Palmoplantar psoriasis is an uncommon clinical form of psoriasis. Although localized to the palms and soles, it has a considerable impact on the patient's function and quality of life.
ObjectivesTo study the effectiveness and safety of psoralen-UV-A (PUVA) therapy in palmoplantar psoriasis and investigate predictors of clinical response.
Material and methodsWe performed a retrospective chart review of all patients with palmoplantar psoriasis treated with topical PUVA therapy at our hospital between 2008 and 2011. Data were collected on effectiveness (using physician global assessment [PGA] scores), safety, and a range of clinical, epidemiological, and treatment-related variables.
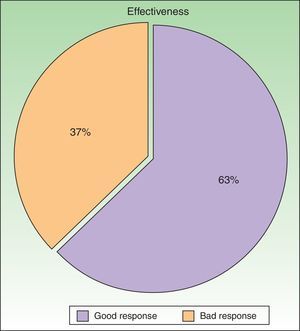
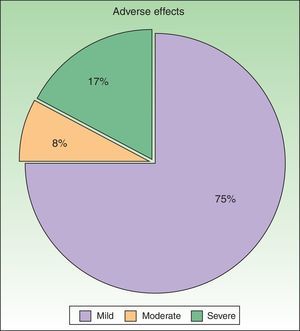
ResultsWe studied 48 patients (33 women and 15 men) with a mean age of 51 years. Treatment was considered to be effective (PGA score of 0 or 1) in 63% of cases. In addition to PUVA, systemic therapy was required in 47.9% of patients; the drug most often used was acitretin. Adverse effects were reported for 25% of patients during treatment. The most common effect was mild erythema, present in 18% of cases.
ConclusionsIn our experience, topical PUVA is an appropriate treatment alternative for palmoplantar psoriasis; it offers similar response rates to systemic treatments, but has a better tolerance and safety profile. Associated systemic treatment, with acitretin in most cases, improved the probability of a satisfactory response to PUVA and should be considered in patients who do not respond adequately after 8 to 10 sessions.
La psoriasis palmoplantar es una forma clínica de psoriasis con una baja prevalencia y una limitada extensión, pero con un marcado impacto en la funcionalidad del paciente y en su calidad de vida.
ObjetivosEstudiar la eficacia, seguridad y factores predictores de respuesta de la PUVA-terapia en el tratamiento de la psoriasis palmoplantar.
Material y métodosEstudio clínico, abierto y retrospectivo en el que se analizaron las historias clínicas de todos los pacientes con psoriasis palmoplantar tratados con terapia PUVA tópica entre los años 2008 y 2011 en nuestro centro. Se recogieron datos sobre eficacia (utilizando el PGA como marcador) y seguridad, así como sobre aspectos clínicos, epidemiológicos y referentes al tratamiento.
ResultadosSe incluyeron un total de 48 pacientes (33 mujeres y 15 hombres) con una edad media de 51 años. El tratamiento se consideró eficaz (PGA 0/1) en el 63% de pacientes. Se requirió el uso de fármacos sistémicos asociados a la terapia PUVA en el 47,9%, siendo el acitretino el fármaco más utilizado. El 25% presentó algún efecto adverso durante el tratamiento, que en su mayor parte consistió en eritema leve (18%).
DiscusiónEn nuestra experiencia la terapia PUVA tópica representa una alternativa adecuada en el tratamiento de la psoriasis palmoplantar, con perspectivas de respuesta similares a las de otros tratamientos sistémicos y con mejor perfil de tolerancia y seguridad. La asociación terapéutica, en particular con acitretino, permite mejorar las posibilidades de respuesta y debería considerarse en caso de respuesta no satisfactoria después de unas 8-10 sesiones.
Palmoplantar psoriasis is an uncommon clinical form of psoriasis. Although localized to the palms and the soles, it has considerable impact on the patient's quality of life.1 Management of the disease is often difficult because in most cases no effective topical or even systemic treatment exists. Topical psoralen–UV-A (PUVA) therapy is one of the treatments most often used because it offers a good balance between efficacy and safety.2 However, very little information is available about the ideal methodology or the existence of predictors of clinical response that would enable clinicians to prioritize the use of this treatment option in selected patients. Likewise, there are only scant data on the usefulness or advisability of combining topical PUVA therapy with systemic agents. We report our experience with topical PUVA therapy in patients with palmoplantar psoriasis in terms of the patients’ epidemiological and clinical profile as well as of treatment outcomes. We also analyzed the data in search of predictors of response and factors associated with the onset of adverse effects.
