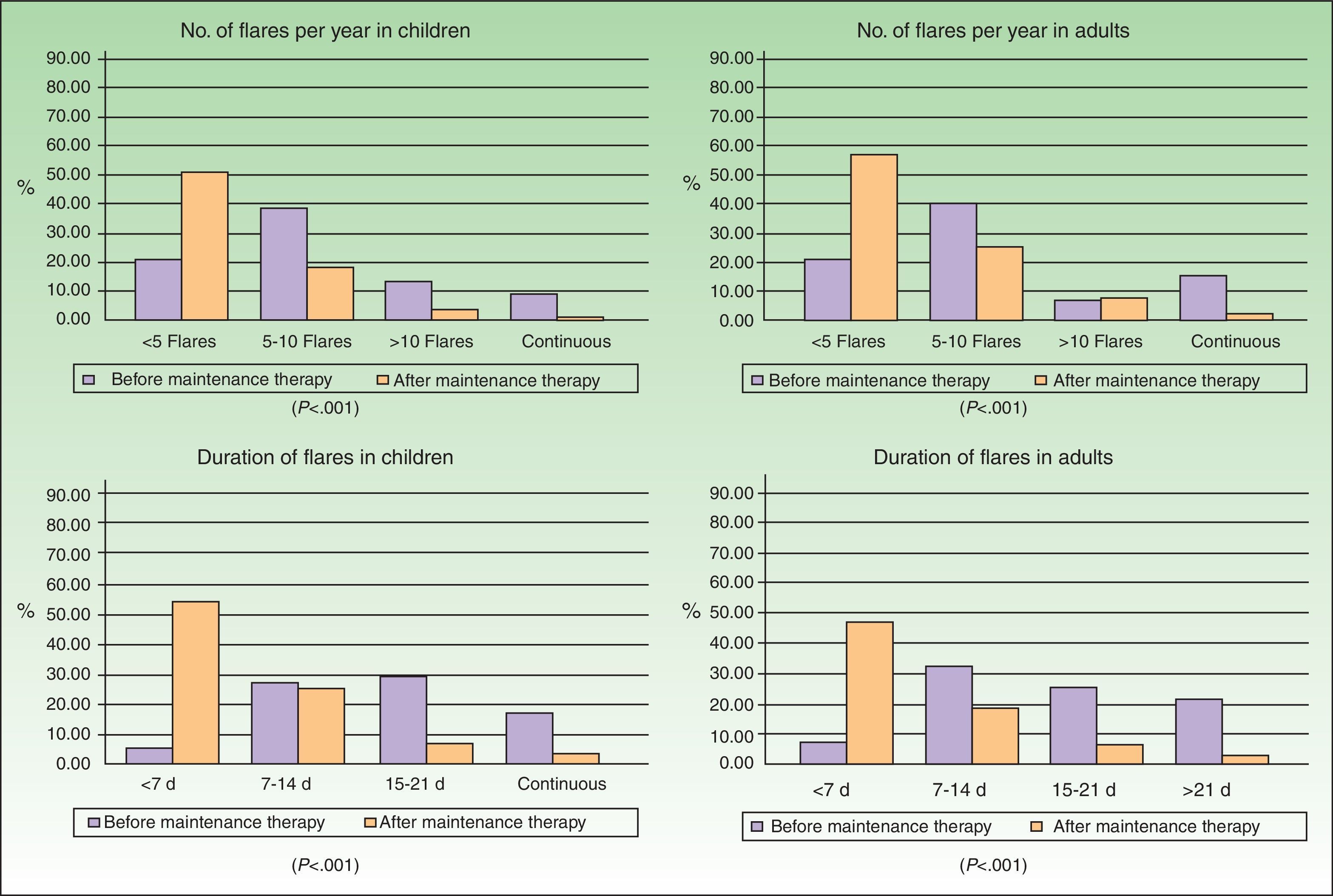
Improvement in the frequency and duration of flares after the start of maintenance therapy was observed in children and adults (Fig. 1) and was statistically significant in both groups (Wilcoxon test, P<.001).
