Although a granular cell tumor (GCT) usually develops on the skin or oral mucosa, it has been described in many other organs. GCT typically presents as a solitary tumor, but multiple lesions can occur. It has also been described in association with other diseases.
ObjectiveTo describe the clinical characteristics of cutaneous and oral mucosal GCTs and explore potential associations with other diseases.
Material and methodsRetrospective study of patients diagnosed with GCT at our hospital between 1995 and 2019. The following information was collected from the patients’ medical records: age, sex, number of GCTs, location, diameter, time to diagnosis, tentative clinical diagnosis, surgical margin status, recurrence, follow-up time, and associated diseases.
ResultsWe detected 89 cutaneous or oral mucosal GCTs in 81 patients (43 women, 38 men) with a mean age of 40.21 years. The mean tumor diameter was 1.34 cm. Five of the 81 patients (6.2%) had multiple GCTs, including noncutaneous tumors. Patients with multiple GCTs were on average younger than those with a single tumor (P = .004). There was only a single case of local recurrence and no cases of distant metastasis. None of the patients had associated diseases.
ConclusionsMost GCTs are benign and local recurrence is uncommon, even in patients with positive margins. Nevertheless, the possibility of multiple tumors affecting the skin, oral mucosa, or internal organs should be borne in mind, especially in young patients.
El tumor de células granulares (TCG) suele desarrollarse en la piel o la mucosa oral pero se ha descrito en muchos otros órganos. Suele ser único pero puede ser múltiple y asociarse a otras enfermedades.
ObjetivosAnalizar las características clínicas de nuestros pacientes con TCG en la piel y la mucosa oral y su posible asociación con otras afecciones.
Material y métodosEstudio retrospectivo de los pacientes con TCG diagnosticados entre 1995–2019. Se revisaron las historias clínicas para obtener los siguientes datos: edad, sexo, localización, número de lesiones, diámetro, tiempo de evolución, diagnóstico clínico de sospecha, estado de los márgenes de resección, desarrollo de recidiva, tiempo de seguimiento y enfermedades asociadas.
ResultadosOchenta y un pacientes presentaron 89 TCG en la piel y la mucosa oral (43 mujeres/38 varones, edad media 40,21años). El diámetro medio fue de 1,34 cm. Contabilizando los tumores extracutáneos 5 de los 81 pacientes presentaban TCG múltiple (6,2%). La edad media de los pacientes con TCG múltiple fue significativamente inferior a la de los pacientes con un único tumor (p = 0,004). Solamente un paciente presentó recidiva local y ninguno desarrolló metástasis a distancia. No hemos detectado ningún caso asociado a otras enfermedades.
ConclusionesLa mayoría de TCG son benignos y a pesar de tener los márgenes afectados no suelen presentar recidivas locales. Sin embargo, hay que tener en cuenta la posibilidad de presentar TCG múltiples tanto en la piel y la mucosa oral como en órganos internos, especialmente en pacientes jóvenes.
Granular cell tumor (GCT) is an uncommon finding that was first described by Abrikossoff en 1926.1 Most cases involve the oral mucosa, skin, and subcutaneous tissue, although GCT has been reported to affect many other organs.2 Its malignant variant is very uncommon.3 The tumors are usually solitary, although they may sometimes be multiple, and in recent years there have been reports of cases associated with Noonan syndrome and neurofibromatosis.4 Our objective was to review the clinical characteristics of patients with GCT on the skin and oral mucosa and assess its potential association with other conditions.
Material and MethodsThe study population comprised all cases coded as GCT on the skin and oral mucosa in the database of the Histopathology Department of Hospital de Bellvitge, L’Hospitalet de Llobregat, Barcelona, Spain between 1995 and 2019. Our institution is an 800-bed teaching institution that provides health care to approximately 1 000 000 people. We retrospectively reviewed the clinical histories to collect the following data: age at diagnosis of GCT or of the first tumor in the case of multiple lesions, sex, location of new lesions, number of lesions, diameter of the tumor, time from onset to diagnosis, suspected clinical diagnosis. We also recorded whether the tumor was removed completely or with positive margins, recurrence, association with neurofibromatosis or other RASopathies, and duration of follow-up. Similarly, we recorded cases coded as GCT in the database of the Histopathology Department and located on any other organ during the same period.
