Part 2 of this series on granulomatous diseases focuses on skin biopsy findings. Whereas the first part treated noninfectious conditions (metabolic disorders and tumors, among other conditions), this part mainly deals with various types of infectious disease along with other conditions seen fairly often by clinical dermatologists.
Ésta es la segunda parte de una serie dedicada a la patología granulomatosa en la biopsia cutánea. Mientras que en la primera parte hablamos, entre otras, de algunas condiciones metabólicas y tumorales, esta segunda parte abordará fundamentalmente patología infecciosa de diversos tipos, junto con otras condiciones relativamente frecuentes en las consultas de dermatología.
Granulomas are among the most common findings in dermatopathology. They are also the most variable, not just because of their morphologic characteristics, but also because of their etiology and clinical and prognostic significance.
The aim of this 2-part article is to provide an overview of the main diseases that produce granulomas observed in skin biopsy specimens. In the first part, we looked at different types of granulomas and giant cells (e.g., foreign body granulomas) and some of the most representative diseases that cause granulomas of the skin, including metabolic, neoplastic, and autoimmune diseases.
In the second part of this review, we look at other types of granuloma-producing diseases, including infections.
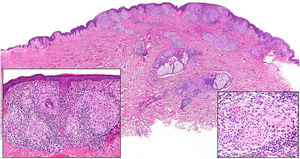
Granuloma AnnulareDefinition. Granuloma annulare (GA) is a relatively common immune-related granulomatous skin disease of multifactorial etiology. It can be localized or generalized, affects patients of all ages, and is associated with systemic diseases. It is a chronic inflammatory condition that is generally slow to resolve.
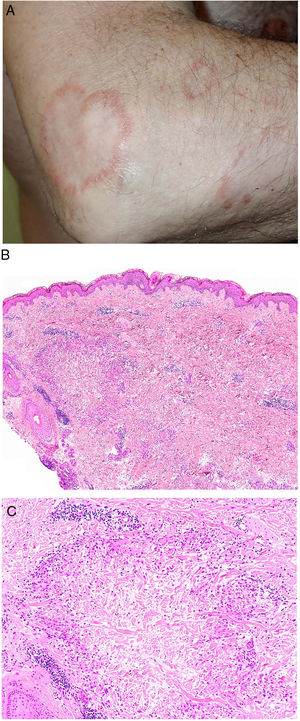
Clinical presentation. GA usually presents as localized or generalized papules, erythematous plaques, or annular nodules with a predilection for the extremities (Fig. 1A).1 Lesions sometimes present as subcutaneous or linear nodules with a perforating ulcerative appearance. Localized forms, in particular papules and subcutaneous lesions, are more common in children and young people, while generalized forms are more common in adults and older patients. Generalized GA can occur in association with various diseases, notably diabetes mellitus, hyperlipidemia, autoimmune diseases, immunodeficiency, hematologic diseases, and, on occasions, cancer. Different morphologic forms of GA in the same patient are not uncommon.
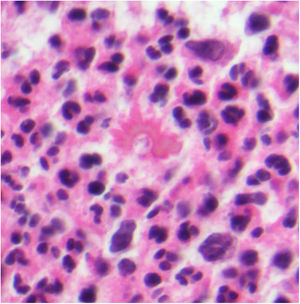
A, Granuloma annulare. An arm showing several erythematous annular plaques with a slightly raised border and clear center. B, Skin showing 2 areas of collagen degeneration and interstitial changes in the reticular dermis surrounded by a band-like inflammatory infiltrate. The superficial dermis and epidermis are preserved. Note also the perivascular lymphocytic crowns and diffuse increase in fibroblast density (hematoxylin-eosin, original magnification ×40). C, Note the central area of disordered collagen accompanied by acid mucin deposits and surrounded by an infiltrate of macrophages, palisading epithelioid cells, fibroblasts, lymphocytes, and occasional eosinophils. Dense perivascular lymphocytic crowns without vasculitis are visible (hematoxylin-eosin, original magnification ×200).
Histopathology. Histology shows one or more areas of collagen degeneration with irregular contours, often in the dermis, surrounded by a granulomatous inflammatory reaction (Fig. 1B).2 The inflammatory infiltrate is composed of palisading macrophages, epithelioid cells, fibroblasts, lymphocytes, and occasional eosinophils. Plasma cells are not present. A basophilic interstitial matrix formed by deposits of acid mucin and/or hypereosinophilic collagen is observed in the center. Colloidal iron is useful for detecting interstitial mucin, as this may be present in very small amounts. Dense perivascular lymphocytic crowns are also observed (Fig. 1C). Elastolysis, and consequently elastolytic granulomas, are not seen in GA. The pronounced degeneration of collagen in perforating GA produces necrotic tissue that is eliminated by hair follicles. Subcutaneous GA may masquerade as a rheumatoid nodule, but can be distinguished by the presence of mucin. There have been rare reports of sarcoid-like GA. GA is the result of an autoimmune inflammatory reaction mediated by CD4+ type 1/17 T helper cells, which activate macrophages (M2) and fibroblasts, triggering the production of acid mucin, metalloproteases, and proinflammatory cytokines, which alter the interstitium and produce collagen degeneration. GA is sometimes associated with impaired innate immunity.3