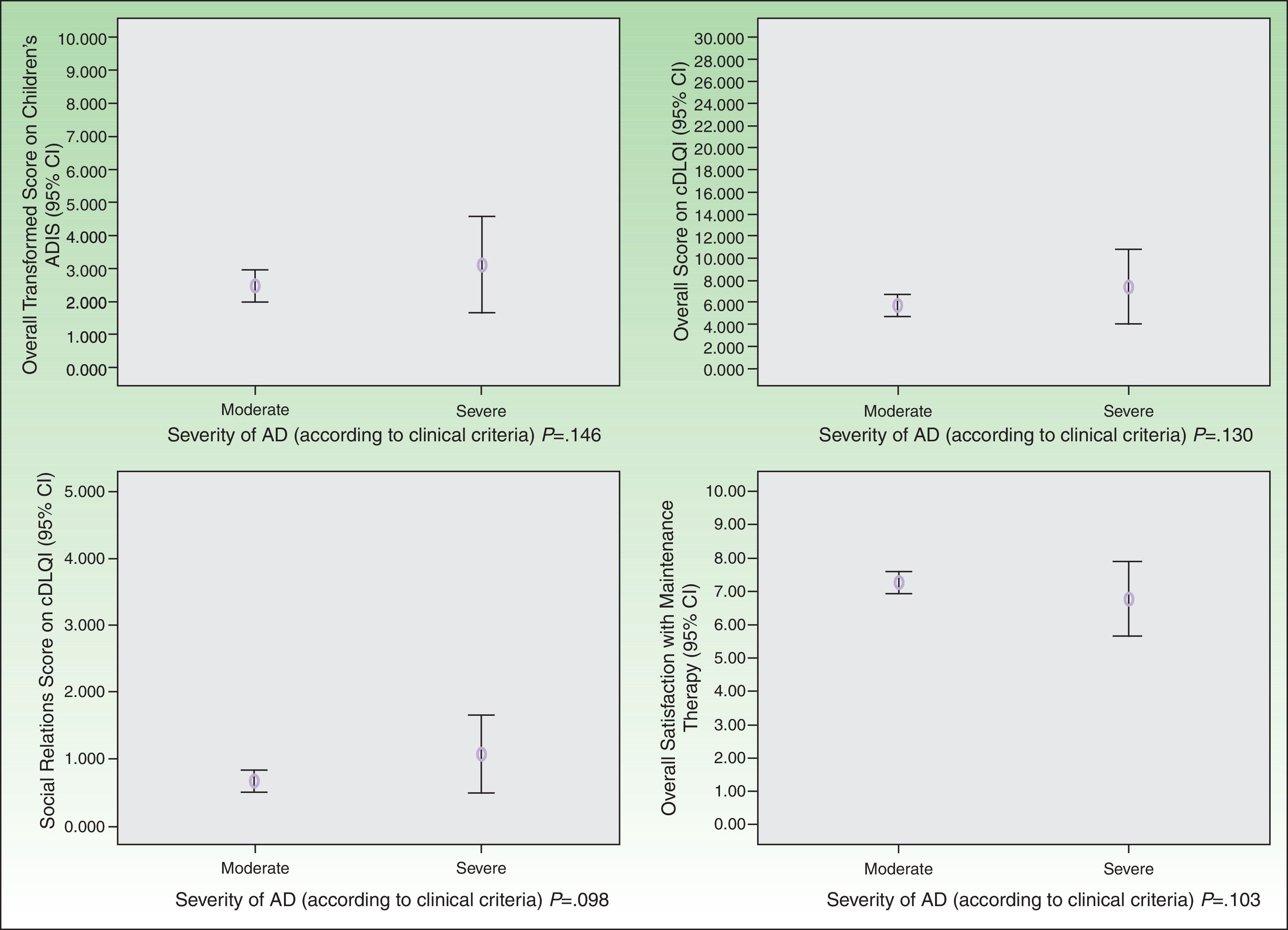
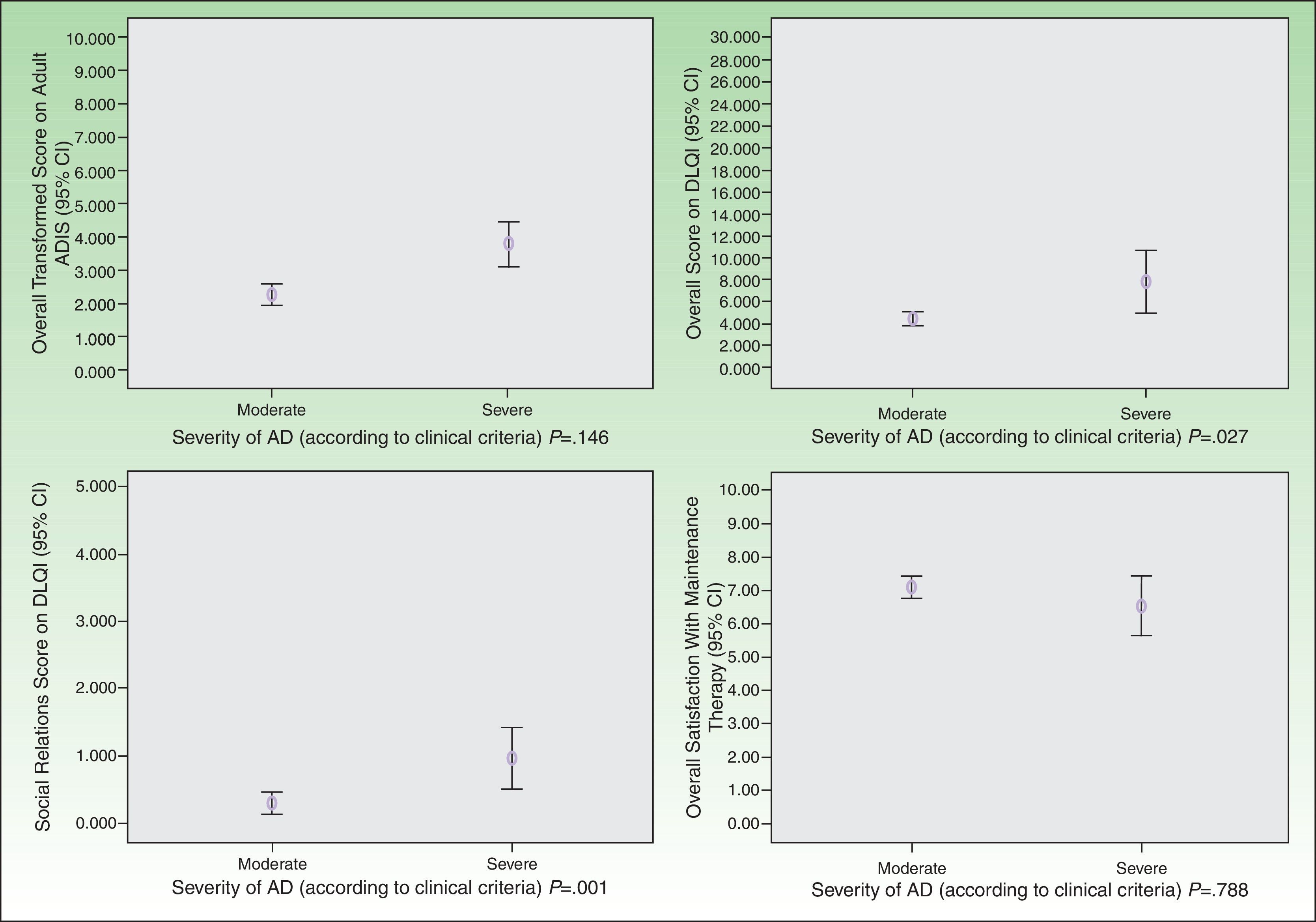
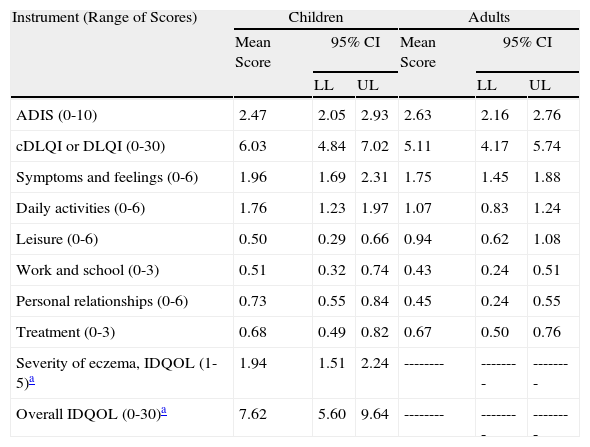
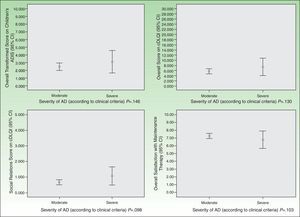
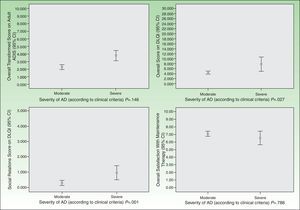
As for HRQOL, the results showed that in most cases AD had a mild impact on the daily lives of patients receiving maintenance therapy (Table 2). However, patients with moderate AD had higher levels of emotional, physical, and social well-being than those with severe AD (Figs. 2 and 3). The differences were statistically significant in adults (Fig. 3).
Health-Related Quality of Life of Patients With Atopic Dermatitis (AD) Receiving Maintenance Therapy.
| Instrument (Range of Scores) | Children | Adults | ||||
| Mean Score | 95% CI | Mean Score | 95% CI | |||