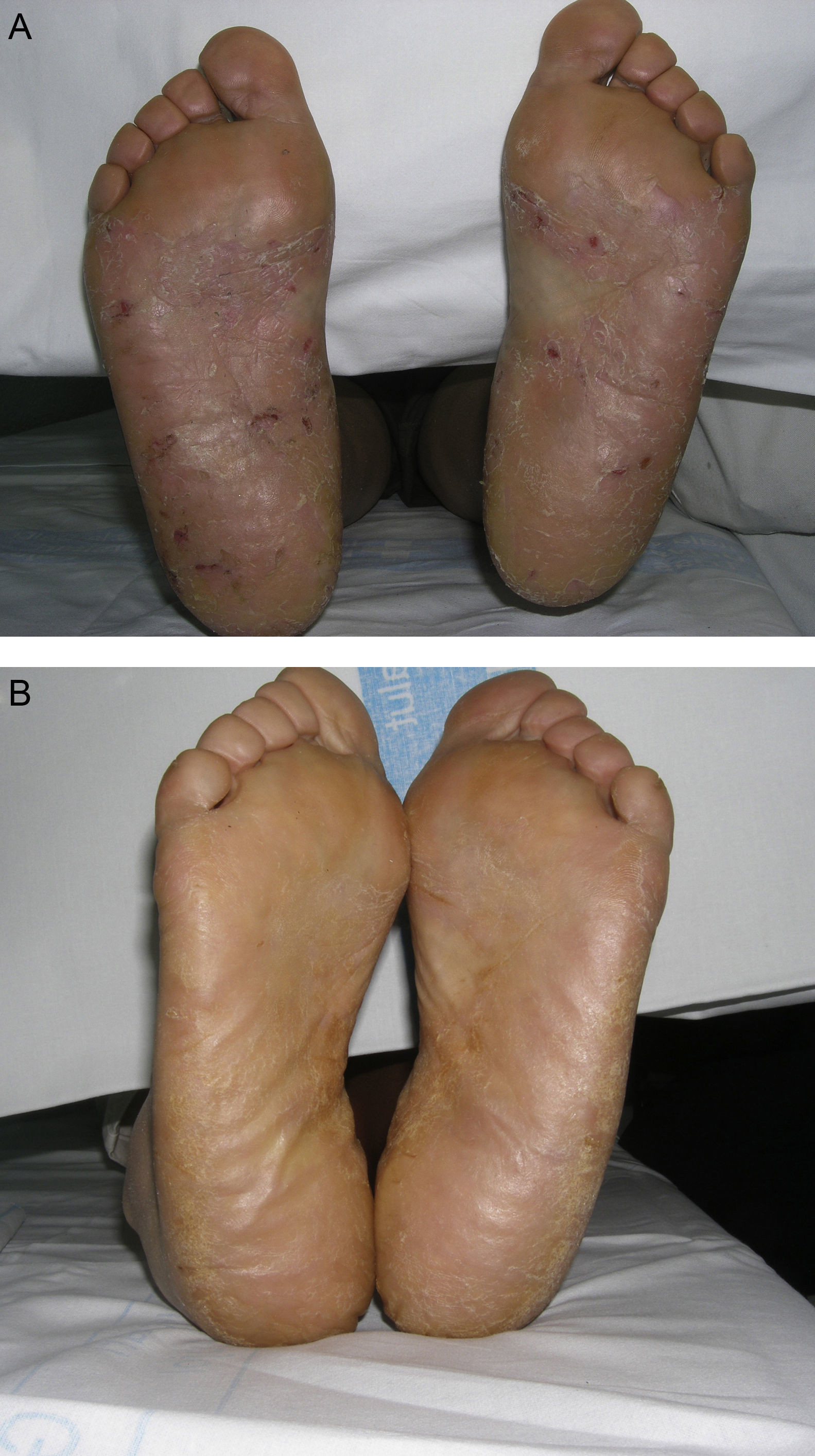
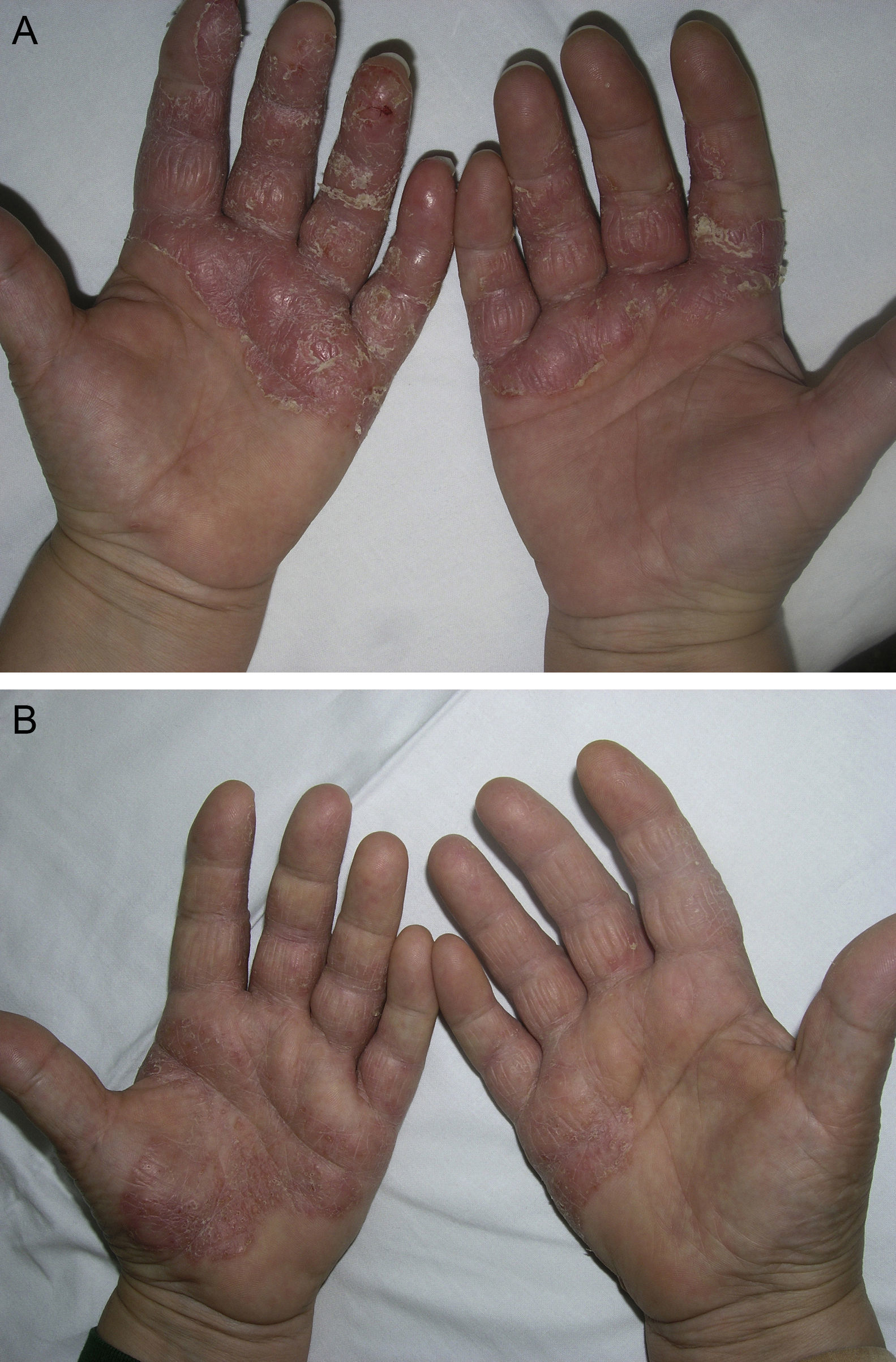
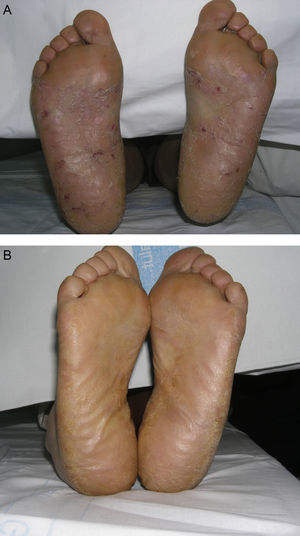
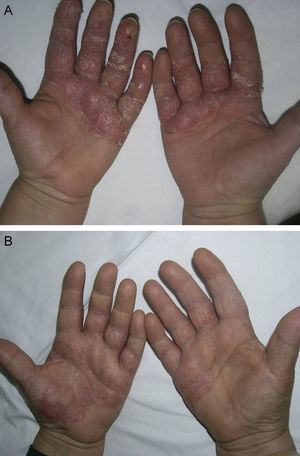
Material and MethodsThis was a retrospective chart review of all the patients with palmoplantar psoriasis treated with topical PUVA therapy, alone or in combination with another treatment, between 2008 and 2011 at our hospital. The diagnosis of palmoplantar psoriasis was clinical. When the differential diagnosis included other conditions (for example, contact dermatitis or hyperkeratotic tinea pedis), additional tests were performed to rule out the other possibilities. If there was any doubt about the diagnosis, the patient was not included in the study. Severity was assessed on the basis of the Physician's Global Assessment (PGA), which was performed at the beginning and end of treatment and recorded in the medical chart. A good response was defined as a score of 0 or 1 on the 5-point PGA scale (0=no lesions, 1=minimal lesions, 2=mild lesions, 3=moderate lesions, 4=severe lesions, and 5=very severe lesions) (Figs. 1 and 2). Since this was a retrospective study, one limitation we had to accept was that no PGA score was available in a small number of patients, in whom the initial assessment of severity in the clinical record took the form of a detailed description or photographic record of the lesions. In such cases, the PGA score for the purposes of our study was derived from the information available. The same circumstance made a detailed assessment of the clinical features impossible; the presence and relative severity of erythema, infiltration, scaling, or pustules were not recorded. To ensure a homogeneous group, patients diagnosed with palmoplantar pustulosis were not included in this study. Data were collected on epidemiological and clinical variables and on response to treatment (Table 1). The data for patients who discontinued treatment for reasons unrelated to the course of the same were included in the epidemiological analysis, but not in the analysis of efficacy (Figs. 1 and 2).
Variables Included in the Study.
| Sex |
| Age |
| Comorbidities (smoking, hypertension, dyslipidemia, and diabetes) |
| Family history of psoriasis |
| Time since onset |
| Prior topical treatment |
| Prior systemic therapy |
| Exclusively palmoplantar involvement vs generalized psoriasis |
| PGA at the start and end of treatment |
| No. of sessions |
| Maintenance regimen (yes or no) |
| Need for adjunctive systemic treatment during PUVA therapy and motive for such therapy |
| Adjunctive systemic therapy |
| Adverse effects |
| Adherence to treatment |
Patients in whom a regimen of topical corticosteroid or keratolytic creams did not produce satisfactory improvement within 4 to 8 weeks were selected for topical PUVA therapy.