The data obtained were analyzed with SPSS for Windows, Version 17.0 (SPSS Inc.). Categorical variables were compared using the Fisher exact test. Continuous variables were compared using the t test when the data were confirmed to be normally distributed. The Mann-Whitney test was performed in the case of nonnormally distributed variables. Statistical significance was set at P < .05. The parameters analyzed were compared by sex, and patient characteristics were compared between those with solitary lesions and those with multiple lesions.
ResultsA total of 89 GCTs were identified on the skin and oral mucosa of 81 patients (43 women, 38 men). At diagnosis of the first GCT, age ranged from 14 to 75 years, with a mean (SD) of 40.21 (14.982) years. The time between onset and diagnosis of the first GCT ranged from 1 to 120 months (mean, 19.02 [25.527] months), and the mean diameter was 1.34 (0.7255) cm.
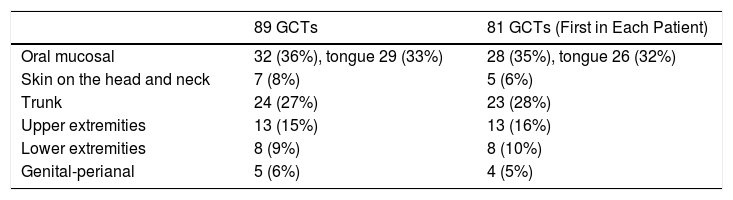
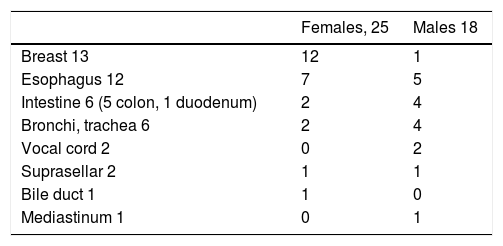
Seventy-eight patients had a single cutaneous-mucosal lesion, and 3 patients had multiple lesions on the skin and oral mucosa (1 patient had 2 lesions, 1 patient had 4 lesions, and 1 had 5 lesions). Table 1 shows the location of the 89 tumors diagnosed on the skin and oral mucosa and the location of the first cutaneous-mucosal lesion in each patient (81 tumors). The lesions were indurated to the touch in 34 cases and painful in 6 (7.4%). The most frequent clinical diagnoses were fibroma-histiocytoma (17 cases), cyst (14 cases), lipoma (4 cases), and GCT (4 cases). During the same period, 43 extracutaneous GCTs were diagnosed in the histopathology department (Table 2). One of the patients with multiple lesions on the skin or oral mucosa also had extracutaneous lesions on the breast; a further 2 patients with a single lesion on the skin or oral mucosa also had extracutaneous lesions (larynx and breast). When extracutaneous tumors were taken into consideration, 5 of the 81 patients had multiple GCTs (6.2%) (Table 3). The mean age of the patients with multiple GCTs at diagnosis of the first cutaneous-mucosal GCT was 21.60 (8.532) years compared with 41.43 (14.524) years for patients with a single GCT on the skin or oral mucosa (P = .004).