| LL | UL | LL | UL | |||
| ADIS (0-10) | 2.47 | 2.05 | 2.93 | 2.63 | 2.16 | 2.76 |
| cDLQI or DLQI (0-30) | 6.03 | 4.84 | 7.02 | 5.11 | 4.17 | 5.74 |
| Symptoms and feelings (0-6) | 1.96 | 1.69 | 2.31 | 1.75 | 1.45 | 1.88 |
| Daily activities (0-6) | 1.76 | 1.23 | 1.97 | 1.07 | 0.83 | 1.24 |
| Leisure (0-6) | 0.50 | 0.29 | 0.66 | 0.94 | 0.62 | 1.08 |
| Work and school (0-3) | 0.51 | 0.32 | 0.74 | 0.43 | 0.24 | 0.51 |
| Personal relationships (0-6) | 0.73 | 0.55 | 0.84 | 0.45 | 0.24 | 0.55 |
| Treatment (0-3) | 0.68 | 0.49 | 0.82 | 0.67 | 0.50 | 0.76 |
| Severity of eczema, IDQOL (1-5)a | 1.94 | 1.51 | 2.24 | -------- | -------- | -------- |
| Overall IDQOL (0-30)a | 7.62 | 5.60 | 9.64 | -------- | -------- | -------- |