In all cases, an oil-in-water emulsion of 8-methoxypsoralen 0.1% was applied to the affected areas 15minutes before the application of UV-A radiation. Treatment was administered using Waldmann 180 and 200 therapy systems (Herbert Waldmann GmbH & Co. KG) fitted with 5 and 14 Philips UVA lamps, respectively. The initial dose was 0.5 J/cm2 for all patients, irrespective of skin phototype. The dose was increased by 0.5 to 1 J/cm2 per session up to a maximum of 6 to 9 J for the soles and of 4 J for the palms, depending on tolerance and clinical course in each case. All the patients received topical treatment, such as potent corticosteroids, vitamin D derivatives, and keratolytic agents, alone or in combination. This was administered after the PUVA sessions. Systemic therapies were also prescribed before or during phototherapy, depending on patient profile and clinical course. In general, the decision to add systemic treatment depended on the patient's characteristics and comorbidities or was taken when the clinical response was unsatisfactory after 8 to 10 sessions of PUVA therapy.
The data collected during the study were analyzed to identify predictors of good response to treatment. Patients were classified into 2 groups based on whether their response to treatment was satisfactory or unsatisfactory. All the variables studied (listed in Table 1) were then compared between the 2 groups.
In the descriptive analysis, the mean or median was used for the quantitative variables and the proportion of each group for the qualitative variables.
In the univariate analysis, the statistical relationship between 2 variables was normally assessed with the χ2 test, but Fisher's exact test was used when a small sample size was expected.
ResultsA total of 48 patients (33 women and 15 men) were included in the study (Table 2). The mean age was 51.3 years (range, 20-84 years). At start of treatment, 40% of the patients were current smokers. The most common comorbidities were hypertension (24.4%), dyslipidemia (50%), and diabetes (12.5%). In most cases (47.1%), the time elapsed since onset of psoriasis was between 1 and 10 years. Psoriasis was confined to the palms and soles in most of the patients (79.1%); a much smaller group (20.3%) had generalized psoriasis with predominantly palmoplantar involvement. The patients with generalized involvement had a psoriasis area and severity index (PASI) of less than grade 3 or 4 in all cases. Both palms and soles were affected in most patients (58.3%), the palms alone were affected in 20.8%, and only the soles in 37.1%. Altogether, 83.3% (n=40) of the patients had received topical treatment before starting the PUVA regimen, and 29.1% (n=14) had also received systemic therapy. The systemic treatments they had received were ciclosporin (14.28%), methotrexate (14.28%), acitretin (28.5%), and biologics (14.28%).
Epidemiological Characteristics and Treatment Efficacy by Treatment Regimen: Monotherapy or Combination Therapy.