Location of Granular Cell Tumor (GCT) of the Skin and Oral Mucosa.
| 89 GCTs | 81 GCTs (First in Each Patient) | |
|---|---|---|
| Oral mucosal | 32 (36%), tongue 29 (33%) | 28 (35%), tongue 26 (32%) |
| Skin on the head and neck | 7 (8%) | 5 (6%) |
| Trunk | 24 (27%) | 23 (28%) |
| Upper extremities | 13 (15%) | 13 (16%) |
| Lower extremities | 8 (9%) | 8 (10%) |
| Genital-perianal | 5 (6%) | 4 (5%) |
Location of the 43 Extracutaneous Granular Cell Tumors.a
| Females, 25 | Males 18 | |
|---|---|---|
| Breast 13 | 12 | 1 |
| Esophagus 12 | 7 | 5 |
| Intestine 6 (5 colon, 1 duodenum) | 2 | 4 |
| Bronchi, trachea 6 | 2 | 4 |
| Vocal cord 2 | 0 | 2 |
| Suprasellar 2 | 1 | 1 |
| Bile duct 1 | 1 | 0 |
| Mediastinum 1 | 0 | 1 |
Two cutaneous neurofibromas were removed in 1 patient, although we did not detect any cases associated with neurofibromatosis or other RASopathies.
Removal of the GCT on the skin and oral mucosa was complete in 39 of the 59 cases for which we have information on the resection margins. Mean follow-up time in these 59 patients was 20.29 (27.704) months (range, 0-144 months). Local recurrence was recorded in only 1 patient.
Mean follow-up time was 17.05 (27.731) months (range, 0-144 months).
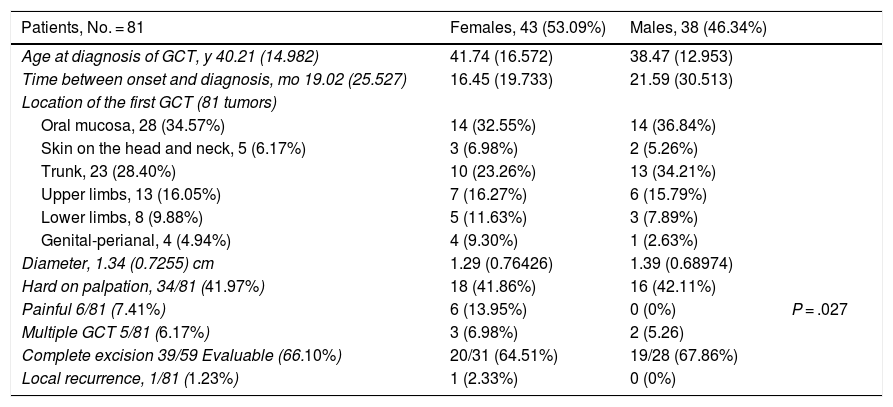
When the clinical characteristics of the 81 patients were compared by sex, statistically significant differences were only observed for painful GCTs, which were more common among females (P = .027) (Table 4).
Clinical Characteristics of the 81 Study Patients by Sex.
| Patients, No. = 81 | Females, 43 (53.09%) | Males, 38 (46.34%) | |
|---|---|---|---|
| Age at diagnosis of GCT, y 40.21 (14.982) | 41.74 (16.572) | 38.47 (12.953) | |
| Time between onset and diagnosis, mo 19.02 (25.527) | 16.45 (19.733) | 21.59 (30.513) | |
| Location of the first GCT (81 tumors) | |||
| Oral mucosa, 28 (34.57%) | 14 (32.55%) | 14 (36.84%) | |
| Skin on the head and neck, 5 (6.17%) | 3 (6.98%) | 2 (5.26%) | |
| Trunk, 23 (28.40%) | 10 (23.26%) | 13 (34.21%) | |
| Upper limbs, 13 (16.05%) | 7 (16.27%) | 6 (15.79%) | |
| Lower limbs, 8 (9.88%) | 5 (11.63%) | 3 (7.89%) | |
| Genital-perianal, 4 (4.94%) | 4 (9.30%) | 1 (2.63%) | |
| Diameter, 1.34 (0.7255) cm | 1.29 (0.76426) | 1.39 (0.68974) | |
| Hard on palpation, 34/81 (41.97%) | 18 (41.86%) | 16 (42.11%) | |
| Painful 6/81 (7.41%) | 6 (13.95%) | 0 (0%) | P = .027 |
| Multiple GCT 5/81 (6.17%) | 3 (6.98%) | 2 (5.26) | |
| Complete excision 39/59 Evaluable (66.10%) | 20/31 (64.51%) | 19/28 (67.86%) | |
| Local recurrence, 1/81 (1.23%) | 1 (2.33%) | 0 (0%) | |
Abbreviation: GCT, granular cell tumor.