Abbreviations: ADIS, Atopic Dermatitis Impact Scale; cDLQI, Children's Dermatology Life Quality Index; DLQI, Dermatology Life Quality Index; IDQOL, Infants’ Dermatitis Quality of Life Index; LL, lower limit; UL, upper limit.
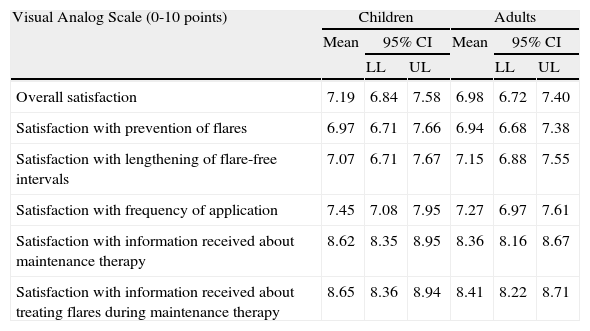
Satisfaction with the maintenance therapy was high in adults and children (Table 3). Both groups were satisfied with the information they received from the dermatologists about the role of maintenance therapy in preventing flares and about how to treat flares when they occur. As for satisfaction as a function of disease severity, patients with moderate AD were more satisfied than those with severe disease but the differences were small and not significant (Figs. 2 and 3).