| Patients on Monotherapy | Patients on Combination Therapy | |
| No. of Patients | 25 | 23 |
| Age | 45 | 57 |
| Sex | 8 men/17 women | 7 men/16 women |
| Smokers | 8 (32%) | 12 (52%) |
| Hypertension | 4 (16%) | 8 (34%) |
| Dyslipidemia | 10 (40%) | 14 (60%) |
| Diabetes | 1 (4%) | 5 (21%) |
| Time since onset | Less than 1 year, 4 (16%); 1-10 years, 14 (56%); over 10 years, 7 (28%) | Less than 1 year, 5 (21%); 1-10 years, 9 (39%); over 10 years, 9 (39%) |
| Good response | 65% | 60% |
| No. of Sessions | 33 | 45 |
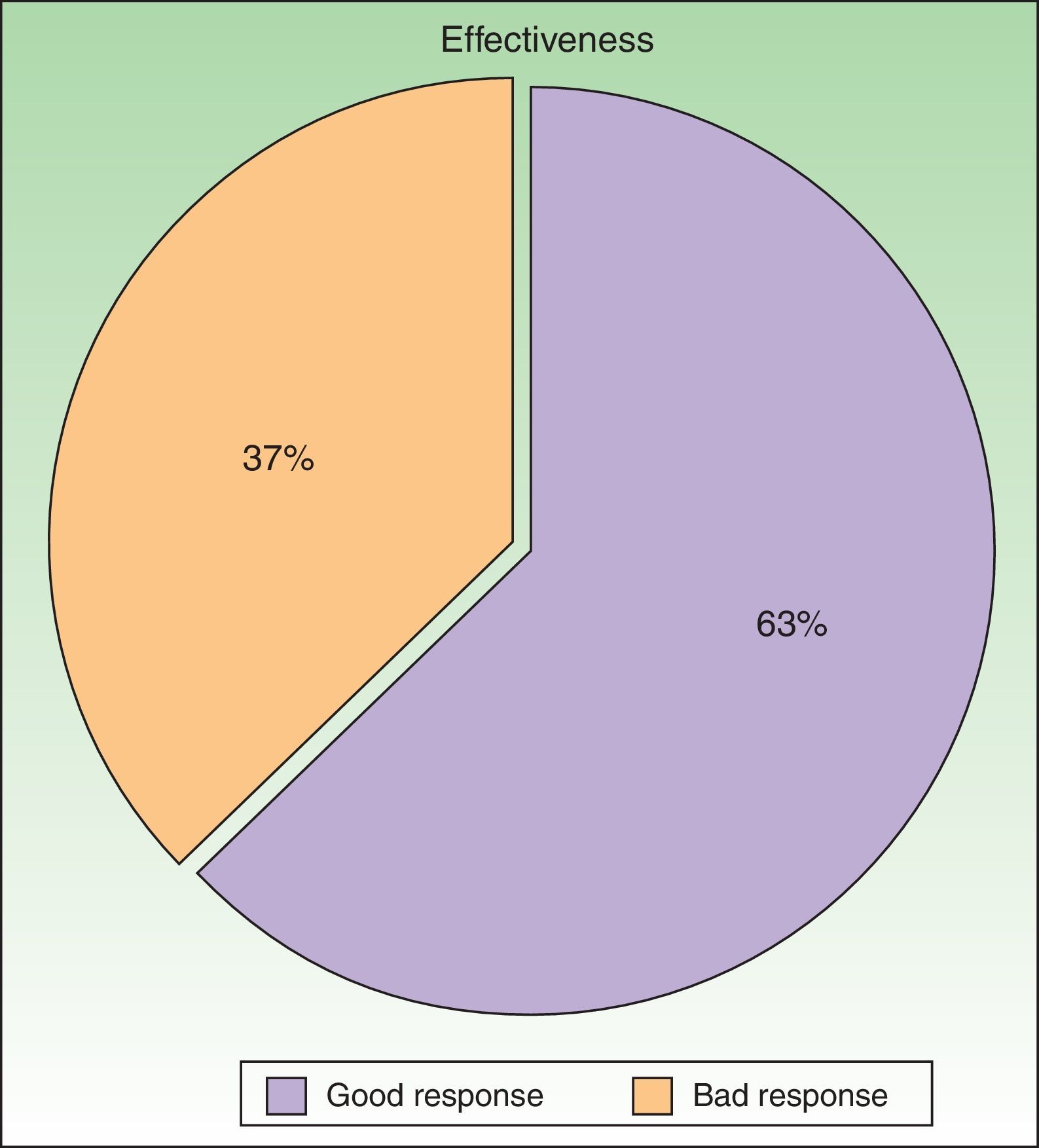
According to the criteria described in the methods section above, a satisfactory response was achieved in 25 patients (62.25%) after a mean of 47 sessions (range, 11-170; median, 33) (Fig. 3). In 23 patients (47.9%), systemic therapy was added during treatment with PUVA because of an inadequate response. The adjunctive systemic treatment prescribed was ciclosporin in 2 patients (9%), methotrexate in 4 (17%), and acitretin in 17 (74%). Acitretin at daily doses of between 10mg and 25mg was the most frequently used adjunctive treatment.
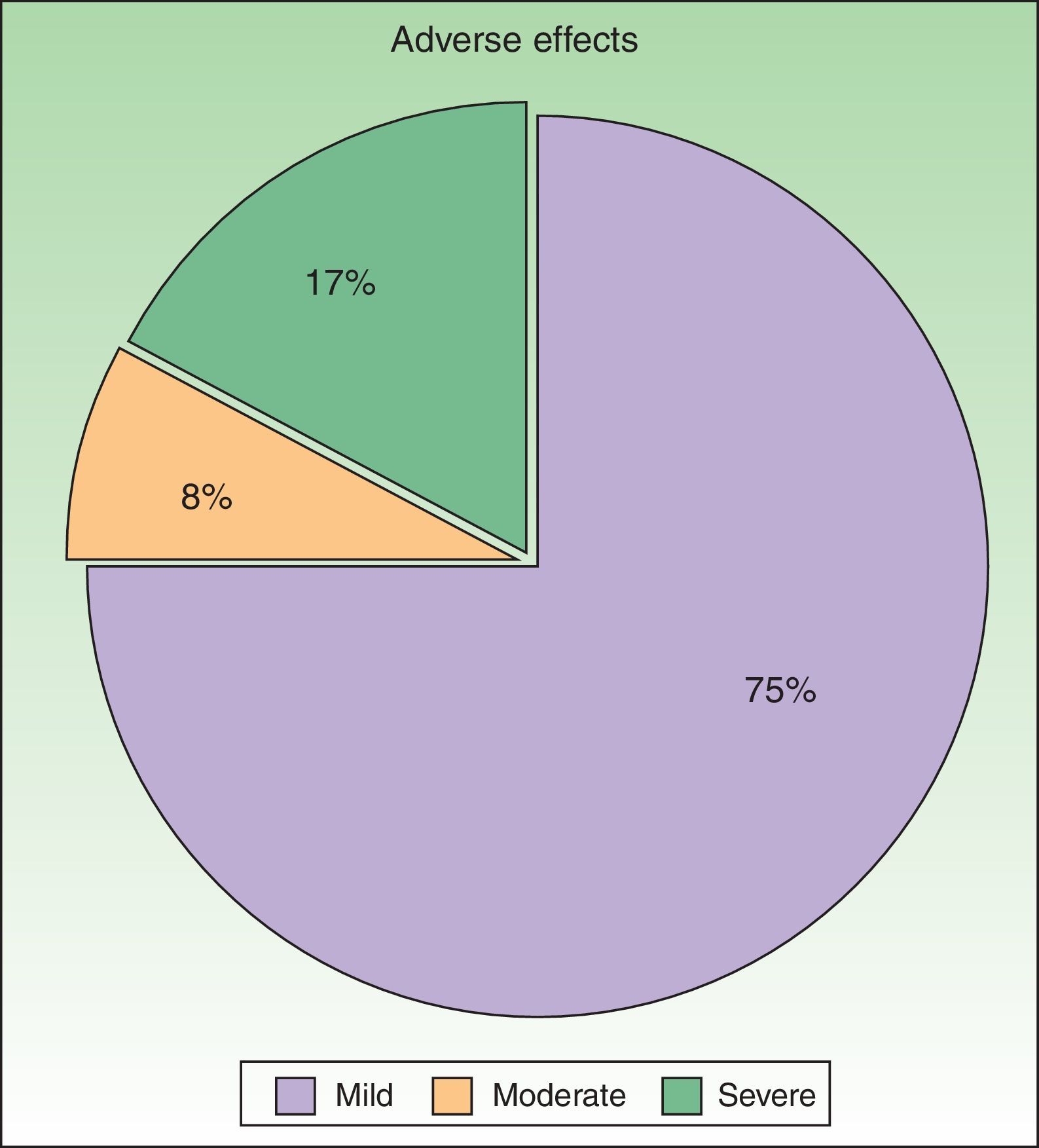
ToleranceOne in 4 patients (25%, n=12) had adverse effects during treatment (Fig. 4). The most common adverse effect was mild erythema (18%, n=9). The incidence of adverse events was higher in patients who had good response to treatment (36% in patients with good response vs 20% in patients with poor response), but this difference was not statistically significant. By contrast, no differences in the number of adverse effects reported were observed between the patients who received combination therapy as compared to those who received PUVA monotherapy (6 patients in each group presented an adverse effect). Most adverse effects were mild or moderate and did not affect the course of treatment or lead to a dose adjustment. Adjustment of the dose was necessary in only 2 patients, and treatment was withdrawn definitively in only 1. No statistical relationship was detected between the incidence of adverse events and response to treatment.
Systemic treatment was generally well tolerated. Acitretin was often associated with adverse effects, such as mucocutaneous xerosis, but these were well controlled with emollients and general measures. In no case was treatment suspended due to adverse effects.
DiscussionIn this series, topical PUVA therapy produced a good clinical response in 6 out of 10 patients with palmoplantar psoriasis and was generally well tolerated.
Very little data are available on the epidemiological profile of patients with palmoplantar psoriasis or whether this profile is similar to that of patients with psoriasis vulgaris. An interesting aspect in our series was the predominance of women (68%), a characteristic not reported by other authors.2 A predominance of women has been reported for the pustular forms of psoriasis, but patients with these forms were excluded from the present study and the clinical characteristics and course of pustular psoriasis differ from those of psoriasis vulgaris.3