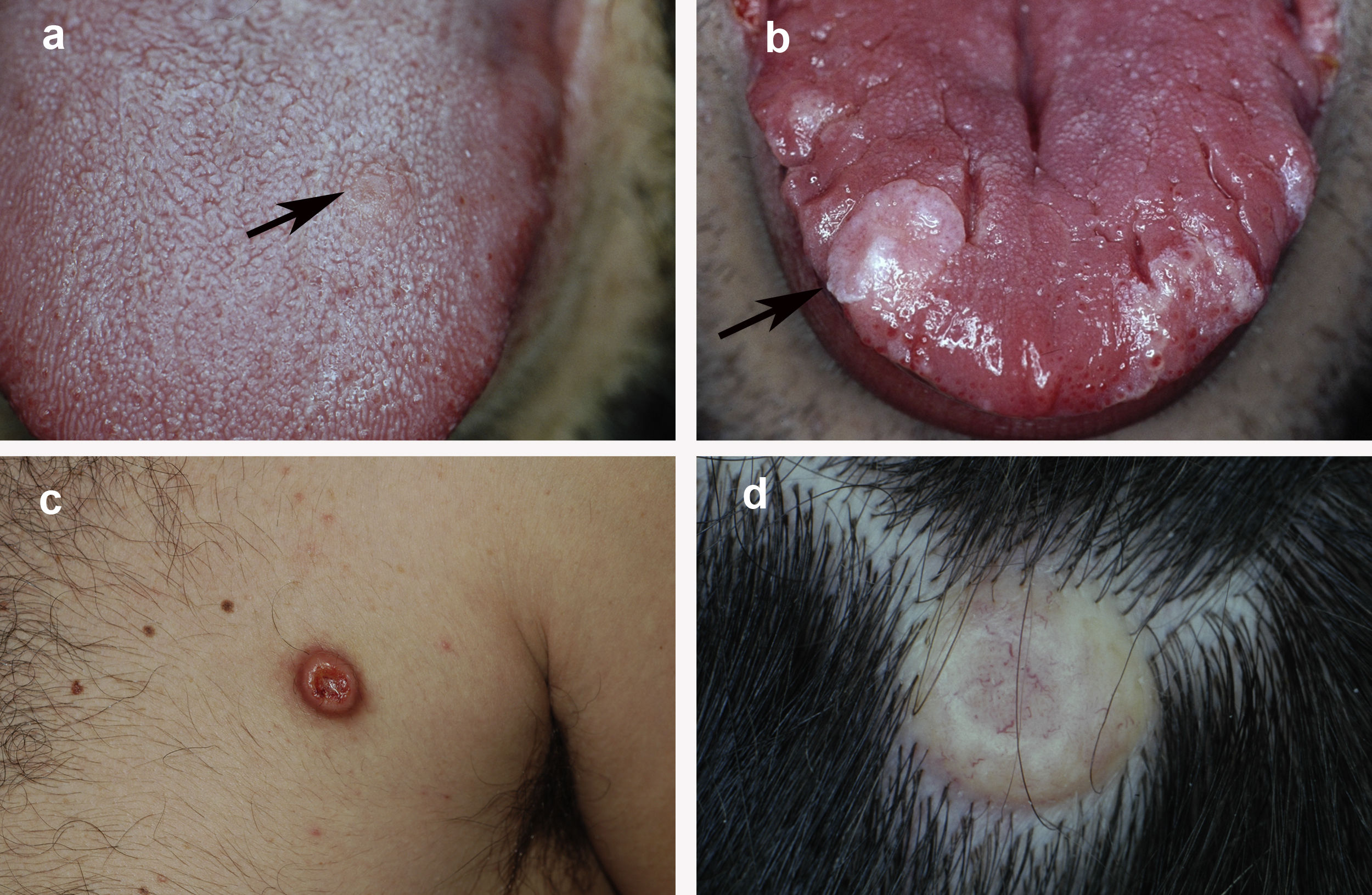
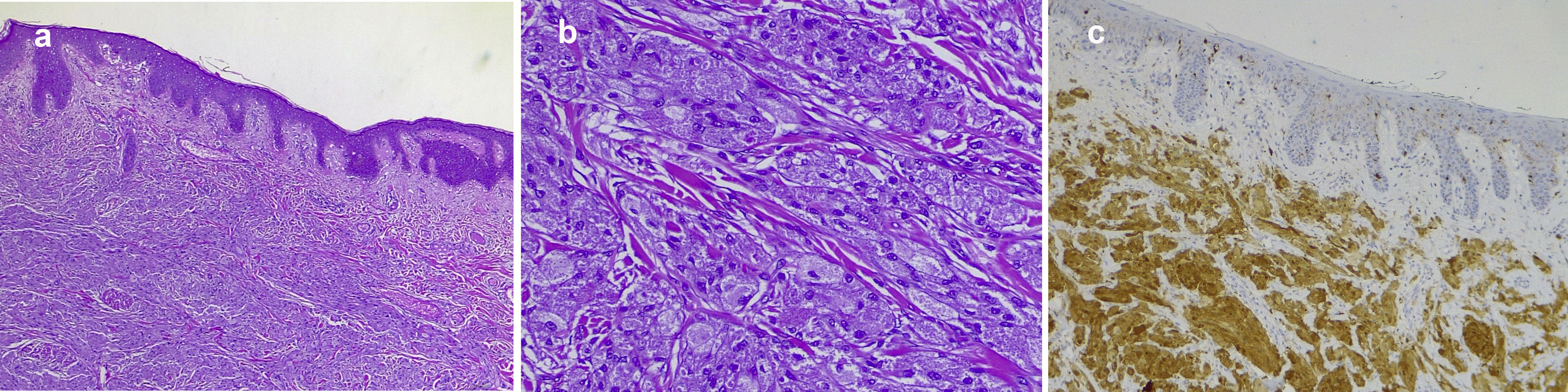
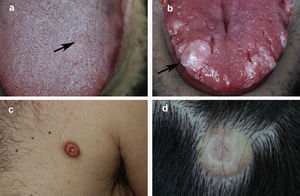
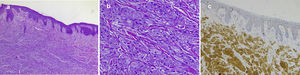
Fig. 1 shows the clinical appearance of the lesions on the tongue and trunk. Fig. 2 shows the histologic image of a typical GCT.
Clinical appearance of granular cell tumor in 4 patients: A, Incipient lesion on the tongue measuring 5 mm in diameter. B, Larger exophytic nodular lesion on the tongue. The lesion is whitish in color owing to the underlying epithelial hyperplasia. C, Nodular erythematous lesion located on the trunk. D, Nodular yellowish lesion on the scalp with telangiectasias.
Typical histopathology image of a cutaneous granular cell tumor: A, Low-magnification image showing dermal proliferation of cells with a granular cytoplasm and some overlying epidermal hyperplasia (hematoxylin-eosin, original magnification, ×40). B, High-magnification image showing detail of the granular cells (hematoxylin-eosin, original magnification, ×200); C, Positive immunohistochemistry staining for protein S100 in granular cells (staining for protein S100 × 100).