Patient Satisfaction With Maintenance Therapy for Atopic Dermatitis (AD).
| Visual Analog Scale (0-10 points) | Children | Adults | ||||
| Mean | 95% CI | Mean | 95% CI | |||
| LL | UL | LL | UL | |||
| Overall satisfaction | 7.19 | 6.84 | 7.58 | 6.98 | 6.72 | 7.40 |
| Satisfaction with prevention of flares | 6.97 | 6.71 | 7.66 | 6.94 | 6.68 | 7.38 |
| Satisfaction with lengthening of flare-free intervals | 7.07 | 6.71 | 7.67 | 7.15 | 6.88 | 7.55 |
| Satisfaction with frequency of application | 7.45 | 7.08 | 7.95 | 7.27 | 6.97 | 7.61 |
| Satisfaction with information received about maintenance therapy | 8.62 | 8.35 | 8.95 | 8.36 | 8.16 | 8.67 |
| Satisfaction with information received about treating flares during maintenance therapy | 8.65 | 8.36 | 8.94 | 8.41 | 8.22 | 8.71 |
Abbreviations: LL, lower limit; UL, upper limit.
As for adherence to medical recommendations, responses to the Morisky Medication Adherence Scale showed that 18.4% of children and 14.9% of adults followed the treatment regimen correctly. More specifically, 49.9% of children and 64.5% of adults reported that they occasionally forgot to apply their treatment. In addition, 34.0% of children and 46.8% of adults said that they stopped using the treatment when they did not have any AD-related symptoms. If we apply less stringent criteria and consider as adherent all patients who said they never forgot apply their treatment and continued to use it when they had no symptoms, then 42.6% of children and 27% of adults adhered to treatment. There were discrepancies between the dermatologists’ perception of patients’ adherence and the patients’ self-reported adherence. The dermatologists considered that the vast majority of their patients (88.7% of children and 88.9% of adults) adhered to the maintenance therapy, but self-reported adherence rates were lower (18.4%-42.6% in children and 14.9%-27.0% in adults). Satisfaction with frequency of application was significantly associated with adherence in adults (adjusted OR, 2.12; 95% CI, 1.38-3.25; P=.001) while low HRQOL was associated with a greater likelihood of nonadherence in children (adjusted OR, 0.529; 95% CI, 0.325-0.859; P=.01).
Regarding the construct validity of the ADIS, a single general factor explained an adequate percentage of the variance in scores (54.04% in the children's ADIS and 48.22% in the adult version). In addition, the scales had good internal consistency (Cronbach α of 0.872 and 0.859 for the children's and adult ADIS, respectively) and all items in both versions were moderately to highly correlated with this single underlying factor (range of correlations for the children's and adult ADIS, 0.701-0.774 and 0.611-0.799, respectively). Finally, the correlation coefficients between scores on both versions of the ADIS and scores on the specific HRQOL questionnaires were moderate to high (range of correlations for children's ADIS and cDLQI, 0.327-0.521; P<.01; range of correlations for adult ADIS and DLQI, 0.553-0.767; P<.01).
DiscussionIn this study, we observed that maintenance therapy has positive effects on the control of AD symptoms. After maintenance therapy was started, the proportion of patients with fewer than 5 flares per year increased significantly. In addition, the number of days the flares lasted decreased significantly in adults and children. This benefit was reflected in greater physical and emotional well-being. In particular, we observed that the impact of AD on patients’ lives was smaller than that reported in previous cohort studies of patients with moderate or severe AD who did not fully adhere to pharmacologic maintenance therapy.11,21 In our study, the mean DLQI and cDLQI scores of patients with moderate or severe AD indicated a mild to slightly moderate impact on HRQOL. Specifically, the impact of AD on HRQOL was mostly mild in patients with moderate disease and moderate in patients with severe disease (Figs. 2 and 3). Despite the small number of patients with severe AD in our sample, these results show the benefit of using pharmacologic maintenance therapy to control AD in clinical practice and are consistent with evidence from more strictly controlled experimental studies.5,6,22–24
Patients were highly satisfied with both the frequency of application of the pharmacologic maintenance therapy and its success in lengthening flare-free intervals. These levels of satisfaction are comparable to, but slightly higher than, the overall levels of satisfaction with treatment for AD flares found in a 2008 study by Schmitt et al.25 in Germany. The same study found that the impression that the treating physician is professionally competent and provides useful information about controlling AD were the strongest determinants of patient satisfaction.25 In the present study, both adults and children placed the highest value on the information they received from their dermatologists about treating flares during maintenance therapy and about controlling the disease with topical pharmacologic agents.