Of note in this series was the comorbidity profile, which was comparable to what might be expected in patients with moderate to severe psoriasis; the percentage of comorbidities was higher than normal for the general population in Spain.4 No comparisons can be made due to the lack of data from other specific studies or screening of patients with palmoplantar psoriasis, but the data collected will be of interest in future studies.
Palmoplantar psoriasis represents a therapeutic challenge. Despite being confined to a small area, this form of psoriasis has a major impact on the patient's quality of life.1 Notwithstanding the limited extent of the disease, this negative impact is considered to justify the use of systemic therapies, which are not without risk. In fact, palmoplantar involvement is classified as an independent criterion of severity in the consensus document of the Psoriasis Group of the Spanish Academy of Dermatology and Venereology, even justifying the use of biologic therapies.5 In our series, the impact of the disease on the patient's quality of life had not been routinely assessed. However, the frequent use of systemic therapy in about half of the patients is an indirect indication of the clinical and psychological impact of the disease in the patients studied.
In most of our patients, lesions were confined to the palms and the soles. The findings in the literature concerning exclusively palmoplantar involvement are very heterogeneous, ranging from 18% to 79% of patients.6,7 Exclusive or clearly predominant involvement of these areas is the hallmark of patients with palmoplantar psoriasis, who may also present minimal lesions in other areas—the elbows for example—which are not noted in the medical record. It is likely that the diagnosis of palmoplantar psoriasis is applied to many of these patients because the limited involvement of other areas of the body is not reflected in their medical record or because these lesions are not noticed.
The absence of lesions in other locations may give rise to doubts about the diagnosis and the clinician may consider the possibility of other dermatoses, such as the hyperkeratotic form of chronic hand eczema.8 Moreover, because of the absence of clear clinical criteria defining chronic hand eczema, some cases in the literature of patients diagnosed with this variant of eczema may actually have had localized forms of psoriasis. This hypothesis is supported by the chronic nature of the condition and the similarity between these 2 entities in terms of therapeutic management and response. In our patients, when atopic or irritant dermatitis was suspected, a specific medical history was obtained and standard or specific patch tests were performed. It is, however, impossible to rule out the possibility that the lesions, in some cases, may have been aggravated by an undetected allergen.