Various cell lines have been proposed as possible causes of GCT. Before immunohistochemistry staining, GCT cells were thought to be myoblasts, probably owing to the fact that in the case of tumors affecting the tongue, infiltration of the striated muscle bundles gave the impression of a muscular origin.2,5 Other cell strains were later proposed as potential causes of the tumor, including histiocytes, neuroendocrine cells, fibroblasts, and undifferentiated mesenchymal cells.1 While the histogenesis of GCT continues to be the subject of debate, there is currently much morphological, immunohistochemical, and ultrastructural evidence in support of Schwann cells as the origin.1
According to the literature, GCT usually develops between the fourth and sixth decades of life, although it can also appear in childhood.1,2 Percentages differ by sex depending on the series, and, while some authors suggest that it is more frequent in females,1,4,6 others have found it to be more common in males.2,7,8 A recent review of almost 1500 published cases revealed that 60.40% correspond to women.9 Our study revealed that GCT was also more frequent in women (53%) and that the mean age at diagnosis of the first GCT was 40.21 years.
GCT is found mainly on the skin and oral mucosa.3,6 The tumor is particularly common on the tongue.3,4,6,7 In our series, 33% of tumors were found on the tongue, followed by the trunk and limbs (Table 1). According to textbooks, GCT usually manifests clinically as a slow-growing round tumor with somewhat undefined margins measuring between 5 mm and 20 mm in diameter.2,4 It may have a verrucous appearance owing to epidermal hyperplasia.2 In the present series, the most commonly reported clinical characteristic was that the lesion was nodular and hard on palpation (34 cases). According to the literature, the color can vary between a normal flesh color and pink or grayish-brown.2 Curiously, the clinical history of 3 of the patients was remarkable for the yellowish appearance of the lesions (Fig. 1D); therefore, we believe that GCT should be included in the differential diagnosis of yellowish nodular lesions. While painful GCTs are considered unusual,2,5 6 of the 81 patients in the present series had painful tumors (7.4%).
As reported elsewhere, the most common presumptive clinical diagnosis in the present series was fibroma-histiocytoma (17 cases).7 Other commonly proposed diagnoses were cyst (14 cases) and lipoma (4). The diagnosis of GCT was suspected in 4 patients, and in all 4 cases the tumor was located on the tongue.
While GCT is usually a solitary lesion, there have been reports of multiple GCTs on the skin and oral mucosa and of cases of GCT on the skin and oral mucosa associated with GCTs affecting internal organs.2–4 The percentage of multiple GCTs varies widely between series (4%-30%).3 However, the highest percentages of multiple GCTs were reported in older series with a limited number of cases.10 In the present study, when the tumors affecting internal organs are included, 5 of 81 patients (6.2%) had multiple lesions (Table 3); this figure is similar to that recorded in a recent review of approximately 1500 cases published in the literature (7.14%).9
Multiple GCTs have been reported to be associated with diseases such as neurofibromatosis and Hodgkin lymphoma.3 They have also been reported in children in association with lentiginosis, congenital heart disease, café-au-lait spots, pulmonary stenosis, and cryptorchidism.4 In recent years, there have been various reports of multiple GCTs in Noonan syndrome,4,11 and, curiously, some of the previous associations are currently classified as RASopathies, together with Noonan syndrome, thus potentially indicating a common pathogenesis. RASopathies are a group of genetic diseases characterized by a mutation affecting the genes that encode proteins from the Ras family, which play a major role in cell differentiation and proliferation. The conditions included in this group are type 1 neurofibromatosis, Noonan syndrome, Legius syndrome, LEOPARD syndrome, and Costello syndrome.12 One of the patients in the present series had undergone removal of 2 cutaneous neurofibromas, although without diagnostic criteria for neurofibromatosis. Similarly, we did not detect any cases associated with Noonan syndrome or familial cases, as in another study carried out in Spain.7
GCTs may also affect internal organs, although this is less common.2,4 The extracutaneous locations reported include soft tissue, breast, thyroid, mediastinum, respiratory tract (larynx, trachea, lung), gastrointestinal tract, bile duct and pancreas, ovary-testicle, urinary tract, heart, and central nervous system.1 In our series, the breast was the most common extracutaneous location, with 13 cases. GCT typically affects the breast in the form of a painless, palpable nodular lesion, which can mimic breast carcinoma in terms of symptoms and radiologic findings.13 Imaging tests can be relatively nonspecific, although the lesion typically presents as a spiculated or infiltrating mass on the mammogram and as a poorly defined irregular mass with posterior acoustic shadowing in ultrasound.13 While GCTs can be benign, the location in the central nervous system is notable owing to its potential severity, as in 2 patients in the present series, who had suprasellar GCTs, that is, 4.7% of the extracutaneous tumors we report.