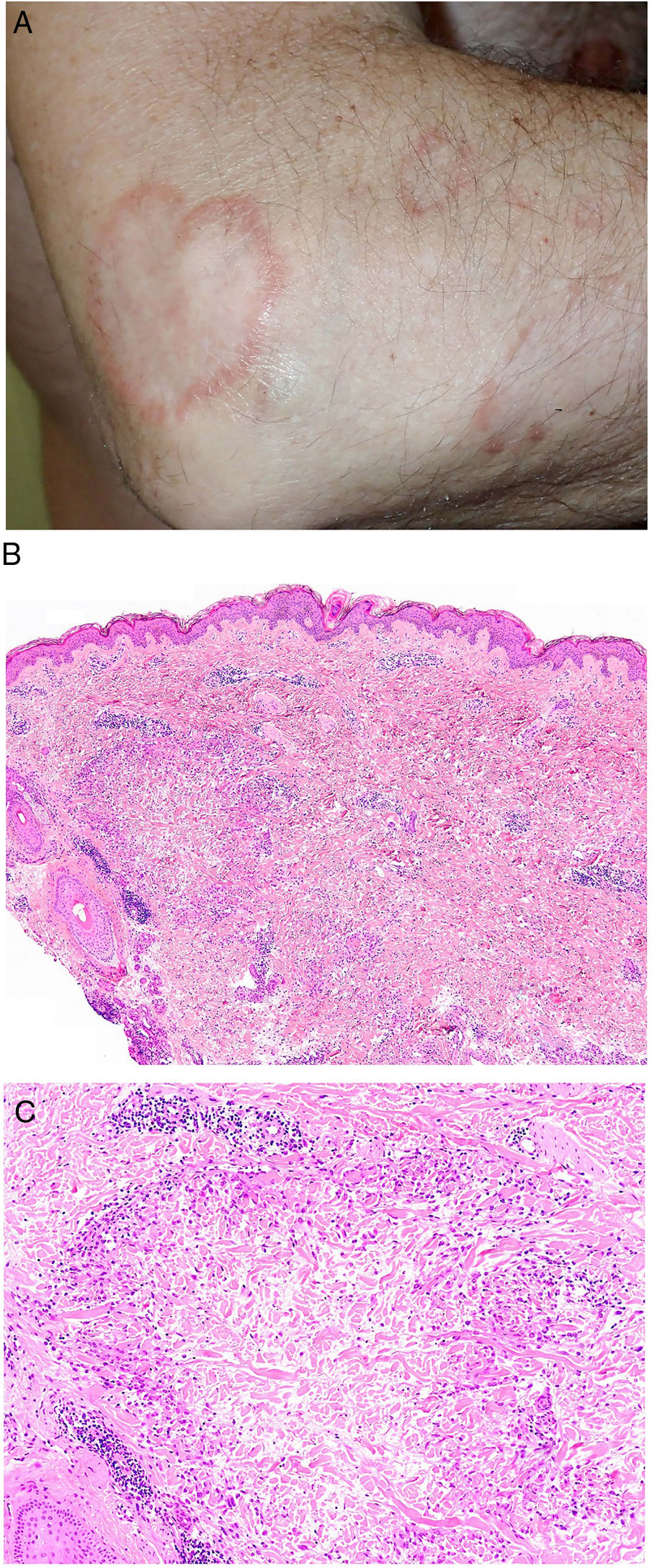
Actinic Granuloma and Annular Elastolytic Giant Cell Granuloma AnnulareDefinition. The terms actinic granuloma4 and annular elastolytic giant cell granuloma annulare (AEGCG)5–7 refer to similar, if not identical, elastolytic granulomatous lesions. While actinic granuloma often refers to lesions located in sun-exposed areas with significant actinic damage, AEGCG is used to describe to similar lesions in non-sun-exposed areas or as an umbrella term to describe both actinic lesions and lesions in non-sun-exposed areas. Because annular lesions are not always present, the term elastolytic giant cell granuloma has been proposed.5,8 Some authors have suggested that AEGCG may be a photoinduced variant of GA, but agreement is lacking.7
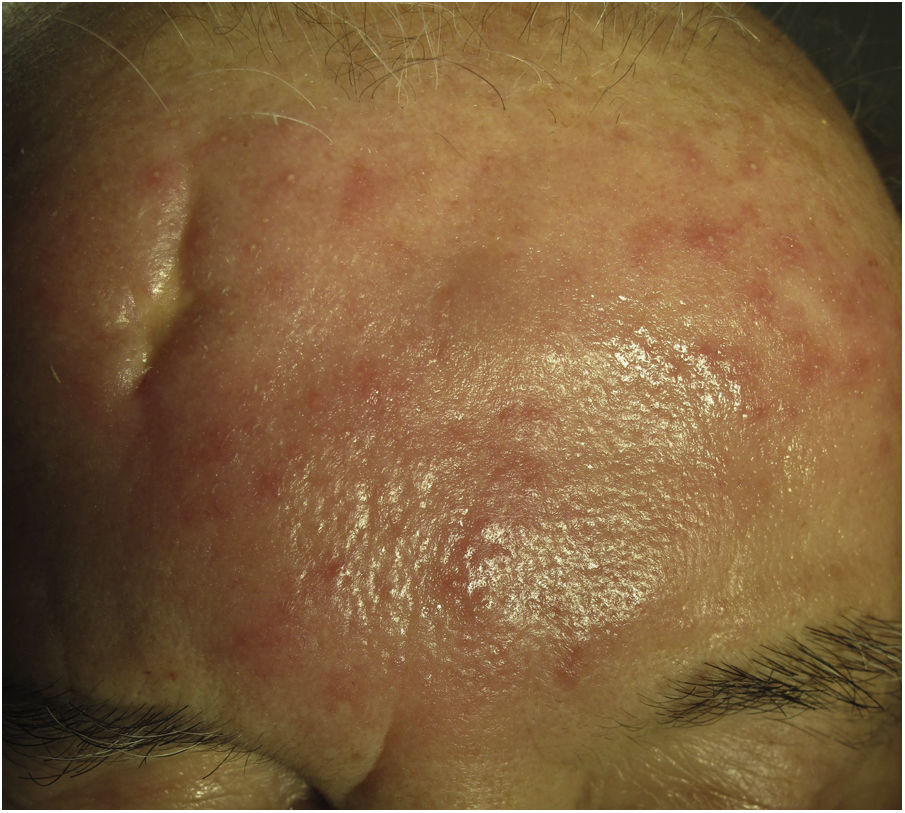
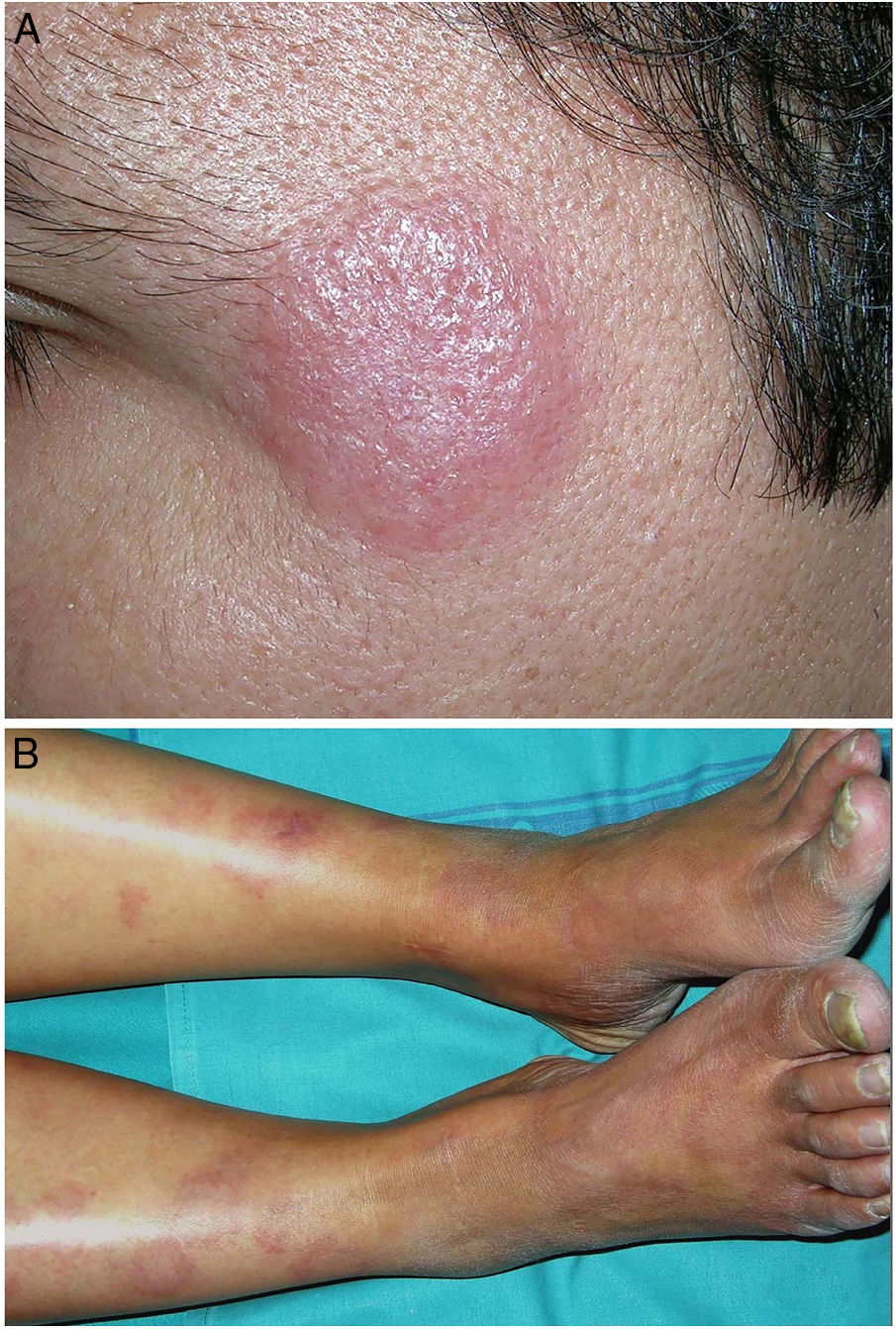
Clinical presentation. Actinic granuloma and AEGCG occur in middle-aged adults, with no clear predilection for men or women.5 Actinic granuloma presents as papules that generally converge to form annular lesions (Fig. 2A). AEGCG, in turn, is characterized by large annular plaques with raised borders and an atrophic center located (according to the strict definition of these entities) in non-sun-exposed areas. There have been reports of reticular and generalized variants and papules that do not evolve to form annular lesions.8 The clinical course is variable, with some lesions resolving spontaneously and others showing resistance to treatment (topical corticosteroids; pimecrolimus; cryotherapy; psoralen with UV-A; oral chloroquine or hydroxychloroquine; systemic corticosteroids, cyclosporine, or clofazimine; fumaric acid; dapsone; tranilast; and isotretinoin).8
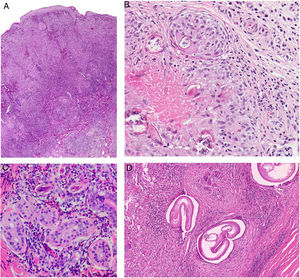
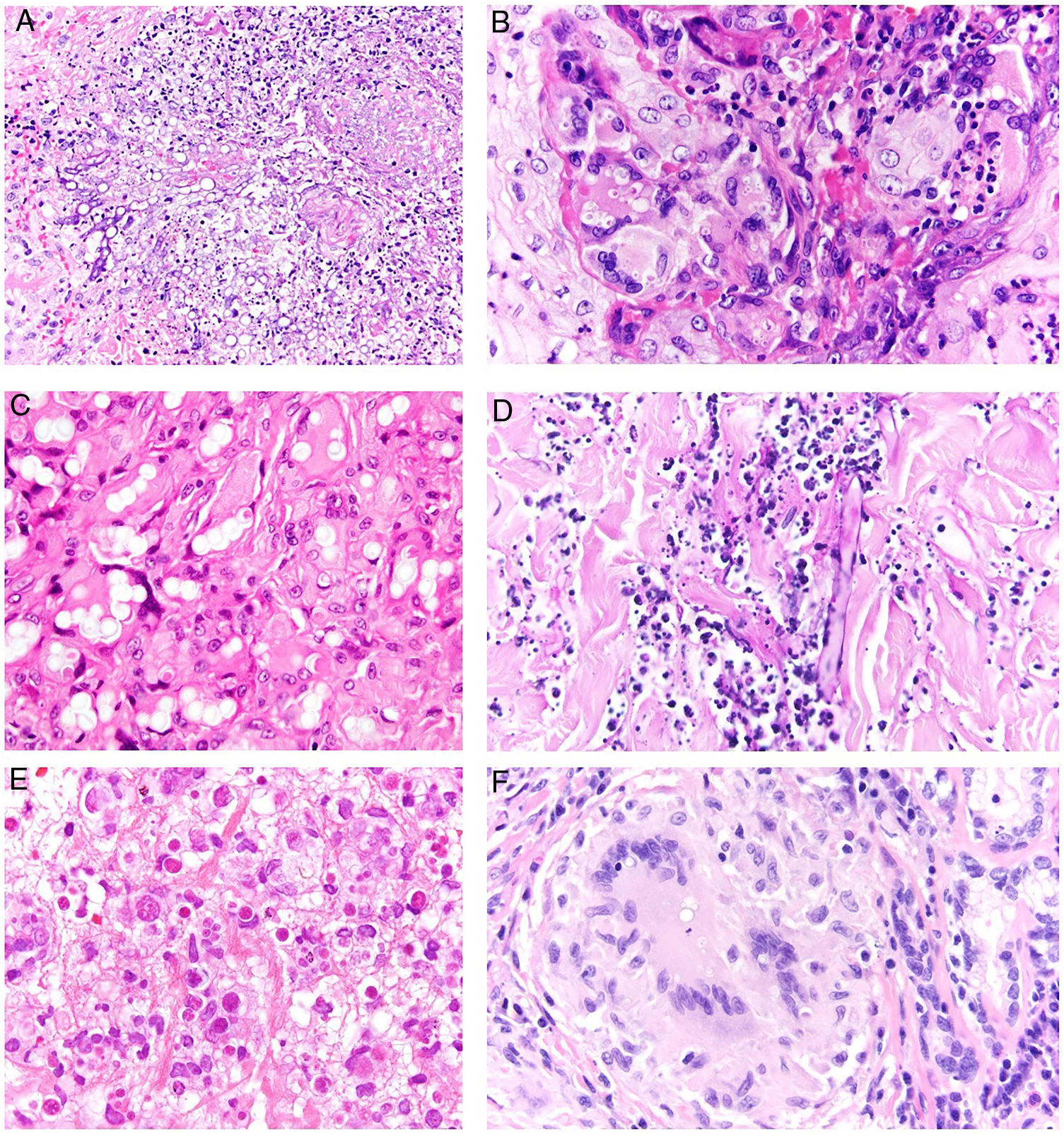
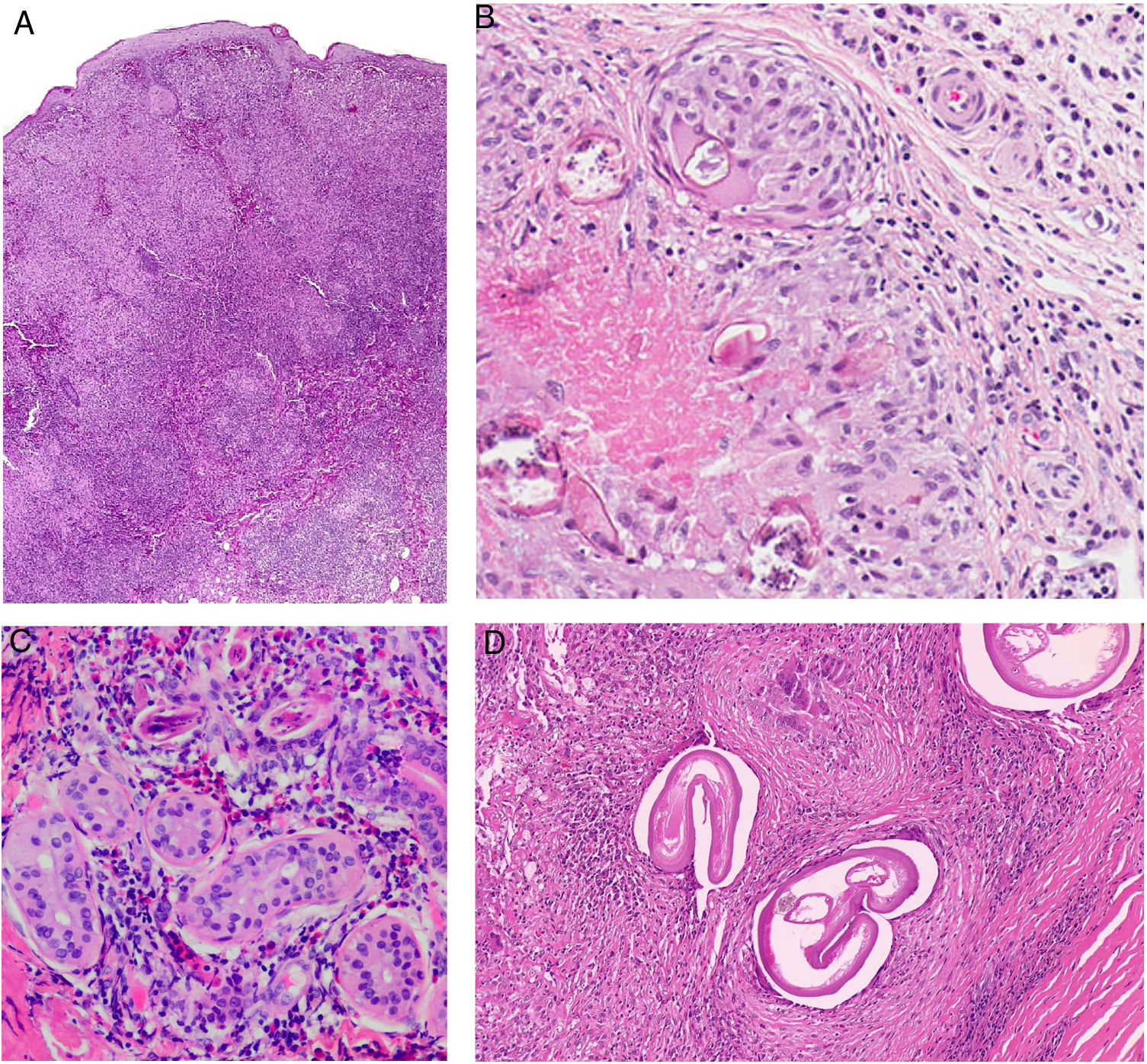
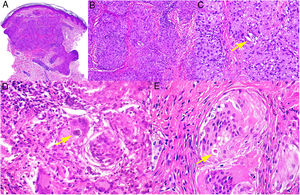
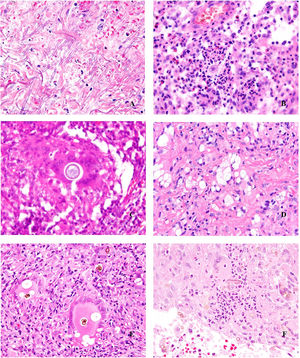
Granuloma annulare. A, Large erythematous annular plaques with a raised border in a sun-exposed area. B, Panoramic histologic view showing an inflammatory infiltrate in the reticular dermis. C-E, Higher-magnification view showing a granulomatous lesion with numerous multinucleated giant cells and elastophagocytosis without palisading, increased mucin, or necrobiosis (hematoxylin-eosin, original magnification ×40 [B], ×400 [C,D], ×600 [E]).
Histopathology. Both actinic granuloma and AEGCG are histologically characterized by dermal granulomatous lesions with multinucleated giant cells (mostly foreign body giant cells with large numbers of nuclei) and signs of multifocal elastophagocytosis (Fig. 2B-E). Mucin deposits, palisading, and necrobiosis are generally not observed.8 There may be a predominance of nonmultinucleated histiocytes or even sarcoid-type granulomas. There have also been reports of a trizonal histologic pattern,4,5 with a giant cell component typically located at the raised erythematous border, a surrounding area of elastosis without an inflammatory component, and a central atrophic area with a marked reduction or absence of elastic fibers. There may be a variable lymphocytic component.7
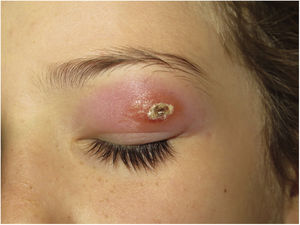
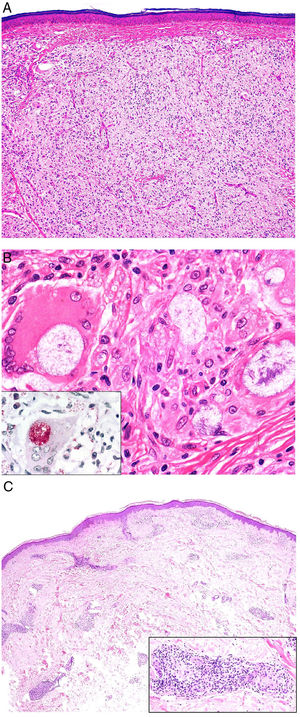
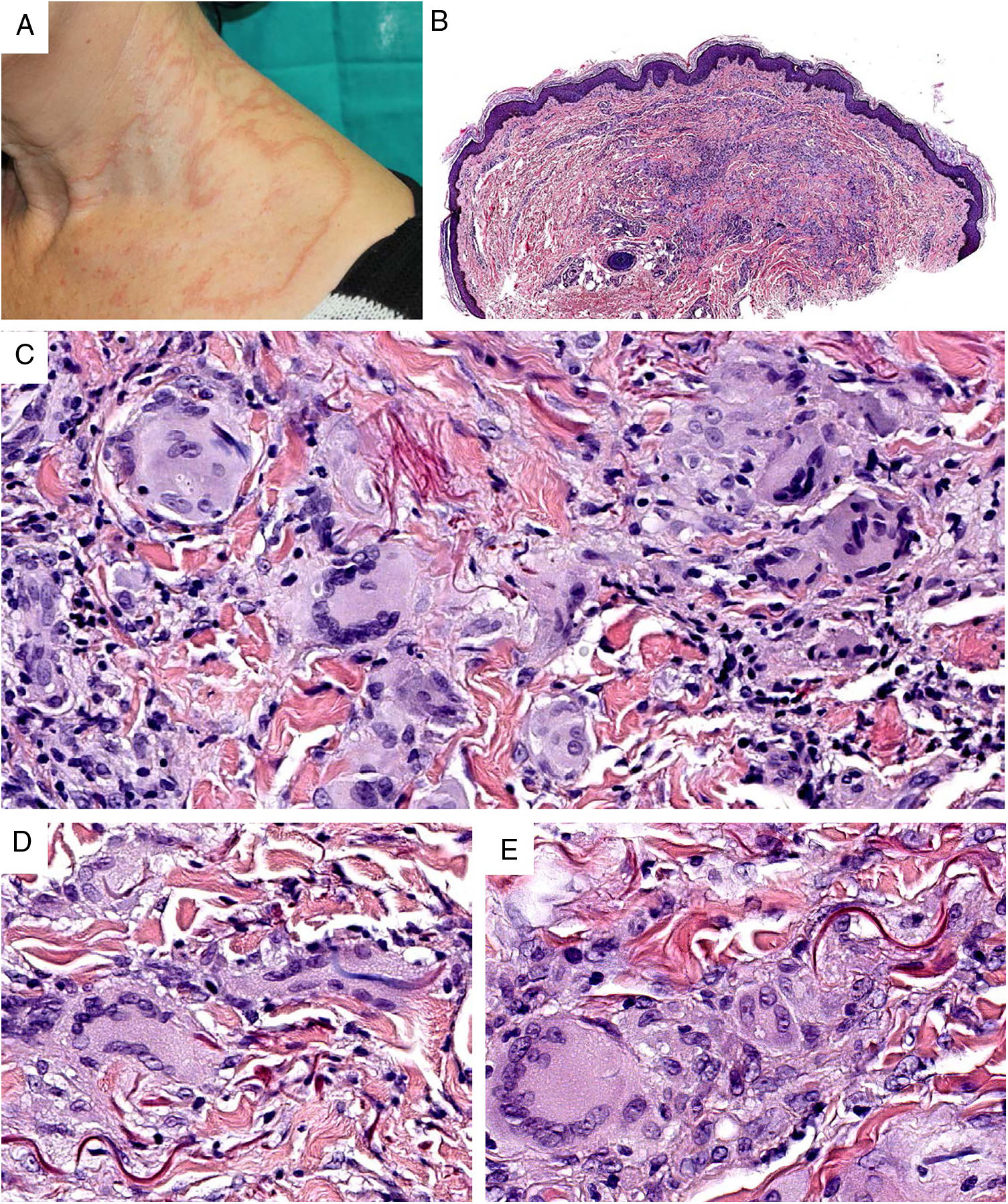
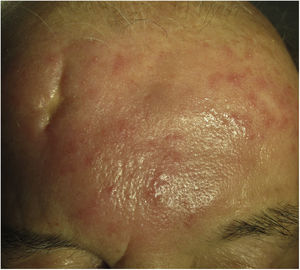
Morbihan DiseaseDefinition. Morbihan disease is a rare disease of unknown etiopathogenesis first described in 1957 by the French dermatologist Robert Degos in a farmer in Morbihan, Brittany.9 Morphologic findings include perilymphatic and intralymphatic granulomas.