The clinical response, assessed using the PGA, was good in our series (0 or 1 in 62.5%). While this result could be considered moderate, it is within the range reported in earlier studies.9–11 The evaluation of the evidence in the literature concerning both therapeutic procedure and outcomes is particularly complex in the case of topical PUVA therapy because of the heterogeneity of the data (Table 3). First, no randomized, placebo-controlled studies have been published that would provide solid evidence to support conclusions; consequently all recommendations are currently based on small series and author experience. Second, there are no uniform criteria for clinical assessment or follow-up, so that in many of the published studies the assessment of outcomes was quantitative (insufficient, moderate, or excellent response, or using a quantitative index) but did not take into account baseline clinical characteristics. The situation is further complicated by the fact that in many of the series reported a number of different skin conditions affecting the palms were studied, including a few cases of psoriasis. Finally, the treatment protocols and equipment used vary from study to study, although most authors used Waldmann 180 and 200 lamps. Despite all these limitations, a common thread emerges. All these studies report a moderate response to treatment in around 50% of cases—although with variable margins—and all indicate the need for prolonged treatment over several months. There is, however, an almost complete absence of data concerning the evolution of the patients’ condition after withdrawal of treatment. A number of authors have studied the use of PUVA therapy in the treatment of palmoplantar psoriasis using topical or oral psoralen or psoralen baths. However, methodological differences and variations in the concentration of psoralen used make it impossible to confirm which procedure yielded the best results, although it is likely that the differences are not great and are linked to the experience of the authors and perhaps to factors unrelated to the actual procedure used. The combination of PUVA and oral acitretin used in our series is common. The recommended dose is around 25mg/d, although once again uniform criteria for recommending such therapy are lacking. Table 3 is a summary of the major studies on topical PUVA therapy in the treatment of palmoplantar psoriasis, in either combination or monotherapy regimens.9,11–14
Summary of Studies on Topical PUVA Therapy in Palmoplantar Psoriasis.
| Cases Treated | Radiation Source | Regimen | Psoralen Administration | Measure of Efficacy | Combination Therapy with Acitretin | Response | Number of Sessions/Cumulative Dose | Tolerance | |
| Spuls et al.11 | 26 (including cases of pustular disease) | Not reported | Initial dose, 0.25%-0.5%. Increments, 0.25%-0.5% | Bath PUVA | Qualitative: no response/significant response | Yes in 12/26, 10-25mg/d | Clearance 5/9. Moderate response 2/9 | Not reported | Not reported |
| Adisen et al.2 | 12 | Waldmann 180/200 | Initial dose, 0.3-0.5 J/cm2. Increments, “according to response and tolerance” | Gel 0.1%/oral | Qualitative: no response/significant response | No | 53% (only in oral PUVA) | Not reported | Not reported |
| Shiener et al.13 | 8 | Waldmann 180/200 | MPD. Increment every 3 sessions, 0.3 J/cm2 to a maximum of 5 J/cm2 | 0.005% gel/bath PUVA | ASI (modified PASI) | No | Reduction in the ASI (modified PASI) from 26-28 to 1.5 | 34 sessions/95 J/cm2 | Not reported |
| Neumann et al.14 | 10 | Waldmann 180/200 | Initial dose, 0.5 J/cm2; maximum, 8 J/cm2 | 0.03% cream | Modified PASI | No | 64.6% | 5 weeks | Erythema/irritation 4/10 |
| Grundmann-Kollmann et al.12 | 4 | Waldmann 180/200 | Initial dose, 0.3-0.5 J/cm2; maximum 3.5-6.6 J/cm2 | 0.0006% cream/bath PUVA | Quantitative marker 0-20 | No | 50%-75% | 18 to 27 sessions | Not reported |
| O’Kane et al.9 | 20 | Not reported | Initial dose 0.3 to 0.5 J/cm2. Weekly or biweekly increments of 0.5 J/cm2 or 20% | Bath PUVA | Qualitative measurement | No | 58% moderate or good response | 19 sessions/Mean cumulative dose 41 J/cm2 | Not reported |
| Carrascosa et al. | 48 | Waldmann 180/200 | Initial dose 0.5 J/cm2. Increments of 0.5 J/cm2 per session | 0.1 O/W emulsion of 8-MOP | PGA | Yes, 10-25 mg/day | 62.5% PGA 0/1 | 47 sessions | Mild in 25% |
Abbreviations: ASI, Area and Severity Index (modified PASI); MOP, methoxypsoralen; MPD, minimal phototoxic dose; O/W, oil-in-water; PASI, Psoriasis Area And Severity Index; PGA, Physician's Global Assessment; PUVA, psoralen UV-A therapy.
Overall, the response to systemic treatments has been assessed much less systematically in palmoplantar psoriasis than in psoriasis vulgaris. Although minimally systematic reviews of systemic therapies in this setting are exceptional, as a whole the results reported are modest and not better than those achieved with phototherapy.1
Acitretin monotherapy is shown to achieve a good response in between 40% and 83%, of patients, although the outcome is better in the case of pustular forms. One meta-analysis situates the likelihood of such a response at 39%, a figure that is probably closer to reality.10 The recommended dose, although variable, ranges from 10 to 35mg/d for both monotherapy and combination regimens with PUVA therapy.