It appears necessary for dermatologists to stress to patients that adherence to therapy and medical recommendations is essential to obtaining clinical benefits and controlling AD.12–14 In our study population, there was room for improvement in adherence to maintenance therapy. The high percentage of patients (34% of children and 47.8% of adults) who stopped using the treatment when the symptoms subsided and who occasionally forgot to use it (49.9% of children and 64.5% of adults) were especially noteworthy. These results are consistent with those of previous studies on topical therapies.12,13,26 We therefore consider that dermatologists should stress the importance of adherence to therapy and adopt patient education strategies.27–29 We believe that outcomes could be optimized by applying long-term AD management strategies of this sort. Moreover, we found that adherence in adult patients was significantly associated with satisfaction with frequency of application, and we believe that this association should be taken into account in efforts to improve adherence.
The main limitation of this study was its cross-sectional design, as this prevented us from recording and measuring the magnitude of clinical changes—related to a reduction in symptom intensity and possible treatment-related adverse effects—and from assessing their relationship with the constructs mentioned above (HRQOL, satisfaction, and adherence). Nevertheless, we achieved our primary objective—to describe HRQOL, patient satisfaction, and adherence in patients receiving maintenance therapy—by applying psychometrically valid scales. In addition, it is important to note that AD severity was assessed on the basis of the dermatologists’ diagnostic criteria within the routine clinical practice of their hospitals. It would have been preferable to complement this assessment with a specific measure of recognized validity30 such as the SCORing Atopic Dermatitis (SCORAD) index. However, we were unable to include this objective instrument in our study because it is not yet widely used in routine clinical practice in Spain.11 Another limitation was the use of indirect measures to detect nonadherence. While we recognize that more accurate results could have been obtained with electronic monitoring systems12,13 or other objective measures, the number of hospitals and patients in this study made the use of such tools unfeasible. We therefore decided instead to include dermatologists’ perception of patients’ adherence in order to incorporate at least 2 assessment methods in this variable. Furthermore, although we are aware that the Morisky Medication Adherence Scale tends to overestimate nonadherence,31 the adherence rates obtained with this test were similar to those found in earlier studies in children.12,26 We included the adherence rates found with the various items of this test in order to provide dermatologists with more information about nonadherence in our patients.
In conclusion, in this study we have confirmed that the ADIS is a short assessment instrument with adequate psychometric properties. Further, the high internal consistency and convergent validity detected between the ADIS and widely validated30 scales such as the DLQI16 and the cDLQI18 support the use of the ADIS in routine clinical practice to obtain additional information about the impact of moderate or severe AD in patients receiving topical pharmacologic maintenance therapy.
Ethical DisclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of dataThe authors declare that they have followed their hospitals’ protocol on the publication of data concerning patients and that all patients included in the study have received sufficient information and have given their written informed consent to participate in the study.
Right to privacy and informed consentThe authors declare that no private patient data are disclosed in this article.
FundingThis study was funded by Astellas Pharma S.A.
Conflicts of InterestDr. Emilio Pedrosa is employed by Astellas Pharma Spain. However, given the aims and scope of this study, the other authors declare that there are no conflicts of interest.
Please cite this article as: Torrelo A, et al. Calidad de vida relacionada con la salud, satisfacción y cumplimiento de los pacientes con dermatitis atópica moderada-grave que siguen un tratamiento farmacológico de mantenimiento. Estudio CONDA-SAT. Actas Dermosifiliogr. 2013;104:409-17.