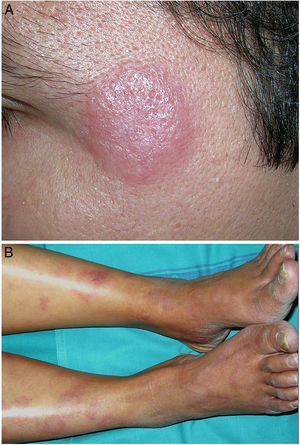
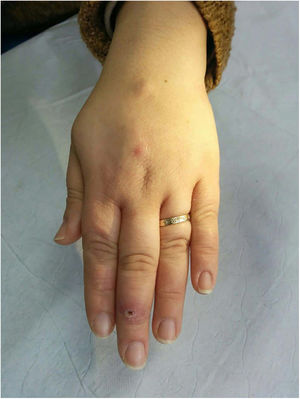
Clinical presentation. Morbihan disease is more common in men aged between 40 and 60 years. It is associated with rosacea in 26% of cases10 and there have been isolated reports of an association with lupus miliaris disseminatus faciei.11 It follows a chronic, recurrent course characterized by symmetric erythema and solid edema involving the upper two-thirds of the face (forehead, glabella, eyelids, nose, and chin) (Fig. 3). Although Morbihan disease is generally asymptomatic, its aesthetics can cause psychosocial disorders, and severe periorbital edema can cause visual field loss. Diagnosis is by exclusion. The differential diagnosis should include lupus erythematosus, dermatomyositis, and chronic actinic dermatitis.
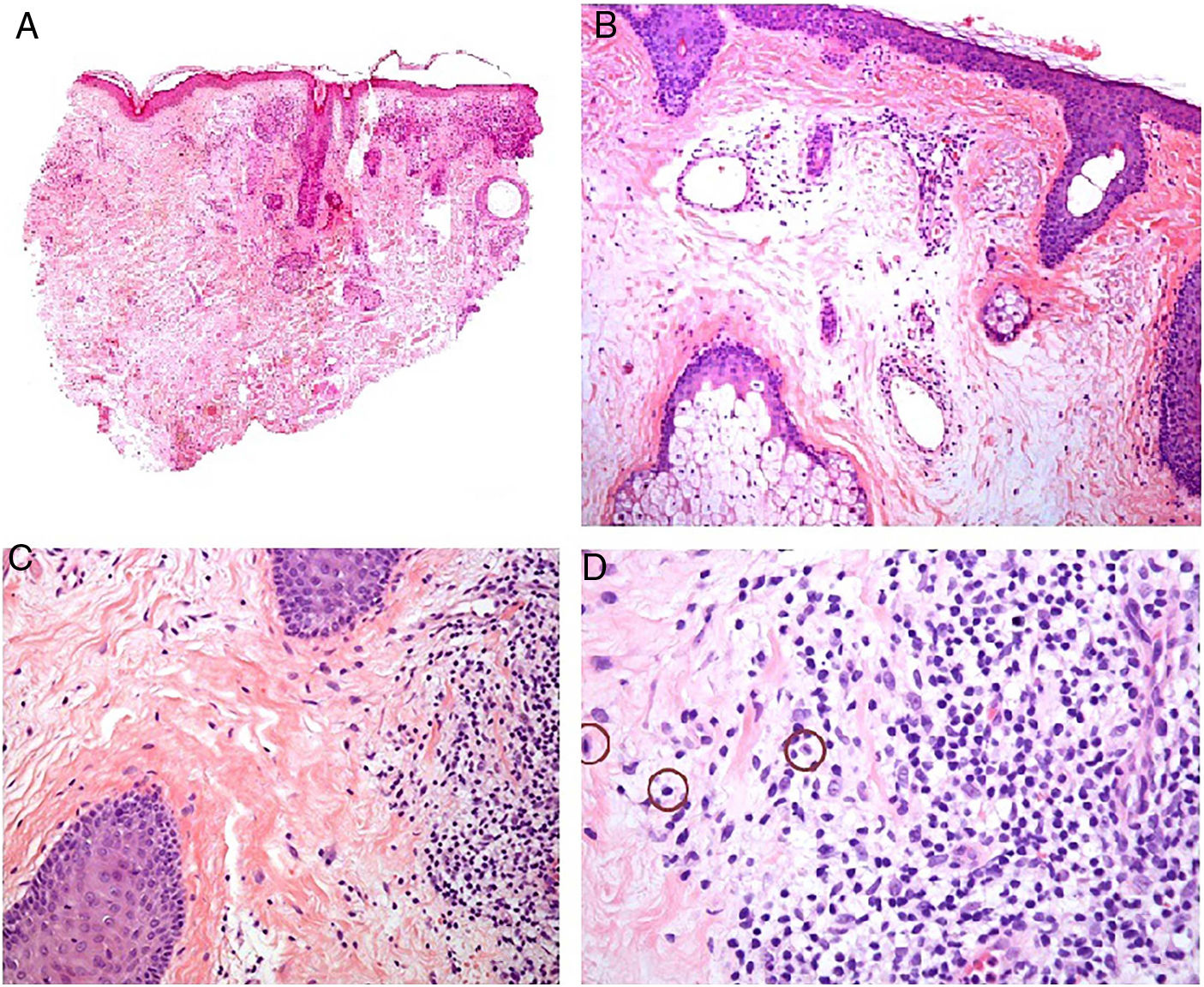
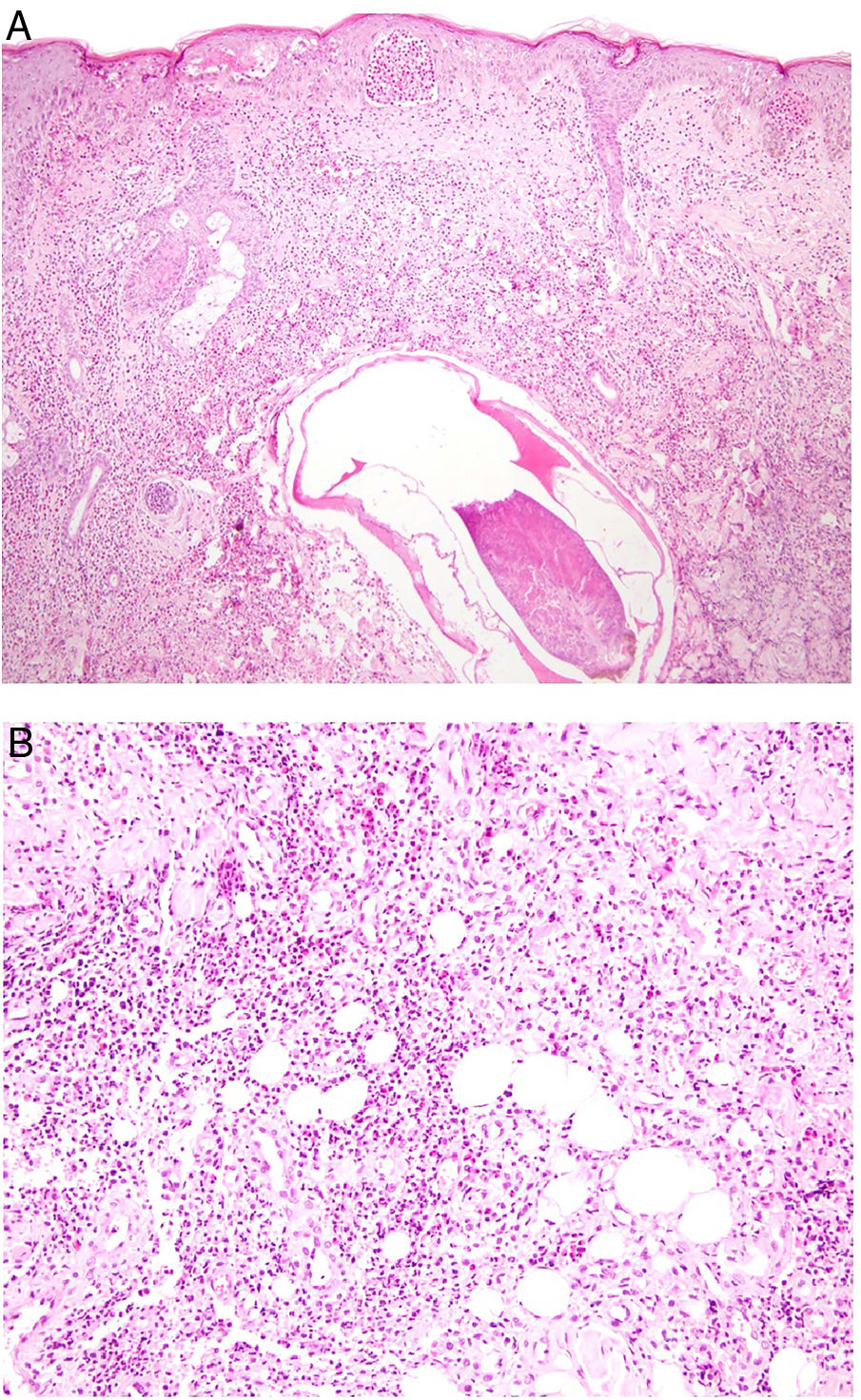
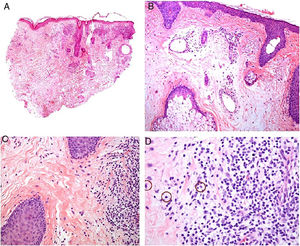
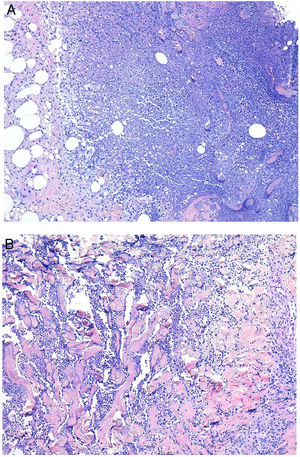
Histopathology. The morphologic findings on histology are quite nonspecific (Fig. 4A), and include dermal interstitial edema (Fig. 4B), vascular ectasia, perifollicular fibrosis, a perivascular and perifollicular lymphohistiocytic inflammatory infiltrate (Fig. 4C) with neutrophils (Fig. 4D) and in some cases mast cells, sebaceous gland hyperplasia, and epithelioid granulomas, which are mainly perilymphatic and intralymphatic12–14 but can be perifollicular. The histologic differential diagnosis should include other more common granulomatous diseases, notably, granulomatous rosacea, lupus miliaris disseminatus faciei, granulomatous periorificial dermatitis, anogenital granulomatosis, sarcoidosis, and Melkersson-Rosenthal syndrome. Most of these entities can be ruled out by integrating clinical and other histologic findings. Finally, although the etiology and pathogenesis of Morbihan disease remain to be elucidated, proposed mechanisms include an imbalance between lymph production and drainage, a loss of capillary and lymph vessel wall integrity associated with chronic inflammation, and alterations to dermal lymphatic vessels as a result of perilymphatic epithelioid granulomas.
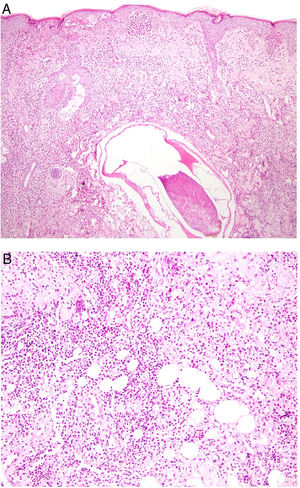
Morbihan disease. A, Panoramic image showing dermal edema with a perifollicular and interstitial inflammatory infiltrate (H&E, original magnification ×20). B, Detail of pronounced dermal edema together with dilated vessels (H&E, original magnification ×100). C, Perivascular lymphohistiocytic infiltrates (H&E, original magnification ×200). D, Detail of several polymorphonuclear neutrophils in the infiltrate (H&E, original magnification ×400). H&E indicates hematoxylin-eosin.
Definition. Sarcoidosis is a multisystem disease of unknown etiology histologically characterized by noncaseating sarcoid granulomas in several organs, such as the lungs, lymph nodes, skin, and spleen.
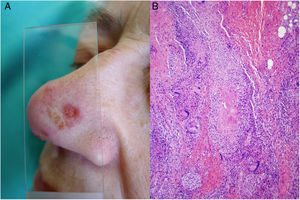
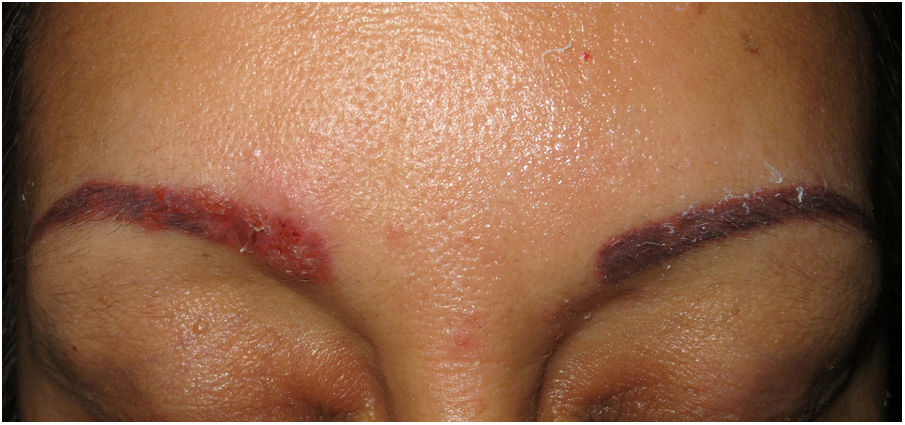
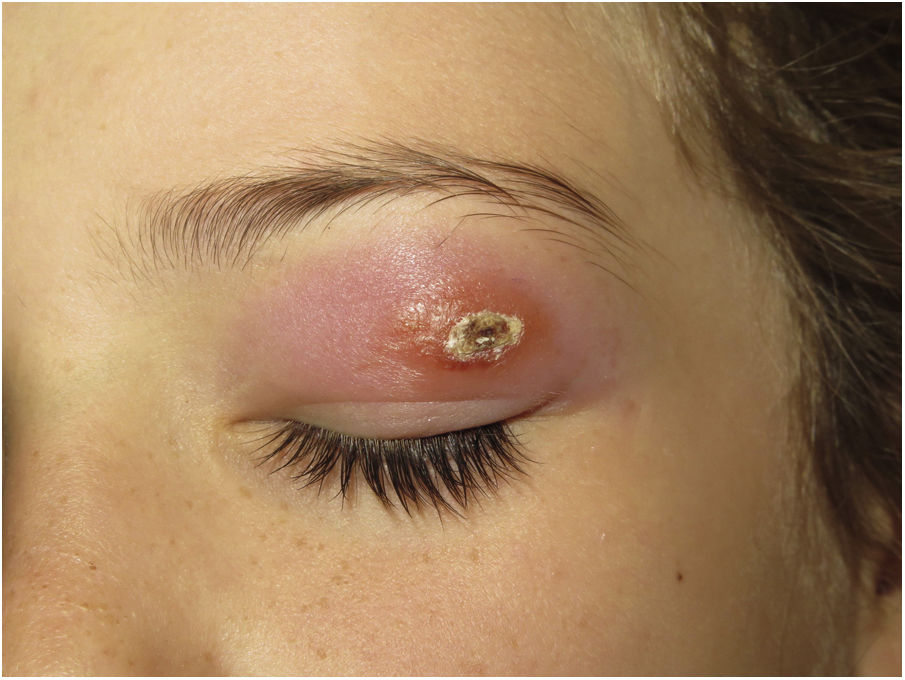
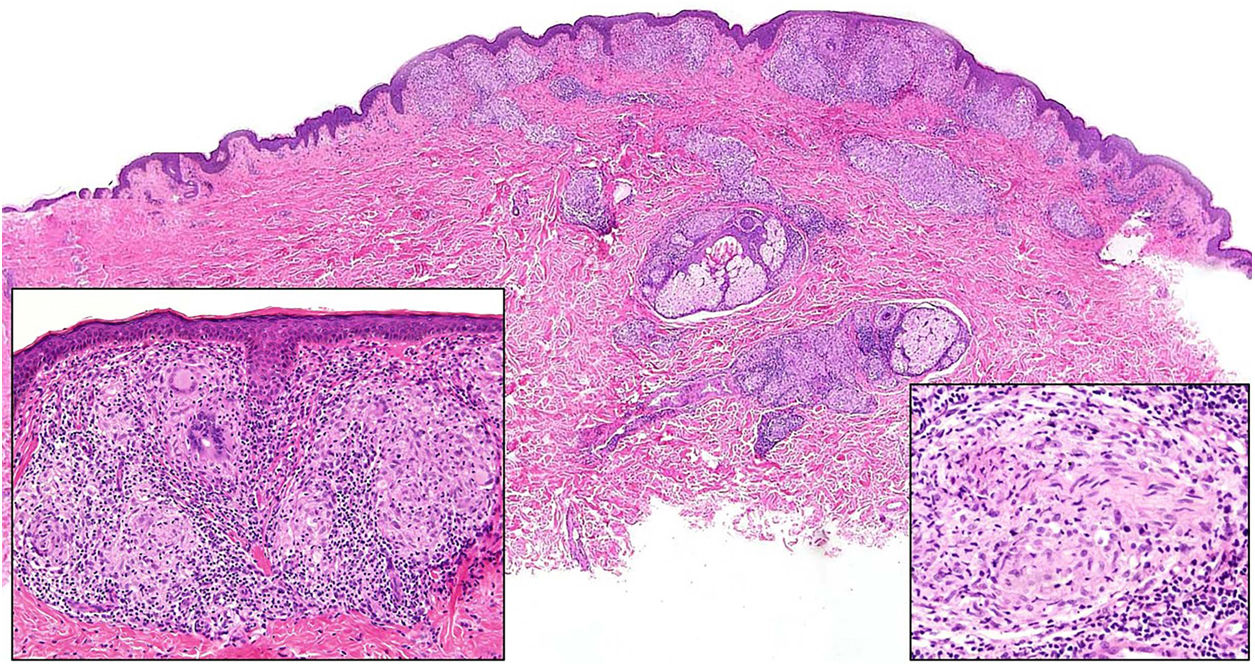
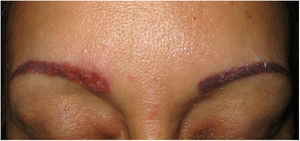
Clinical presentation. Skin lesions in sarcoidosis are classified as specific or nonspecific. Specific lesions include granulomas, which are clinically highly variable and frequently consist of macules, papules, and plaques of varying shapes and sizes.15 The lesions sometimes arise in scars or at sites of trauma (e.g., tattoos, surgery, laser therapy, venipuncture)15 (Fig. 5). Red-violaceous plaques in the center of the face, a manifestation known as lupus pernio, are characteristic of sarcoidosis. Nonspecific lesions do not have granulomas and include erythema nodosum, calcifications, prurigo, and erythema multiforme.