Methotrexate and ciclosporin are 2 of the drugs most often used to treat psoriasis vulgaris with satisfactory results. By contrast, these treatments achieve only modest results in patients with palmoplantar psoriasis, with the few studies in the literature reporting percentage data citing a response rate between 35% and 45% for methotrexate and 66% for ciclosporin.
The use of biologic drugs, which has brought about a qualitative leap forward in the treatment of moderate and severe forms of plaque psoriasis, has had only very limited impact in the palmoplantar forms of the disease. In a series of 49 patients treated with adalimumab, Leonardi et al.15 reported a 51% improvement at week 28 as assessed by PGA with, in general, very good tolerance. In a similar study, Bissonette et al.16 treated 24 patients with infliximab, obtaining a significant improvement in 35%. In the latter study, the effectiveness of the treatment was assessed by classifying the lesions on the hands and feet with a modified palmoplantar PASI scale. In a recently published study on the use of ustekinumab and etanercept in a small series of patients, excellent results were reported in about 35% of patients at 16 weeks.17,18
In our series, the most common adjunctive therapy was acitretin (70%). The use of acitretin in conjunction with systemic PUVA therapy has been accepted as a way to speed up the response to treatment and improve the likelihood of achieving a response. A recent systematic review demonstrated that the combination of acitretin and PUVA therapy produced a synergistic effect, particularly evident in the palmoplantar forms of psoriasis.19
While the response to treatment was not better overall in the patients who received combination therapy, it should be noted that in this series acitretin was prescribed before the start of phototherapy to patients with particularly severe lesions or added to the regimen during PUVA therapy when the response to treatment was unsatisfactory. In view of this circumstance, it is reasonable to assume that adjunctive therapy with acitretin allowed us to obtain satisfactory results in a subset of patients in whom topical PUVA therapy alone would have failed. It should be noted that the doses of acitretin used in most of the patients treated, although in the therapeutic range, were relatively low. While the use of low doses could have been a factor in the suboptimal response achieved in some cases, it also prevented the development of the adverse effects associated with long-term treatment, which are in general not very serious but nonetheless uncomfortable. In our series, no patients discontinued therapy with acitretin due to mucocutaneous adverse events or laboratory abnormalities. When combination therapy is deemed appropriate, our usual strategy is to start the patient on a low dose and only consider an increase if the response to treatment is unsatisfactory or when excellent tolerance makes such an increase appear advisable.
The mean age of the group that received combination therapy in our series was higher than that of the series as a whole. Such therapy was also more frequent in patients who had a longer history of psoriasis and more comorbidities. Although we have no objective criteria to support the hypothesis, it would appear likely that the patients who received combination therapy had previously tried out all the different types of topical treatment and monotherapy with PUVA. The criteria used for the clinical assessment (a PGA score of 3 or higher) does not allow us to establish differences between the two groups.
Topical PUVA therapy was generally well tolerated. Although the incidence of adverse effects reported in both our study and others is about 25%, most of the adverse effects reported are mild and do not interfere with the treatment protocol or patient adherence to treatment.9,10 In our series, only 4% of patients discontinued treatment due to erythema or phototoxic effects associated with 8-methoxypsoralen. Factors such as the recommendations in place regarding the treatment protocol and nursing follow-up could encourage acceptance of treatment.
To make it possible to tailor the treatment strategy to each patient, it would be of great interest to discover whether any clinical or epidemiological variables exist that could predict a good response to treatment. With this in mind, we tried to identify predictors of response in this series of patients, comparing the clinical characteristics of each case with the course and outcome of treatment. The only relevant association identified was that the mean age of the patients with a better response was higher than that of the group as a whole. It is possible that this finding may be related to the fact that this group of patients also received adjunctive treatment with acitretin.
Our study undoubtedly has limitations, which we readily acknowledge, such as its retrospective nature, the defects related to the assessment of response (PGA), and the circumstances encountered in everyday clinical practice that may have influenced the treatment regimen, such as the adjustment of the number of sessions received by a particular patient for personal or work reasons. However, it should be noted that the clinical assessment was always performed by the same dermatologists, who were responsible for the phototherapy unit, and that the protocol applied and the equipment used were the same in all cases.
In conclusion, in our experience, topical PUVA is an appropriate treatment option for palmoplantar psoriasis, offering similar response rates to systemic treatments, but with a better tolerance and safety profile. Combination therapy, particularly with acitretin, improved the likelihood of response—even in cases in which single-drug therapy with acitretin had failed. Adjunctive therapy should be considered when the response to PUVA is considered unsatisfactory after several sessions.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Ethical DisclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of dataThe authors declare that they have followed the protocols of their workplace on the publication of patient data and that all patients included in the study received sufficient information and gave their written informed consent to participate in the study.
Right to privacy and informed consentThe authors declare that no private patient data are disclosed in this article
Please cite this article as: Carrascosa JM, et al. Eficacia y seguridad en terapia con psoralen-UVA (PUVA) tópica en psoriasis palmoplantar. Experiencia en una serie de 48 pacientes. Actas Dermosifiliogr. 2013;104:418-25.