Histologically, GCT is a nonencapsulated tumor formed by large polyhedral cells with a small central hyperchromatic nucleus and a cytoplasm with abundant eosinophilic granules owing to the accumulation of secondary lysosomes in the cytoplasm.2,3 The cytoplasmic granules are periodic acid–Schiff–positive and diastase-resistant.3 Dermal tumors frequently extend to the superficial hypodermis.3 The tumor cells often reveal large eosinophilic granules surrounded by a transparent halo known as pustulo-ovoid bodies of Milian.2,3 One study reported the presence of these bodies in 47% of tumors, and the authors stated that the number increased with the age of the tumor.7 The overlying epithelium is often characterized by prominent pseudoepitheliomatous hyperplasia that could be confused with squamous carcinoma if the biopsy is taken superficially.2,3 In one series, overlying epidermal hyperplasia was detected in 58% of tumors.7 Immunohistochemistry staining is positive for protein S100, CD68 antigen, and specific neuronal enolase.3 Some tumors may be S100-negative and are known as nonneural GCTs.1,3 These tumors were recently reported to overexpress ALK and cyclin D1 and are probably a different entity.14
Malignant GCT is extremely rare. In fact, some authors only consider a case to be malignant if the tumor metastasizes3; according to this criterion, less than 2%-2.5% of GCT are malignant.1,9,15 Fanburg-Smith et al.16 proposed 6 criteria for classifying GCT as benign, atypical, or malignant as follows: necrosis, spindling, vesicular nuclei with large nucleoli, more than 2 mitotic figures per high-power field, high nuclear-to-cytoplasmic ratio, and pleomorphism. Tumors that did not fulfill any of these criteria were considered benign, those that fulfilled 1 or 2 criteria were considered atypical, and those that presented 3 or more were classed as malignant. Malignant tumors grow more quickly and can lead to metastasis, especially to regional lymph nodes, lungs, liver, and bone.1 In a recent study of 113 cases of malignant GCT, the mean age was 49.2 years, and the 5-year survival rate was 62.8%.17 No cases of malignant GCT were detected in our study.
Simple excision is the treatment of choice in benign GCT,2 although local recurrences are possible. As expected, recurrences are more common in cases with positive surgical margins, although some studies have also found local recurrences of resected tumors with tumor-free margins.6 We found that while excision was incomplete in 20 of 59 cases of GCT affecting the skin and oral mucosa for which information on surgical margins was available, only 1 local recurrence was detected.
ConclusionsAs in previous studies, the most common location of GCTs in the present series was the oral mucosa (especially the tongue) and skin, although the tumor can also affect internal organs. When GCTs affecting internal organs are taken into account, 5 of our patients had multiple GCTs (6.2%). While some cases have been associated with neurofibromatosis, Noonan syndrome, and other RASopathies, we believe that this finding is uncommon in the Spanish population, since we did not observe it in any of the patients in the present series. Most GCTs are benign, and despite having positive margins, they do not generally recur locally. However, the possibility of multiple GCTs on the skin and oral mucosa, as well as on internal organs, must be taken into account. Since patients with multiple tumors are younger at diagnosis of the first GCT than patients with a single lesion, it is important to remember that the probability of multiple lesions is greater in young patients.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Marcoval J, Bauer-Alonso A, Llobera-Ris C, Moreno-Vílchez C, Penín RM, Bermejo J. Tumor de células granulares. Estudio clínico de 81 pacientes. Actas Dermosifiliogr. 2021;112:441–446.