Histopathology. The most characteristic histologic finding in sarcoidosis is the presence of "naked" sarcoid granulomas, which are noncaseating epithelioid granulomas surrounded by a small number of peripheral lymphocytes (Fig. 6A,B). They are usually located in the reticular dermis, and occasionally extend into the subcutaneous tissue. The epidermis may have a normal appearance, but sometimes shows atrophy, hyperplasia, or ulceration.15–18 Other less characteristic findings are perifollicular and perineural granulomas, tuberculoid granulomas, focal fibrinoid necrosis, dermal mucin, lichenoid damage, and vasculitis.16,17 Intracytoplasmic inclusions (Schaumann bodies and asteroid bodies) may be occasionally observed, but while characteristic, they are not pathognomonic.17,18 Schaumann bodies are concentric, basophilic lamellar structures formed by calcium and proteins (Fig. 6D), while asteroid bodies are spiculated radial eosinophilic structures that appear to be formed by cytoskeletal filaments (Fig. 6E). Observation of foreign bodies in the granulomas (e.g., silica particles) is not uncommon16,18 (Fig. 6E) and has given rise to the concept of silica granulomas, which are thought to occur in patients with sarcoidosis who are more predisposed to the formation of granulomas in response to the accidental implantation of silica particles in the dermis.19–21
Sarcoidosis. A, Epithelioid granulomas occupying the superficial and mid dermis with few peripheral lymphocytes (H&E, original magnification ×20). B, Epithelioid granulomas composed of cells with abundant eosinophilic cytoplasm (H&E, original magnification ×100). C, Silica particle (arrow) in a sarcoid granuloma (H&E, original magnification ×200). D, Schaumann body (arrow) (H&E, original magnification ×400). E, Asteroid body (H&E, original magnification ×2400). H&E indicates hematoxylin-eosin.
Definition. Interstitial and palisaded neutrophilic granulomatous dermatitis is an entity, or rather a reactive histologic pattern, that is usually observed in systemic diseases, mostly of an autoimmune nature.
Clinical presentation. It is a self-limiting condition that starts with symmetric, erythematous annular lesions, papules and nodules, or plaques,3,22,23mainly located on the extensor surface of the extremities and trunk.3,24 It occurs in association with other conditions such as arthralgia and pruritus,3 and is observed in autoimmune diseases,3,22,25 such as lupus erythematosus, inflammatory bowel disease, rheumatoid arthritis,26 granulomatosis with polyangiitis,27 and certain hematologic malignancies, such as leukemia, multiple myeloma, and Hodgkin lymphoma.25,28 Its association with drugs is unclear.3,22
Histopathology. Histology shows a diffuse, superficial and/or deep dermal inflammatory infiltrate under a normal epidermis,23 with central palisading neutrophils and histiocytes around an area of degenerated collagen with scant mucin (Fig. 7A,B). Other findings include karyorrhexis with variable leukocytoclastic vasculitis22 and occasional eosinophils.23,26
A, Interstitial granulomatous dermatitis. Palisading histiocytes around an area of necrosis with neutrophils (hematoxylin-eosin, original magnification ×100). B, Interstitial granulomatous dermatitis. Detail of neutrophils dissecting an area of degenerated collagen (hematoxylin-eosin, original magnification ×100).
Definition. The deep mycoses are deep skin infections caused by fungi. Skin infection can occur by direct inoculation (e.g., trauma, wound infection) or spread from other organs, such as the lungs or central nervous system.
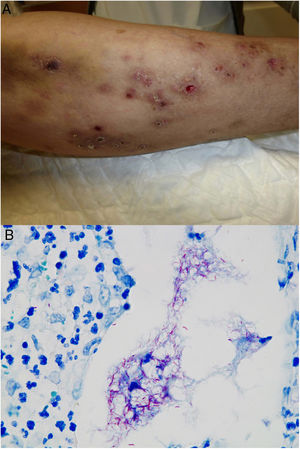
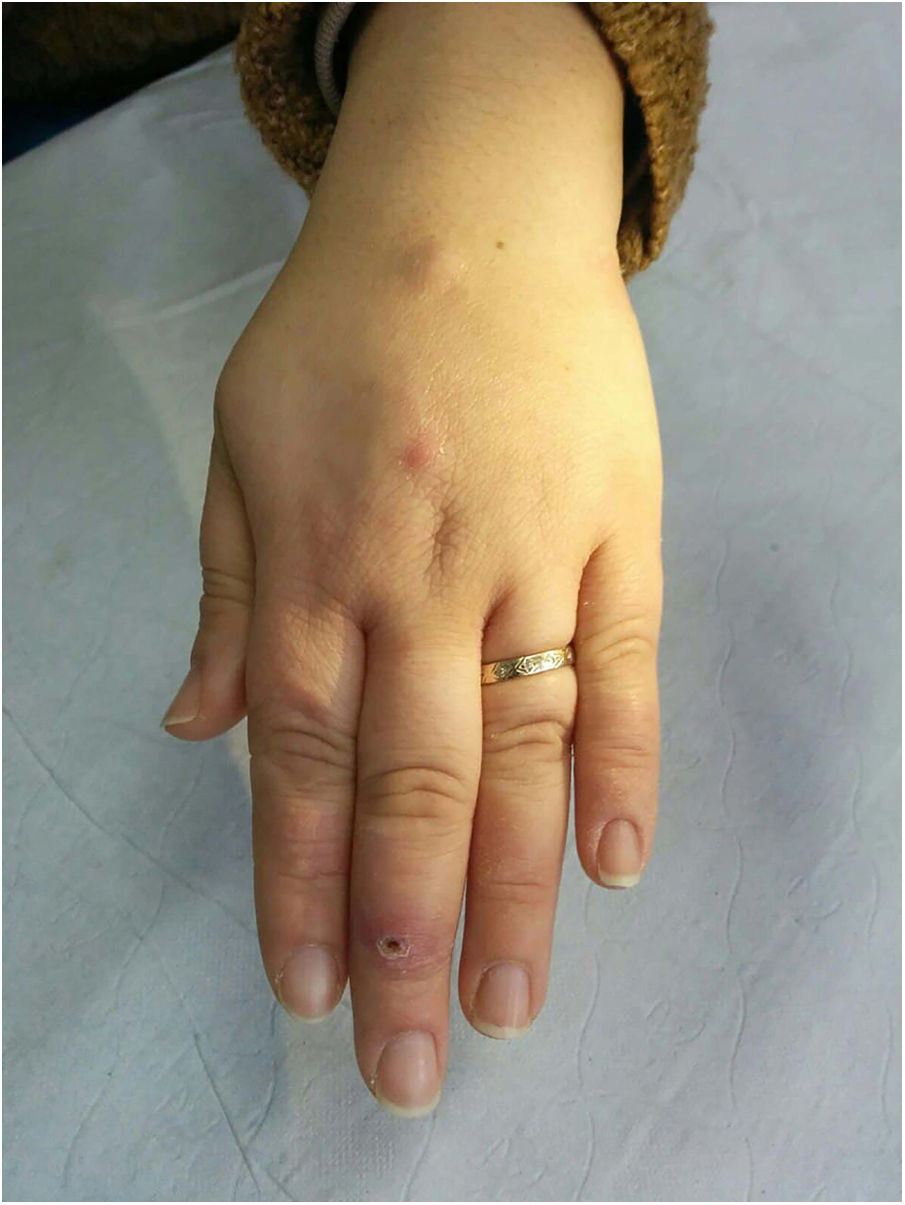
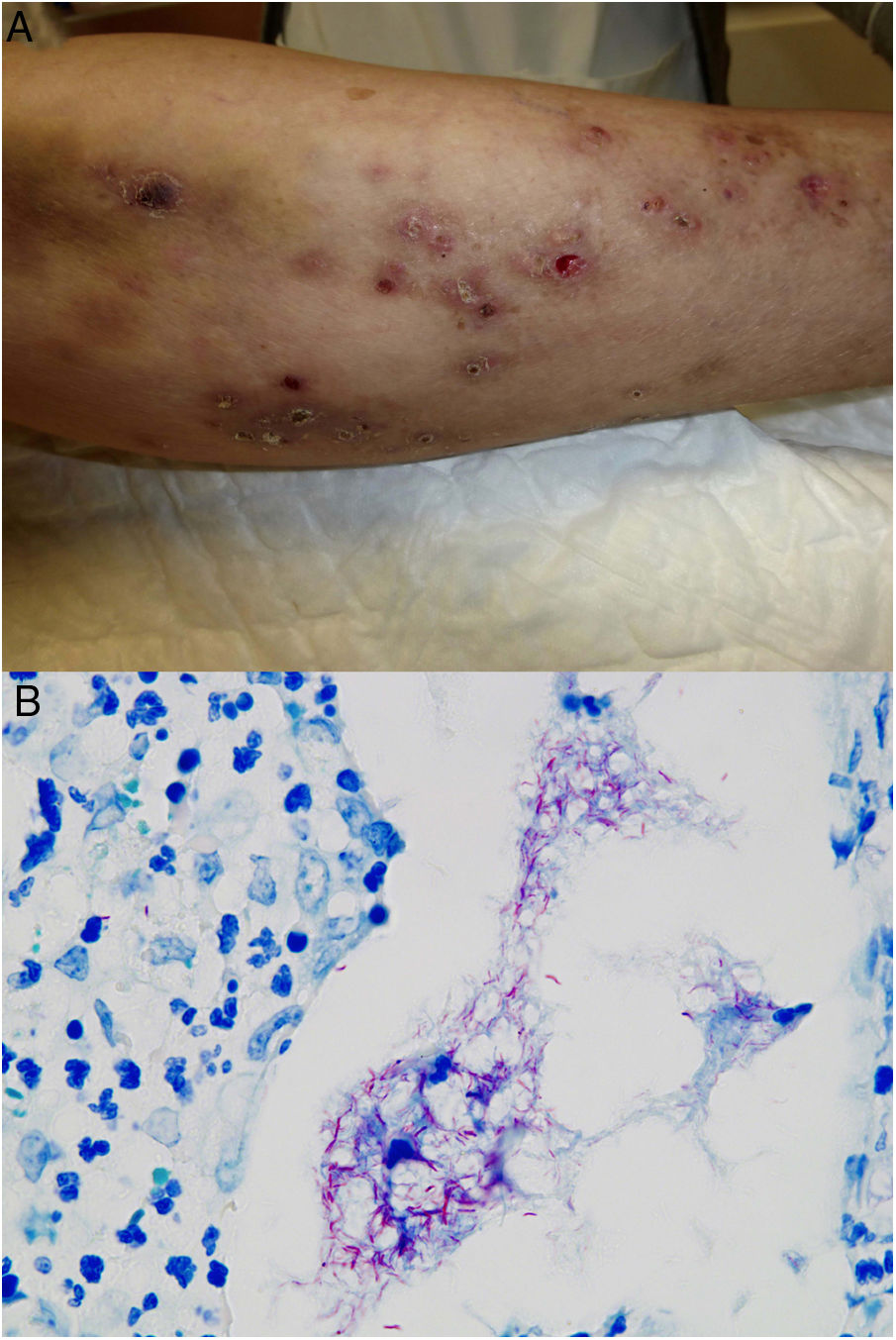
Clinical presentation. Skin lesions caused by deep fungal infections can be highly variable depending on the species involved, and include macules, papules, plaques, pustules, nodules, scars, areas of panniculitis, intertrigo, and tumors, with or without ulceration. Some lesions may even acquire a verrucous appearance that can be quite pronounced. Fungi can sometimes be eliminated through lesions in the form of particles visible to the naked eye (e.g., “sulphur" granules in the case of eumycetoma).29 Certain infections in addition to sporotrichosis can produce a clear sporotrichoid pattern, characterized by linearly distributed lesions along the course of the lymphatic vessels (Fig. 8). Other infections can result in somewhat more characteristic lesions, such as keloid lesions in lobomycosis or molluscum-like lesions in coccidioidomycosis. The clinical presentations are so varied that infections such as histoplasmosis have been called fungal syphilis.
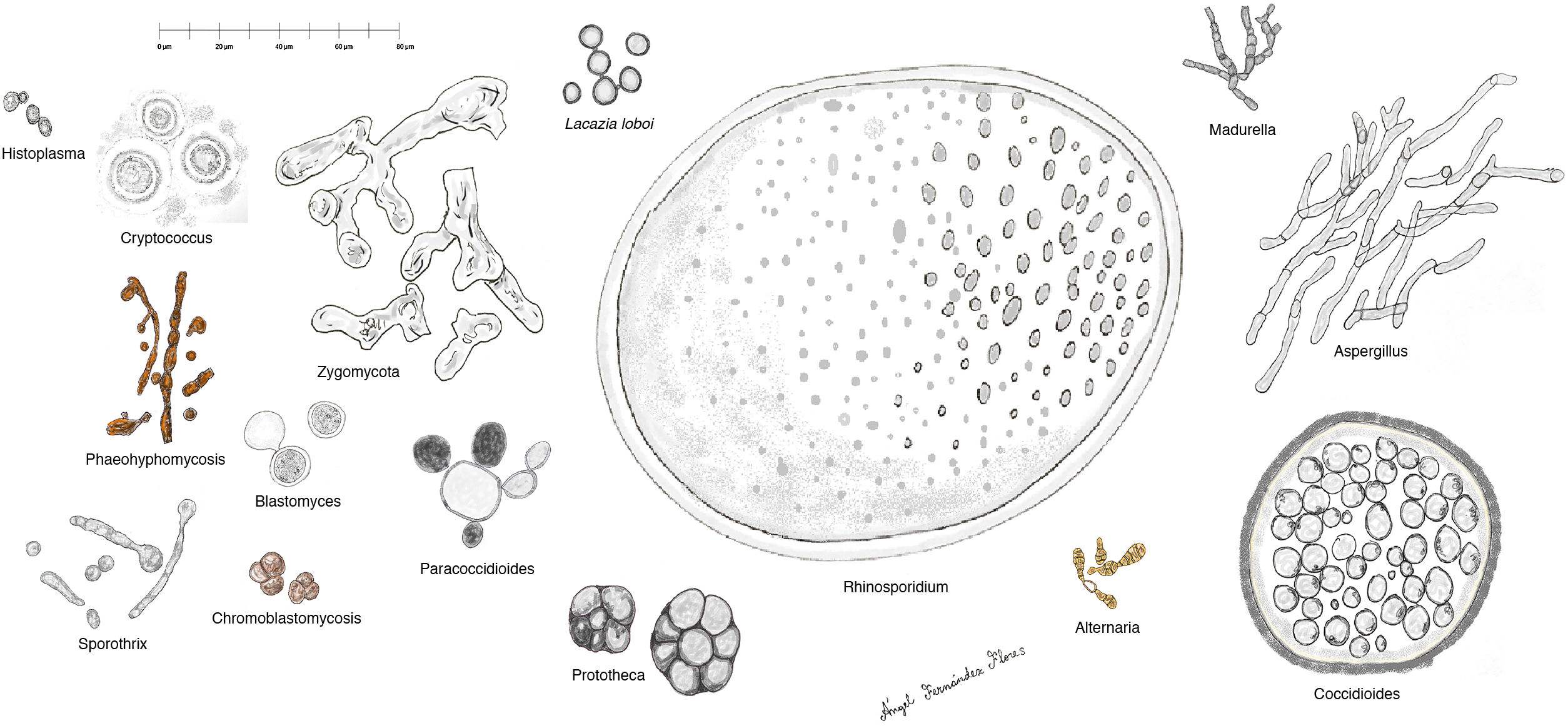
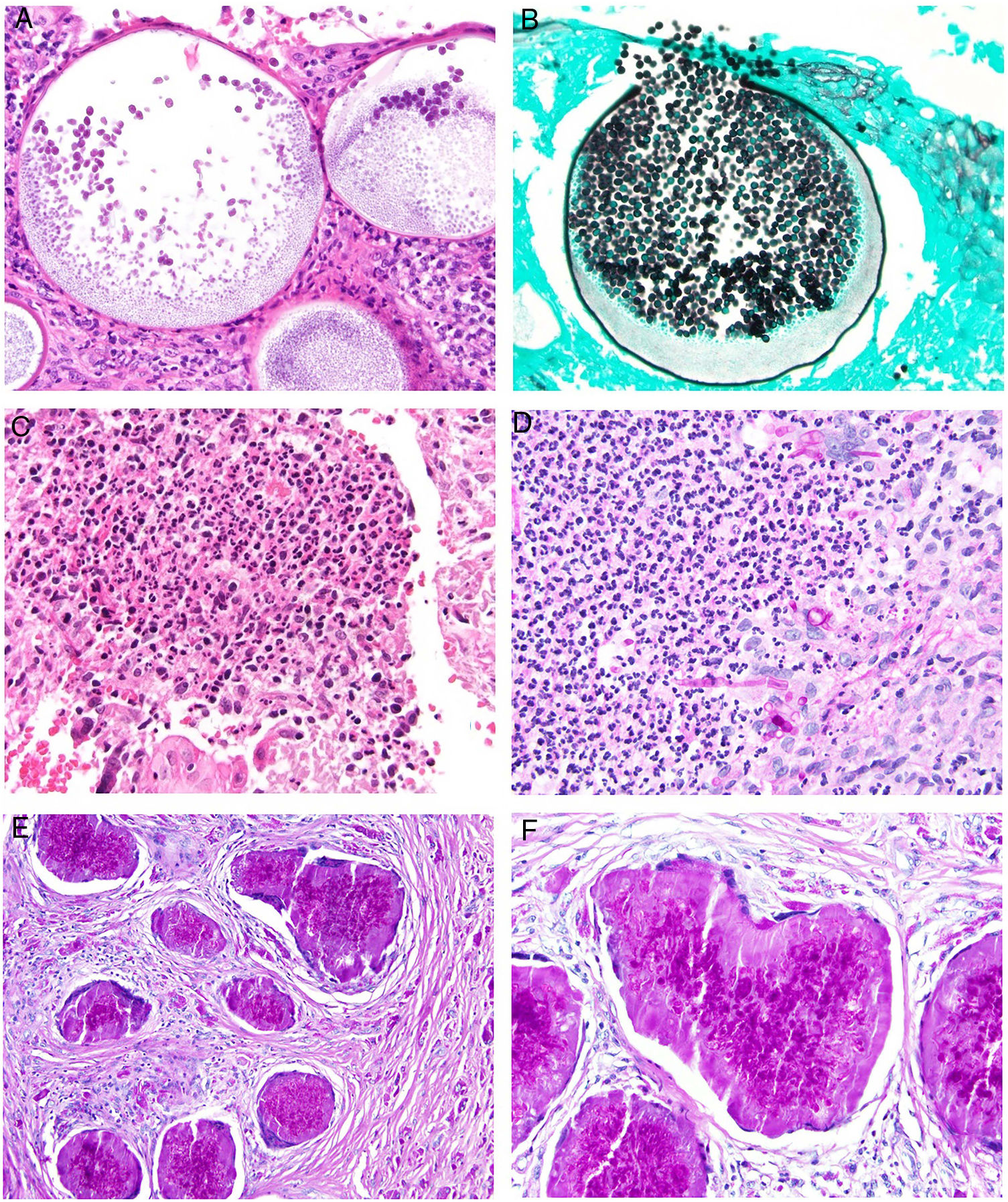
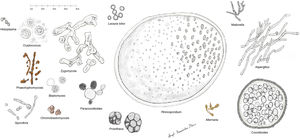
Histopathology. The fungi responsible for the deep mycoses are distinguished by their morphology and size (Fig. 9).30 The main agents that affect humans are shown in Fig. 9, which includes Prototheca, an alga, for comparative purposes. Infections caused by pigmented fungi are easily identifiable (chromoblastomycosis, phaeohyphomycosis, and alternariosis). Most fungi responsible for the deep mycoses are easily identifiable by hematoxylin-eosin staining as they have distinctive appearances, sizes, and shapes (Figs. 10–12), but special techniques are sometimes needed for better visualization. The most widely used stains are periodic acid-Schiff (PAS) and Grocott. Some fungi (e.g., Lacazia) are birefringent under polarized light.
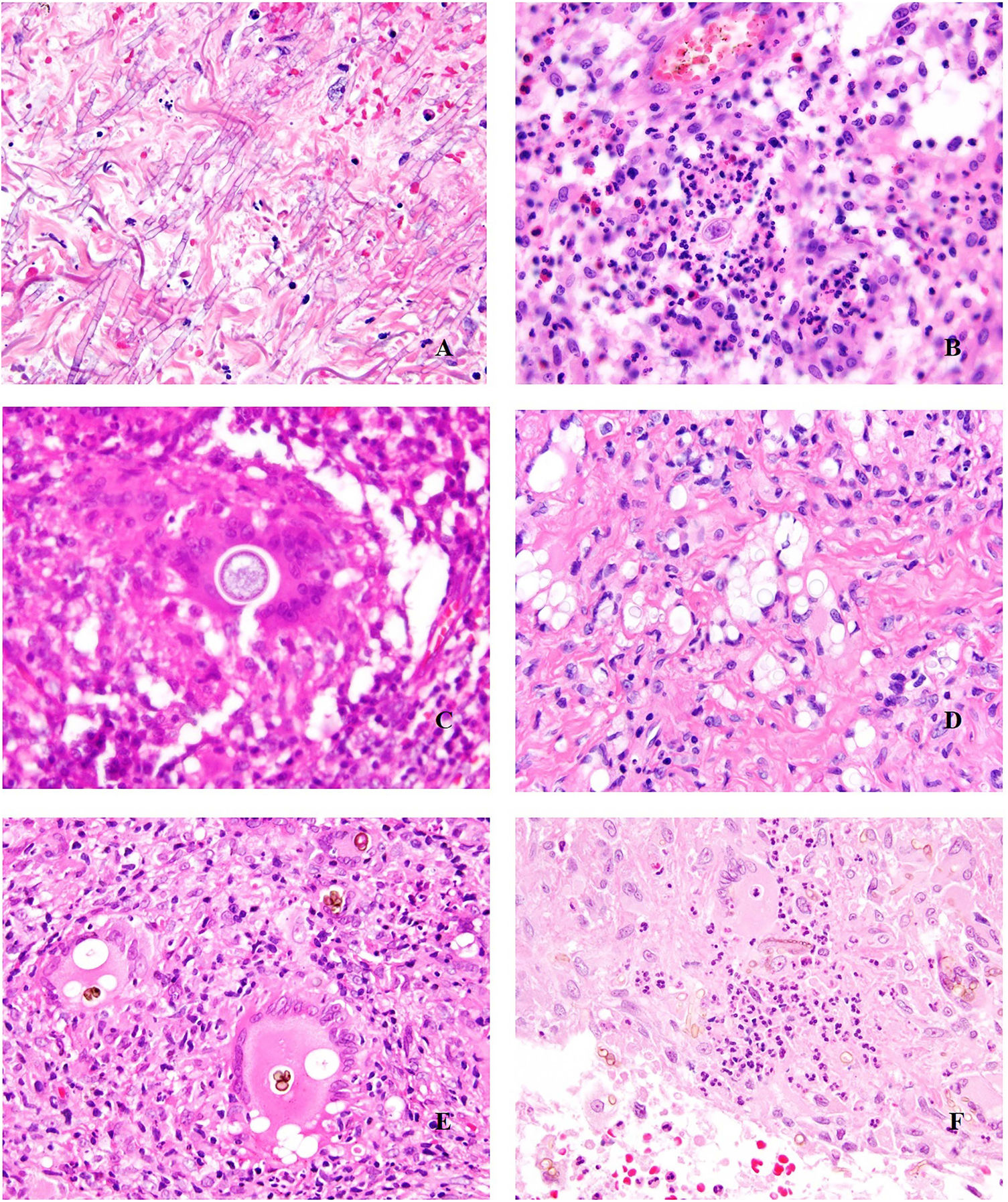
Deep skin mycosis. A, Aspergillosis (H&E, original magnification ×600). B, Blastomycosis (H&E, original magnification ×600). C, Coccidioidomycosis (H&E, original magnification ×600). D, Cryptococcosis (mucicarmine, original magnification ×200). E, Chromoblastomycosis (H&E, original magnification ×600). F, Phaeohyphomycosis (H&E, original magnification ×600). H&E indicates hematoxylin-eosin.
Deep skin mycoses. A, Fusariosis (H&E, original magnification ×400). B, Histoplasmosis (H&E, original magnification ×600). C, Lobomycosis (H&E, original magnification ×600). D, Mucormycosis (periodic acid-Schiff, original magnification ×200). E, Protothecosis (H&E, original magnification ×600). F, Paracoccidioidomycosis (H&E, original magnification ×600). H&E indicates hematoxylin-eosin.
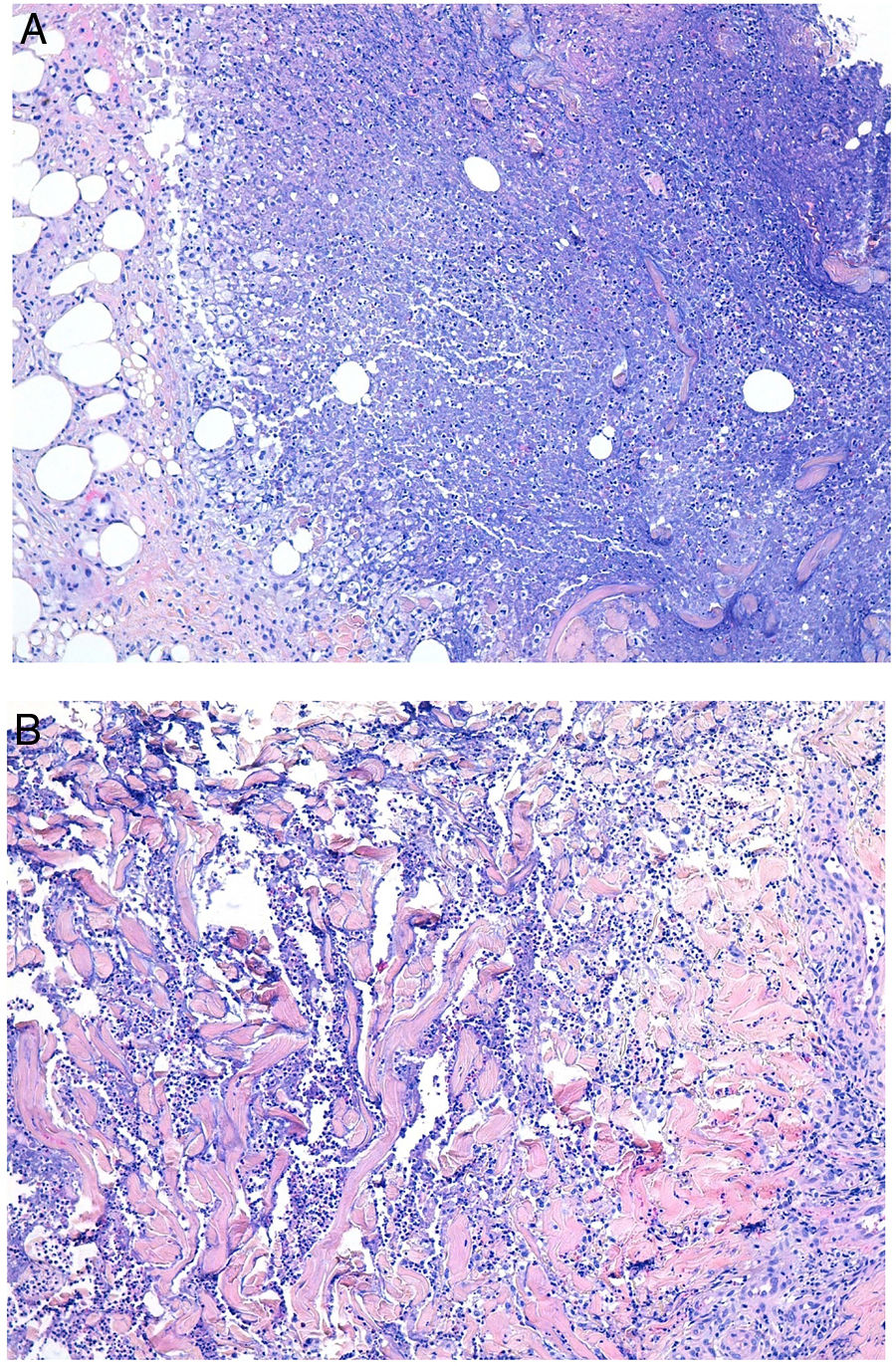
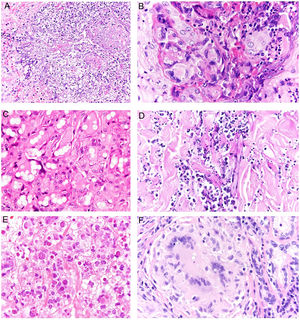
Deep skin mycosis. A,B, Rhinosporidiosis (A: H&E, original magnification ×400; B: Grocott, original magnification ×400). C, Sporotrichosis (H&E, original magnification ×600). D, Alternariosis (PAS × 600). E,F, Eumycetoma (PAS × 200 [E] and ×600 [F]). H&E indicates hematoxylin-eosin; PAS, periodic acid-Schiff.
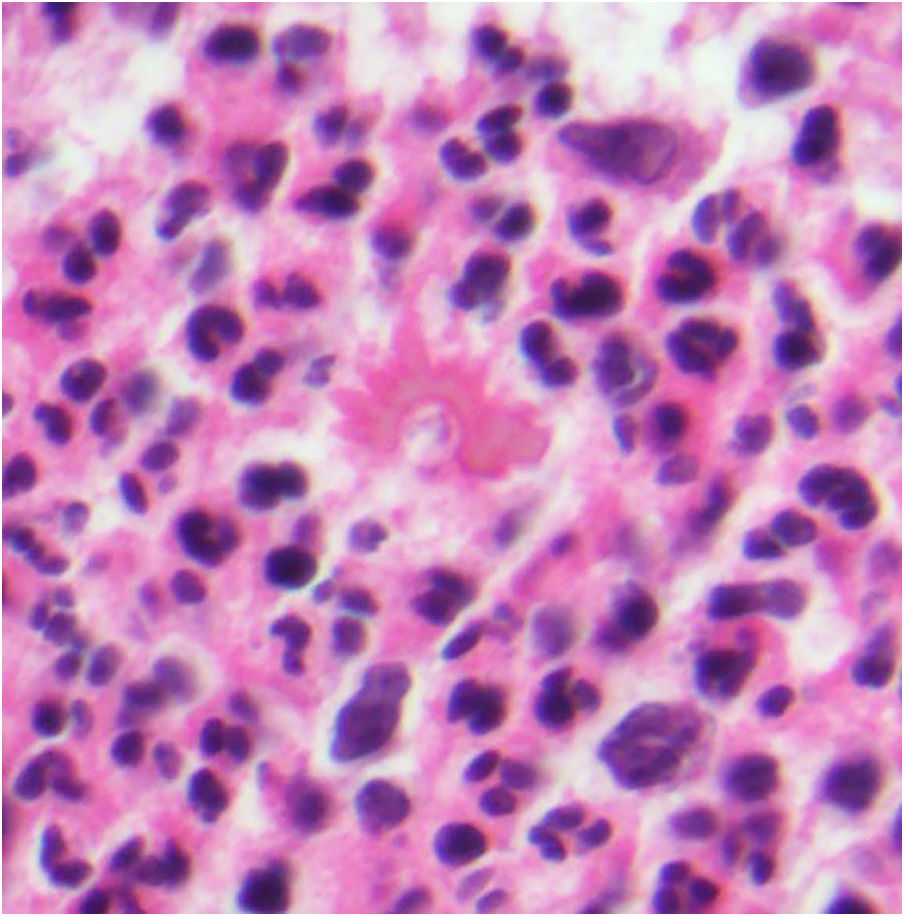
Several fungi trigger the formation of intense antibody deposits, visualized as a crown of eosinophils radiating around the fungus. This phenomenon is known as the Splendore-Hoeppli phenomenon (Fig. 13).
Deep skin fungal infections are usually accompanied by a suppurative granulomatous response (abscess formation), with fungi usually observed in the center of the granuloma (suppurative area). Suppurative granulomas are not pathognomonic, as they are seen in other types of infections (e.g., those caused by atypical mycobacteria or Tularemia species). Sarcoid and caseating necrotizing granulomas may also be observed, making it less likely to suspect a fungal infection. Some fungi (such as Coccidioides) can induce a background mucinous response.
Hyperplasia of the overlying epidermis, sometimes with a verrucous appearance, is quite common. Fungi may be eliminated through the skin. Granulomas may not always form in immunocompromised individuals, who show a predominantly neutrophilic response and are more prone to spread of the infection.
Mycobacterial InfectionsDefinition. Mycobacterial infections are caused by organisms of the genus Mycobacterium. The classic distinction is between Mycobacterium tuberculosis, responsible for tuberculosis, and non-tuberculous mycobacteria, which are grouped in the atypical category. Leprosy, which is caused by Mycobacterium leprae, is considered in a separate section in this article.
Cutaneous tuberculosis is uncommon and accounts for less than 2% of extrapulmonary forms of tuberculosis. Its incidence, however, has increased together with that of other forms of tuberculosis in relation to human immunodeficiency virus (HIV) infection and an increase in multidrug-resistant strains. Nontuberculous mycobacteria are a large, heterogeneous group of ubiquitous opportunistic pathogens. Their incidence is difficult to estimate, although it appears to be increasing in both immunosuppressed and immunocompetent individuals.31–34 The main causes of cutaneous tuberculosis are Mycobacterium marinum (swimming pool granuloma), Mycobacterium fortuitum-chelonae complex, and Mycobacterium ulcerans (Buruli ulcer), which is extremely rare in our setting.34
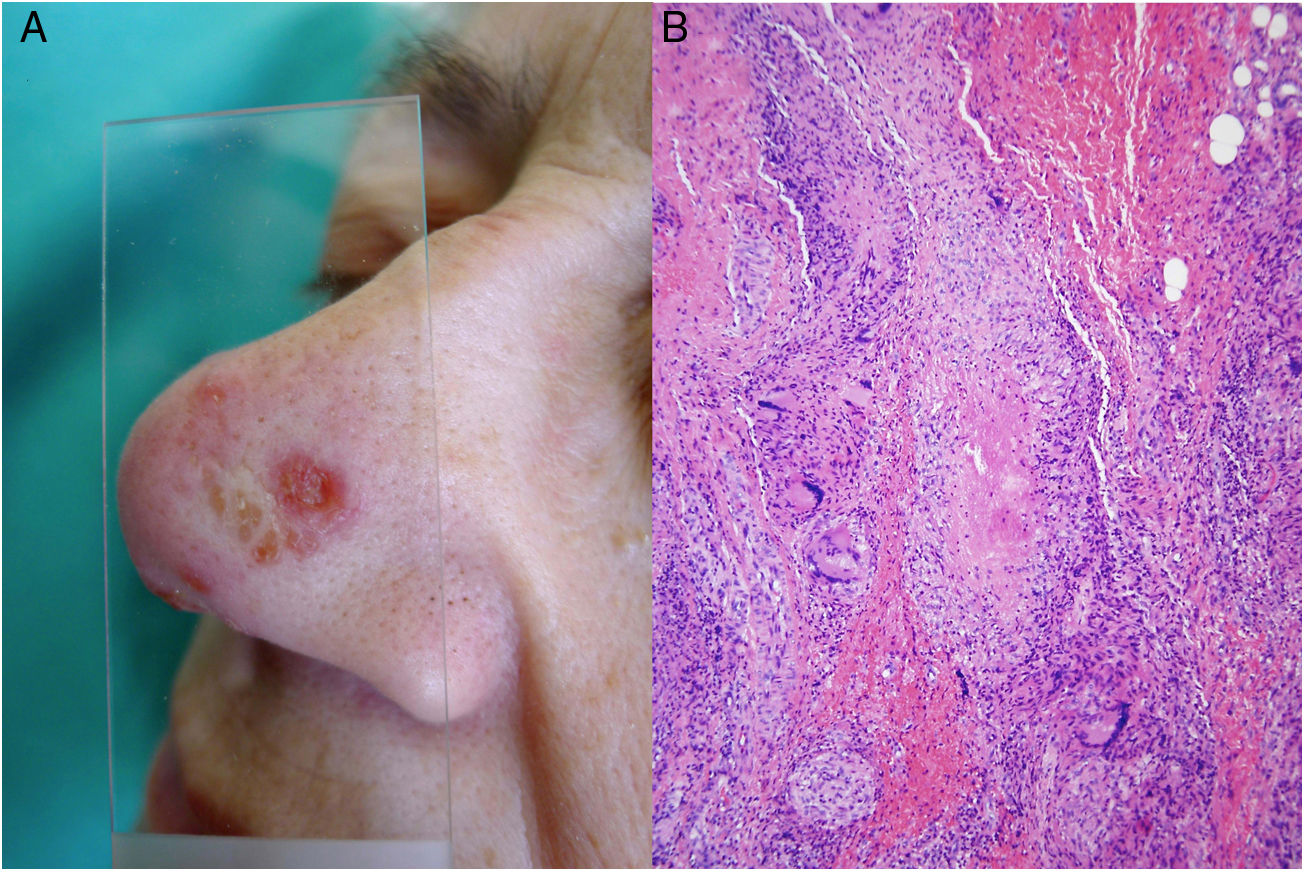
Clinical presentation. Cutaneous tuberculosis is characterized by 2 types of lesions35–39: true tuberculosis (linked to the presence of bacilli in lesions and a direct consequence of infection) and tuberculid lesions (a hypersensitivity reaction to antigens from the mycobacteria). It is usually classified according to the mode of acquisition, which can be exogenous (inoculation causing tuberculous chancre or tuberculosis verrucosa cutis) or endogenous, occurring by contiguity (scrofuloderma, tuberculosis cutis orificialis, and some cases of lupus vulgaris) or hematogenous spread (lupus vulgaris [Fig. 14A], miliary tuberculosis, and tuberculous gumma). Tuberculids include erythema induratum, papulonecrotic tuberculid, and lichen scrofulosorum.36–39
Mycobacterium tuberculosis. A, Lupus vulgaris lesion on the nasal dorsum (most common location) in a patient with pulmonary tuberculosis. Note the erythematous plaque formed by several papules and the characteristic apple jelly appearance observed by diascopy (Photograph courtesy of Dr Sánchez-Aguilar). B, Histologic appearance of a typical tuberculosis lesion: granulomas with central caseous necrosis and abundant peripheral lymphocytes (hematoxylin-eosin, original magnification ×40).
The clinical appearance of lesions caused by atypical mycobacteria is very broad and in many cases does not help establish a diagnosis (Fig. 15A). M marinum infections develop 1 to 2 weeks after sustaining a wound in an aquatic environment and often spread in a sporotrichoid-like pattern.34,35,40M fortuitum-chelonae infections are the result of wound contamination, following, for example, surgery, injections, mesotherapy, or acupuncture.35,40 Outbreaks have been described in association with the use of contaminated tattoo ink.41
Example of Mycobacterium chelonae infection. A, Painful, suppurative nodular lesions with superficial ulcers and crusting (photo courtesy of Dr Rodríguez Blanco). B, M chelonae infection. Microorganisms sometimes tend to form clusters in areas of suppuration, where they can be demonstrated by Ziehl-Neelsen staining (Ziehl-Neelsen, original magnification ×1000).
Histopathology. All cutaneous forms of M tuberculosis infection are characterized by the presence of tuberculoid granulomas (Fig. 14B), which consist of aggregates of epithelioid macrophages and Langhans giant cells, variable degrees of central caseous necrosis, and abundant peripheral lymphocytes. The granulomas do not always show necrosis or suppuration, and, depending on the host’s immune status, there may even be predominant necrosis and an almost inexistent granulomatous response.36,39,42 Diagnosis is based on identification of the microorganisms involved, as morphologic findings are not diagnostic. Microorganisms can be detected histopathologically, using Ziehl-Neelsen staining, for example, but this is positive in less than 5% of cases, as skin lesions usually have a low bacterial burden, and a minimum of 1000 to 10 000 colon-forming units per milliliter are normally required for detection.43,44 Alternative techniques include microbiological techniques such as smears, culture, and polymerase chain reaction (PCR) analysis of paraffin-embedded biopsy tissue. The main entity to consider in the differential diagnosis, apart from other granulomatous infections, is sarcoidosis, which can be distinguished by the absence of necrosis and peripheral lymphocytes.
Erythema induratum is essentially a form of lobular panniculitis characterized by granulomatous inflammation and small vessel vasculitis within the lobules. Papulonecrotic tuberculids involve extensive dermal necrosis with areas of granulomatous inflammation and vasculitis.36,37
In the category of atypical mycobacteria, M marinum infection histologically resembles a granulomatous inflammatory reaction that is normally confined to the dermis and often shows central suppuration and secondary epidermal changes.32,33M fortuitum-chelonae lesions show varying degrees of acute inflammation followed by the formation of granulomas, which are generally poorly defined. In some cases, aggregates of bacilli will be seen in areas of suppuration (Fig. 15B). These changes are nonspecific and are also observed in infections caused by other species that are less likely to affect the skin.33,44
LeishmaniasisDefinition. Leishmaniasis is caused by flagellate parasites of the Leishmania genus and is usually transmitted by sandfly bites. The main reservoirs are wild or domestic mammals, particularly dogs.
Clinical presentation. Leishmaniasis can be cutaneous, mucocutaneous, or visceral. The clinical forms are highly variable and depend on the characteristics of the parasite, the vector, the host’s immune response, and the location of the lesions.45 Cutaneous leishmaniasis (usually confined to the face, scalp, arms, or other exposed areas) presents as a self-limiting, slow-growing, hard, crusted papule (Fig. 16). Disseminated cutaneous forms occur in immunosuppressed individuals. There are also recurrent forms and post kala-azar dermal leishmaniasis. Mucocutaneous leishmaniasis, which is mostly caused by Leishmania braziliensis, causes lesions involving the oral and nasopharyngeal mucosa. With the exception of dark pigmentation, skin involvement is rare (5%) in visceral leishmaniasis (kala-azar), in which parasites are distributed throughout the reticuloendothelial system, causing fever, hepatosplenomegaly, and severe systemic disease.
Several of the more than 20 Leishmania species that are potentially pathogenic to humans tend to show visceral tropism (e.g., Leishmania donovani and Leishmania infantum), but the majority show tropism for the skin or mucocutaneous surfaces. Exclusive cutaneous involvement may be seen in infections caused by L infantum, the predominant species in Spain and the western Mediterranean basin.46
Histopathology. Histologic findings are correlated with clinical presentation and immune response. In patients who mount a poor immune response, histology typically shows innumerable amastigotes and abundant histiocytes, but no other inflammatory cells. Immunocompetent patients tend to have small or moderate numbers of amastigotes, in addition to granulomas, and, on occasions, necrosis.
In acute forms of leishmaniasis, histology shows a dense, diffuse, predominantly histiocytic dermal infiltrate together with abundant lymphocytes and plasma cells. The epidermis may show marked hyperplasia, with hyperkeratosis or pseudoepitheliomatous changes. In other cases, atrophy or ulceration will be seen.
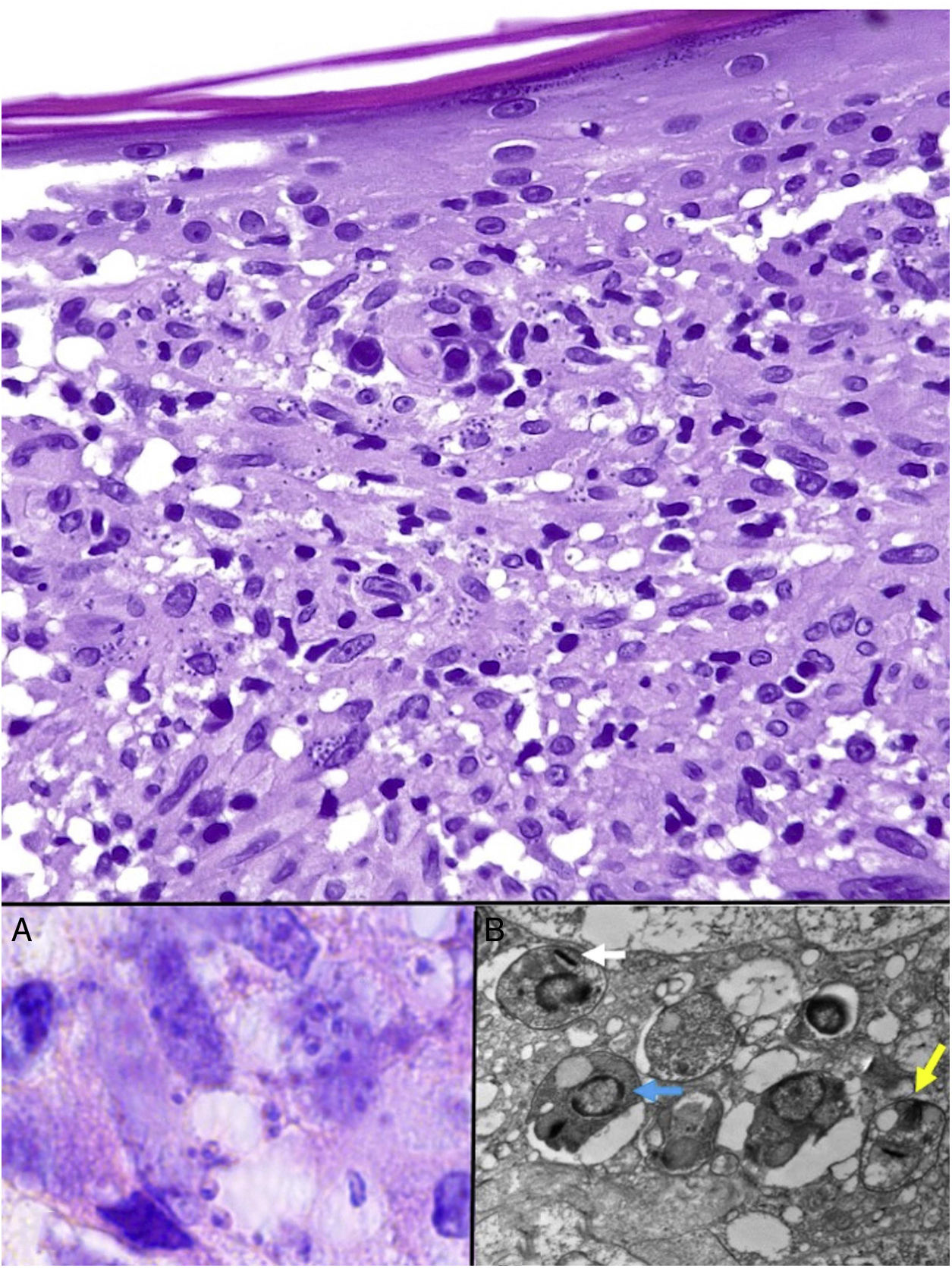
Leishmania amastigotes (the intracellular form of the parasite) are predominantly observed at the intracytoplasmic level. They can be clearly visualized by staining with hematoxylin-eosin, or, when inside macrophages, Giemsa. They may also be observed in cytological smears obtained by scraping, touch imprint, or fine needle aspiration. Their bodies are round or oval and measure 2 to 5 μm in diameter. They show a peripheral enhancement pattern corresponding to the kinetoplast and are typically arranged at the periphery of the cytoplasm (Fig. 17, box A). Electron microscopy can distinguish between the nucleus, kinetoplast, and internal flagellum (Fig. 17, box B).47 They are not encapsulated and therefore stain negative for PAS and Grocott, distinguishing them from Histoplasma capsulatum. The promastigote form (which is longer, larger, and has an external flagellum) is confined to the intestine of the vector and is therefore not seen in lesions.
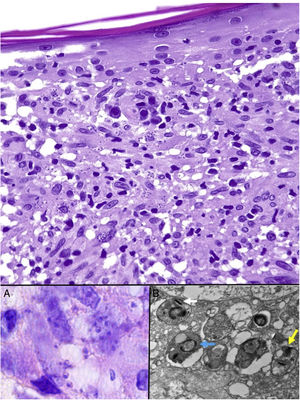
Leishmaniasis. Subepidermal granulomatous infiltrate with atrophy of the epidermis. Abundant Leishmania amastigotes in the upper dermis (H&E, original magnification ×200). A, 2-μm intracellular amastigotes with peripheral enhancement and a tendency to align along the periphery of the cytoplasm (marquee sign) (H&E, original magnification ×1000). B, Transmission electron microscopy image (×8000) showing the nucleus (blue arrow), the kinetoplast (white arrow), and inner flagellum (yellow arrow). H&E indicates hematoxylin-eosin.
In more chronic forms of leishmaniasis, confluent, poorly defined, epithelioid granulomas with a disordered appearance (messy granulomas)48 are observed and usually occupy the full thickness of the dermis (Fig. 18A). Additional findings include Langhans giant cells, an increased number of plasma cells, and a decreased number of amastigotes, such that they may be difficult to discern. Amastigotes tend to be located in the uppermost areas of the dermis, at the subepidermal level (Fig. 17).
A, Chronic form of leishmaniasis. Confluent, poorly defined, epithelioid granulomas with a disordered appearance (messy granulomas)48 that usually occupy the full thickness of the dermis (hematoxylin-eosin, original magnification ×20). B, Vulvar bilharziasis cutanea tarda. Area of the reticular dermis showing an epithelioid granuloma with central necrosis around 7 round or oval structures. One of these, the most intact, has a lateral spicule, which is characteristic of Schistosoma hematobium. C, Swimmer’s itch due to cercariae. This is not a granulomatous reaction. Note the 3 longitudinal fragments of cercariae (species not identifiable) close to the eccrine glands and surrounded by a lymphohistiocytic infiltrate with numerous eosinophils. D, Onchocercoma. Note the granulomatous infiltrate with neutrophils and sclerosis, as well as several giant foreign body cells around the cross section in 3 areas. Note also the characteristic retraction artifact around the thick eosinophilic cuticle of the 3 filariae.
Recurrent leishmaniasis shows confluent non-necrotizing epithelioid granulomas in which it is very difficult or even impossible to identify amastigotes. Other cells present are lymphocytes, plasma cells, and eosinophils.
Immunohistochemistry is a useful diagnostic aid when there are few parasites. Monoclonal anti-Leishmania antibodies exist,49 but they are not widely available and their use is not standardized. Staining for CD1a with clone MTB1 may also be helpful, as it is positive for amastigotes, possibly due to transfer from Langerhans cells. CD1a preferentially stains amastigotes in the upper dermis, with less intense patterns observed in the deeper layers.50 Not all Leishmania species, however, show positive results. In addition, other clones of the same antibody may be negative. PCR is the most sensitive method for detecting Leishmania species, with a sensitivity rate of close to 100%.51
In diffuse cutaneous leishmaniasis, the dermis is diffusely occupied by macrophages that do not form granulomas and clear cytoplasm containing innumerable amastigotes (Fig. 18A). Lymphocytes and plasma cells are scarce, while epidermal changes are variable.
The histologic differential diagnosis is broad and depends on the form of presentation. It must include other granulomatous diseases and depends on the correct identification of amastigotes. The dense inflammatory infiltrate can cause confusion with lymphoma. Intracellular parasites are also observed in rhinoscleroma, histoplasmosis, inguinal granuloma, and blastomycosis, each with distinct morphologic and staining characteristics. Established or chronic Leishmania lesions present abundant plasma cells, requiring the exclusion of secondary or tertiary syphilis. Epidermal hyperplasia can sometimes mimic squamous cell carcinoma. Other granulomatous diseases, such as lupus vulgaris, leprosy, and sarcoidosis should also be considered in the histologic differential diagnosis.52
Diseases Caused by ParasitesGranulomas secondary to parasite infestations account for up to 16.7% of all granulomas, and include, among others, schistosomiasis and granulomas secondary to hydatid cysts.53 In the section on helminth infections, we have included infections caused by trematodes (schistosomiasis, paragonimiasis, and fascioliasis), nematodes (cutaneous larva migrans, onchocerciasis, filariasis, gnathostomiasis, loiasis, dracunculiasis, strongyloidosis, ascariasis, streptocerciasis, dirofilariasis, and trichinosis) and cestodes (sparganosis, cysticercosis, and echinococcosis). The focus is on infections in which histology typically shows granulomas.
Cutaneous SchistosomiasisDefinition. Cutaneous schistosomiasis is an endemic infection in tropical and subtropical regions caused by species of the genus Schistosoma, which are commonly found in snails. The infection is transmitted by contact with contaminated water. Of the 5 species that can affect humans, the 3 most common are Schistosoma hematobium (located in Africa and the Middle East and responsible for urinary schistosomiasis), Schistosoma japonicum (located in Japan and Southeast Asia), and Schistosoma mansoni (more common in Africa, South America, and the Caribbean).
Skin lesions are associated with anthropophilic Schistosoma parasites, that is, parasites whose larval form (cercaria) can pierce the skin, producing adult worms that mate and release eggs to the exterior through the intestine or bladder. These skin lesions can be classified as 1) bilharziasis cutanea tarda, which is a granulomatous reaction to adult worms and eggs; 2) hypersensitivity reactions (cercarial dermatitis or swimmer's itch); or 3) transient cutaneous responses to the release of eggs into the bloodstream (not involving intralesional parasites), with fever, urticaria, and sometimes, purpura (Fig. 18B).54 Swimmer's itch may also be caused by anthropophilic schistosomes, which, unable to mature and complete their life cycle in humans, die in the skin, producing a local inflammatory reaction only (Fig. 18C). In this next section, we will focus on cutaneous schistosomiasis (bilharziasis), which is typically granulomatous.
Clinical presentation. Schistosomiasis presents as papillomatous papules or plaques that can mimic genital warts associated with human papillomavirus infection.55 If detected, it is important to rule out parasites in the bladder and intestine.
Histopathology. Although papillomatosis and parakeratosis may be observed, the most characteristic histologic finding is a granulomatous inflammatory infiltrate with eosinophils and plasma cells around Schistosoma eggs. S hematobium eggs have a terminal spike, S mansoni eggs have a lateral spike, and S japonicum eggs have no spikes. In the case of swimmer's itch, the presence of nonpathogenic metacercariae unable to survive triggers a more intense reaction, producing greater spongiosis and a denser eosinophilic and neutrophilic infiltrate (Fig. 18C).
Onchocerca InfectionsDefinition. Onchocerca infections are a group of infections caused by nematodes (roundworms) of the Filarioidea genus with a threadlike body and a mouth surrounded by papillae. They are usually transmitted by mosquitoes. In this section, we will review the subcutaneous forms, which include onchocerciasis and loiasis or eye worm. Onchocerciasis, which is caused by Onchocerca volvulus and transmitted by blackflies of the Simulium genus, is characterized by the formation of granulomas.
Clinical presentation. Onchocerciasis produces significant xerosis and signs of premature skin aging, accompanied by intense itching, isolated smooth-surfaced papules and hyperkeratotic macules, leopard skin (areas of depigmented and repigmented skin), and subcutaneous nodules called onchocercomas, which tend to arise on bony prominences.
Histopathology. Microfilaria may be observed in the skin, alongside edema, myxoid deposits, dilated lymph vessels, and an inflammatory neutrophilic and eosinophilic infiltrate. Histologic examination of an onchocercoma will show numerous organisms surrounded by a granulomatous inflammatory infiltrate (Fig. 18D). This infiltrate is usually separated from the thick cuticle of the filaria by a retraction artifact caused by the presence of phagocytes unable to attach themselves to the cuticle.
GnathostomiasisDefinition. Gnathostomiasis is an emerging parasitic disease caused by the third-stage larvae (L3) of nematodes of the genus Gnathostoma (order Spirurida), which have a complex life cycle involving a first intermediate host, a copepod, which is ingested by fish and freshwater amphibians, in which L2 parasites develop into L3 larvae capable of movement. From these second intermediate hosts, they can pass into dogs, cats, or pigs, where they trigger the formation of granulomas in the mucosa of the gastrointestinal tract. L1 aquatic larvae will then be eliminated in stools and ingested by copepods, where they will continue their cycle. Gnathostomiasis is more common in areas where fish or amphibians are consumed raw (e.g., sushi or ceviche).
Clinical presentation. Gnathostoma parasites cannot complete their life cycle in humans, so once ingested, they travel through the mesenteric plexus to the subcutaneous tissue, where they cause migratory panniculitis. A history of eating raw fish and observation of tissue or peripheral blood eosinophilia are highly suggestive of gnathostomiasis.56 Migratory panniculitis may also be seen in subcutaneous toxocariasis, loaiasis, and even Strongyloides stercoralis infection, cutaneous larva migrans and, exceptionally, in association with ectopic migrating flukes such as Fasciola hepatica and Paragonimus species.
Histopathology. Although granulomas may form around Gnathostoma larvae (Fig. 19A), histology typically shows eosinophilic infiltrates of varying intensity, intermixed with lymphocytes, neutrophils, histiocytes, and even plasma cells (Fig. 19B). The worms may sometimes be found in the deep dermis or panniculus adiposus and have a diameter of approximately 200 to 300 μm.
Differential diagnosis. Gnathostoma worms are similar in size to Spirometra worms, which cause sparganosis if ingested in raw meat or fish or if present in water or topical products applied to the skin. The larvae, which are white, flat and have a scalloped appearance, can live in the stomachs of carnivorous mammals, from where they may migrate to the skin, producing nodules that measure between 0.5 and 5 cm and resemble lipomas or fibroids. The incubation period is between 6 and 11 days.57 These nodules can open to the surface, allowing the larvae to escape. Histology often shows a granulomatous reaction around the larvae, which are easy to distinguish from Gnathostoma larvae, as they lack genital and intestinal structures and have a loose central stroma and discontinuous smooth muscle fibers.57 A palisaded granulomatous reaction with mucin, similar to that observed in GA, has been reported in sparganosis. Unlike in other parasitic diseases, surgical removal of the worm is common. Identification of the larvae is diagnostic.58
DirofilariasisDefinition. Dirofilariasis is caused by nematodes of the genus Dirofilaria; the larvae of these nematodes are transmitted by mosquito bites and can affect the lung (Dirofilaria immitis) and other organs, including the skin (Dirofilaria repens).59
Clinical presentation. The cutaneous manifestations of Dirofilaria repens are single or multiple erythematous nodules with a soft consistency caused by the degeneration of immature larvae, triggering a foreign body granulomatous reaction, with palisaded granulomas, necrobiotic collagen, and a mixed inflammatory infiltrate comprising eosinophils, plasma cells, lymphocytes, and histiocytes.
Histopathology. The histologic findings of dirofilariasis are similar to those of necrobiosis lipoidica, although in serial tissue sections, it is common to see spaces in the upper area of the subcutaneous tissue with cuticular striations and spines and a central area with filarial viscera.59
StrongyloidiasisDefinition. Strongyloidiasis is primarily a gastrointestinal parasitic disease but it can affect the skin. Skin lesions are more common in immunosuppressed patients and are clinically relevant as they are the most common purpuric lesions observed in hyperinfestations and are associated with a poor prognosis.60
Clinical presentation. Cutaneous strongyloidiasis can present as fast-moving larva currens (5–15 cm/h) or purpuric macules that converge centrifugally to form larger lesions in the abdominal and thigh areas.
Histopathology. Strongyloides larvae can be detected in capillaries or among collagen fibers in the reticular dermis and may be surrounded by an inflammatory granulomatous infiltrate, although this is rare and has been attributed to the death of the larvae.61
Differential diagnosis. Cutaneous larva migrans, which occurs following the penetration of Ancylostoma braziliense into the skin, produces a characteristic, linear, serpentine lesion that follows the trajectory of the larva, which advances several centimeters a day. Histology does not normally show granulomas, but rather spongiosis and a lymphocytic inflammatory infiltrate rich in eosinophils. Strongyloides L3 larvae are similar in size to Ancylostoma braziliense larvae (about 3 keratinocytes), but the former are typically found in the dermis, while the latter, although less common, are typically found in the epidermis.62 Size is useful for differentiating Strongyloides larvae from L3 Gnathostoma larvae, which with a diameter of 200 to 300 μm, are considerably larger.
CysticercosisDefinition. Cysticercosis is an infectious disease caused by the larval stage of Taenia solium. It is particularly concerning when it affects the central nervous system. It is estimated that 2% of seizure emergencies in developing countries are caused by this infection.
Clinical presentation. Cutaneous cysticercosis presents as subcutaneous nodules (a single nodule or < 10) in 88% of cases.63 The nodules are mainly located on the trunk and contain a yellowish liquid.
Histopathology. When larvae are present, histology will show a cystic cavity surrounded by a vesicular wall. The lumen, which is scalloped in appearance, contains round nodular structures that correspond to invaginated scolices.63 Granulomas are more common following rupture of the cyst.
Echinococcosis or Hydatid DiseaseDefinition. Echinococcosis, or hydatid disease, is caused by larval stages (metacestodes) of parasites of the genus Echinococcus.
Clinical presentation. As in cysticercosis, echinococcosis cysts can clinically masquerade as an infundibular cyst or lipoma64 and tend to grow at a rate of 1 mm to 5 cm a year.
Histopathology. Hydatid cysts have 3 main layers: an outer layer, the pericyst, formed by host cells; a middle layer; and a thick, translucent inner germinal layer, from which the scolices arise.64 Since the lesions lack an epithelial lining, but contain multiple layers of elongated epithelioid histiocytes, they are pseudocysts. Histiocytes may stain positively with CD68, and lymphocytes and eosinophils are usually present. Cyst rupture leads to a granulomatous inflammatory reaction with fibrosis and multinucleated histiocytes, neutrophils, eosinophils, and, on occasions, leukocytoclastic vasculitis.
LeprosyDefinition. Leprosy is a chronic infection caused by the obligate intracellular parasites Mycobacterium leprae and Mycobacterium lepromatosis. It predominantly affects the skin, upper respiratory tract, and peripheral nerves. It is still endemic in many countries, including India, Indonesia, and Brazil. Most cases in Europe are imported.65,66
Clinical presentation. The clinical forms of leprosy range from tuberculoid or paucibacillary leprosy at one end of the spectrum (few lesions and a competent immune system) to lepromatous or multibacillary leprosy at the other (numerous lesions and a deficient immune system).67 In between lies indeterminate leprosy, which, depending on the patient's immune system, can progress to tuberculoid or lepromatous leprosy.
Indeterminate leprosy manifests as 1 or more hypopigmented or erythematous macules, while tuberculoid leprosy presents as 1 or more well-demarcated erythematous-brownish plaques with a loss of sensation (Fig. 20A). Lepromatous leprosy, in turn, is a systemic disease that mainly presents with skin manifestations consisting of abundant, poorly demarcated plaques or nodules with pronounced diffuse infiltration and a progressive loss of sensation. In between are various dimorphic (borderline) lesions, with intermediate manifestations and lesions that are often annular (Fig. 20B).68 Histoid leprosy is a rare nodular variant. Finally, there are a series of lepra reactions that represent acute episodes within this slow-progressing disease. Type I reactions are type IV hypersensitivity reactions, while type II reactions are the result of immune complex vasculitis.
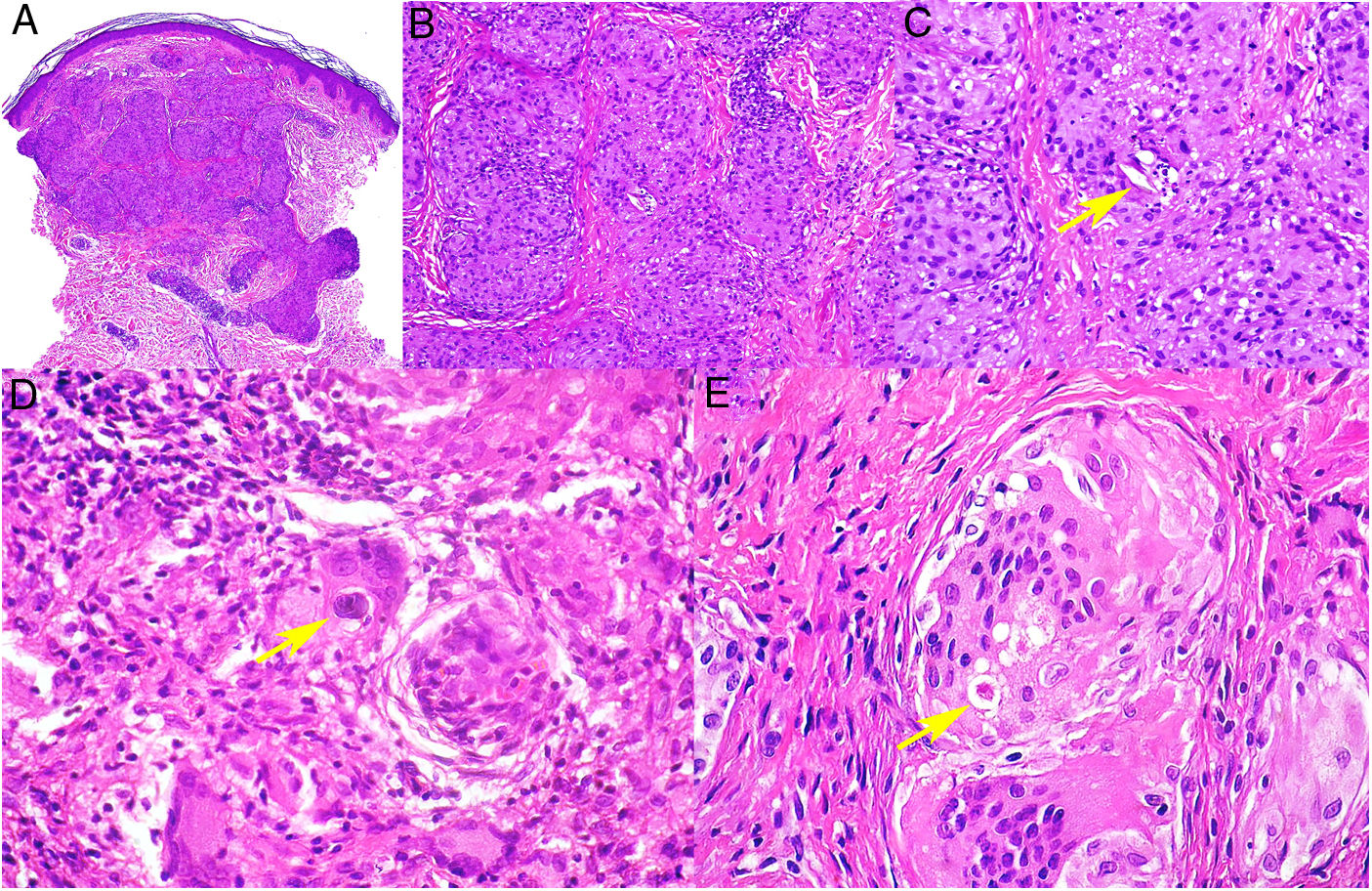
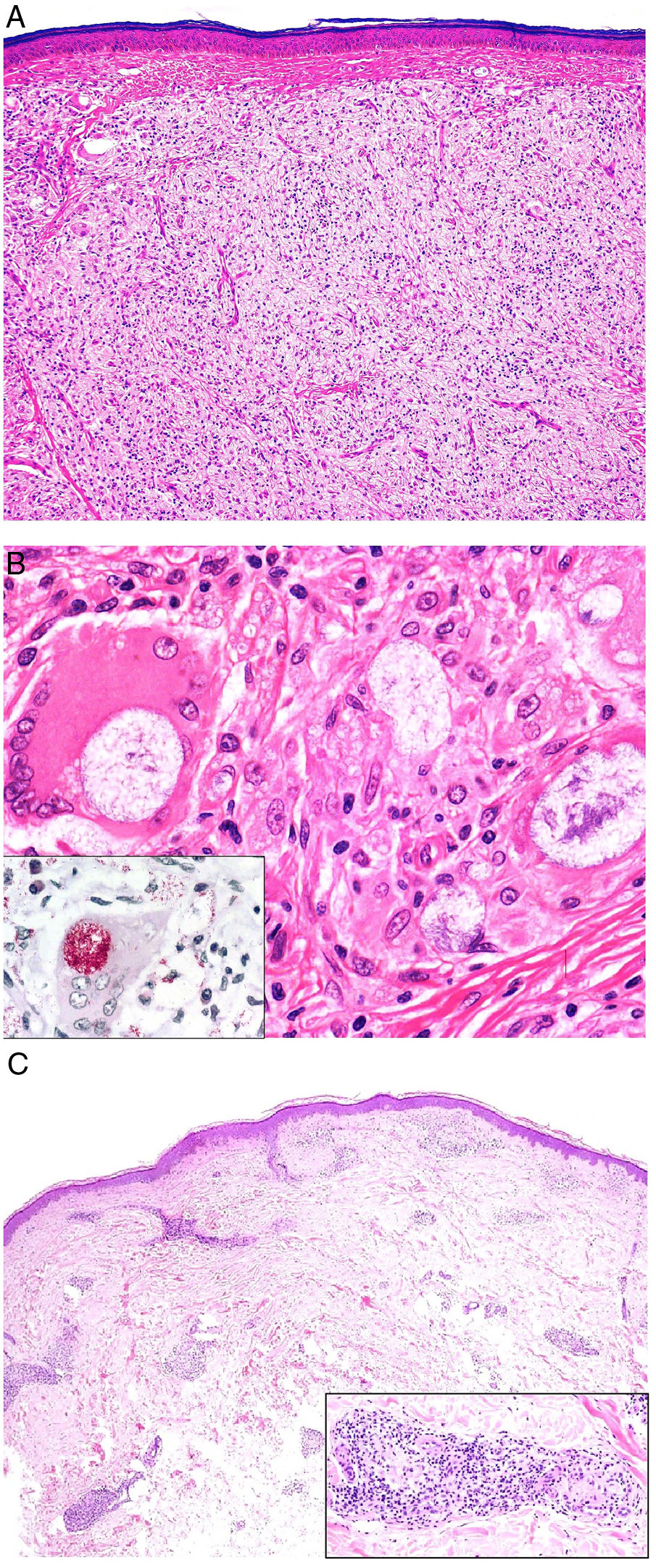
Histopathology. In indeterminate leprosy, histology shows a slight lymphohistiocytic infiltrate surrounding vessels, appendages, and nerves. Tuberculoid leprosy is characterized by non-necrotizing epithelioid granulomas with multinucleated giant cells and numerous lymphocytes; the infiltrate is often "sausage-shaped", as it follows the course of nerves and appendages. It does not normally contain bacilli (Fig. 21). The infiltrate in lepromatous leprosy contains some lymphocytes, but it is predominantly a nodular or diffuse dermal histiocytic infiltrate under a Grenz zone (Fig. 22A). The histiocytes, which are S-100 positive, have a broad granular cytoplasm, which in more advanced lesions is foamy; the nerves are usually intact in early-stage lesions, but in later stages they may show concentric fibrosis. The appendages are not visible. Histology shows large numbers of bacilli, which often form amphophilic groups called globi (Fig. 22B).65,67,68 Borderline forms show features in between those observed in tuberculoid and lepromatous leprosy (Fig. 22C). Histoid leprosy is characterized by predominantly spindle-shaped nodules formed by histiocytes containing numerous bacilli.67
Leprosy. Image of tuberculoid leprosy specimen from the patient in Fig. 20A showing a “sausage-shaped” lymphohistiocytic infiltrate along the course of the neurovascular bundles (H&E, original magnification ×20). The lower left inset shows epithelioid granulomas surrounded by lymphocytes (H&E, original magnification ×100). The lower right inset shows a nerve in the center of the granuloma (H&E, original magnification ×100). H&E indicates hematoxylin-eosin.
A, Lepromatous leprosy. Image showing a sheetlike histiocytic infiltrate under a Grenz zone (H&E, original magnification ×40). B, Higher magnification view showing numerous foamy histiocytes and multinucleated giant cells with globi in their cytoplasms (H&E, original magnification ×200). The lower left inset shows numerous bacilli stained using the Job-Fite technique; some of these are grouped into globi in the macrophages (Job-Fite, original magnification ×200). C, Dimorphic leprosy. Biopsy specimen from the patient in Fig. 19B, with a superficial and deep perivascular and periadnexal inflammatory infiltrate (H&E, original magnification ×20), composed of lymphocytes and histiocytes, as shown in the lower right inset. H&E indicates hematoxylin-eosin.
Type I (reversal) reactions are characterized by a lymphocytic-granulomatous infiltrate around neurovascular bundles. Type II reactions (erythema nodosum leprosus), in turn, consist of leukocytoclastic vasculitis alongside other findings of leprosy, including globi. Lucio phenomenon is a variant of a type II reaction that consists of significant inflammation with arterial thrombi, infarctions, and abundant microorganisms.68
Mycobacterium leprae complex bacteria are gram-positive, acid-fast bacilli that can be detected with Fite or Ziehl-Neelsen staining and PCR.65
FundingNo funding was received for this study.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Aróstegui Aguilar J, Diago A, Carrillo Gijón R, Fernández Figueras M, Fraga J, García Herrera A, et al. Granulomas en dermatopatología: principales entidades. Parte II. Actas Dermosifiliogr. 2021. https://doi.org/10.1016/j.ad.2021.04.001
This article is an initiative of the Dermopathology Research Group of the Spanish Academy of Dermatology and Venereology (AEDV) and the Spanish Pathology Society (SEAP). All the authors contributed equally to this work, regardless of the order of their surnames.

![Granuloma annulare. A, Large erythematous annular plaques with a raised border in a sun-exposed area. B, Panoramic histologic view showing an inflammatory infiltrate in the reticular dermis. C-E, Higher-magnification view showing a granulomatous lesion with numerous multinucleated giant cells and elastophagocytosis without palisading, increased mucin, or necrobiosis (hematoxylin-eosin, original magnification ×40 [B], ×400 [C,D], ×600 [E]). Granuloma annulare. A, Large erythematous annular plaques with a raised border in a sun-exposed area. B, Panoramic histologic view showing an inflammatory infiltrate in the reticular dermis. C-E, Higher-magnification view showing a granulomatous lesion with numerous multinucleated giant cells and elastophagocytosis without palisading, increased mucin, or necrobiosis (hematoxylin-eosin, original magnification ×40 [B], ×400 [C,D], ×600 [E]).](https://static.elsevier.es/multimedia/15782190/0000011200000008/v2_202110201006/S1578219021002109/v2_202110201006/en/main.assets/thumbnail/gr2.jpeg?xkr=ue/ImdikoIMrsJoerZ+w9/t1/zx4Q/XH5Tma1a/6fSs=)
![Deep skin mycosis. A,B, Rhinosporidiosis (A: H&E, original magnification ×400; B: Grocott, original magnification ×400). C, Sporotrichosis (H&E, original magnification ×600). D, Alternariosis (PAS × 600). E,F, Eumycetoma (PAS × 200 [E] and ×600 [F]). H&E indicates hematoxylin-eosin; PAS, periodic acid-Schiff. Deep skin mycosis. A,B, Rhinosporidiosis (A: H&E, original magnification ×400; B: Grocott, original magnification ×400). C, Sporotrichosis (H&E, original magnification ×600). D, Alternariosis (PAS × 600). E,F, Eumycetoma (PAS × 200 [E] and ×600 [F]). H&E indicates hematoxylin-eosin; PAS, periodic acid-Schiff.](https://static.elsevier.es/multimedia/15782190/0000011200000008/v2_202110201006/S1578219021002109/v2_202110201006/en/main.assets/thumbnail/gr12.jpeg?xkr=ue/ImdikoIMrsJoerZ+w9/t1/zx4Q/XH5Tma1a/6fSs=)