Epidermal necrolysis (EN) is a rare mucocutaneous reaction usually induced by drugs. It is known as Stevens-Johnson syndrome if <10% of the body surface area is affected and toxic epidermal necrolysis if >30% is affected. EN is associated with high mortality, which can be accurately predicted based on the score for toxic epidermal necrolysis (SCORTEN).1 There is consensus regarding patient hospitalization and management in a specialist intensive care unit, which is associated with improved survival.1 Treatments with corticosteroids, classical immunosuppressants, anti-tumor necrosis factor α (anti-TNF-α) agents, intravenous immunoglobulins (IVIG), and plasmapheresis have been described, despite a lack of clear evidence supporting their efficacy.1,2
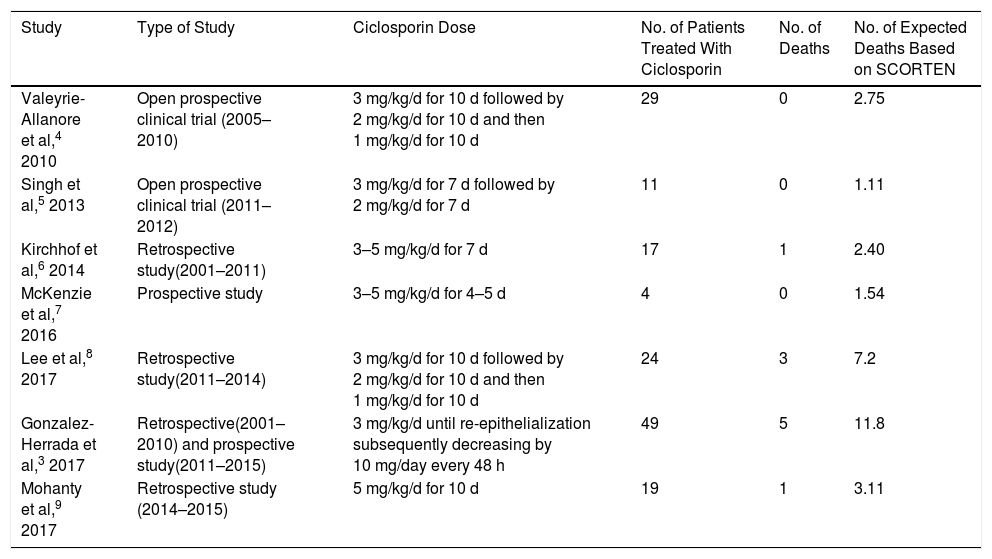
González-Herrada et al3 recently published the results of a study conducted in 2 burn units in Madrid. In the first unit, at the Hospital Universitario de Getafe (HUG), EN patients were mainly treated with ciclosporin (3 mg/kg/d) until re-epithelialization [Table 1]). In the second unit, at the Hospital Universitario La Paz (HULP), patients were mainly treated with IVIG without ciclosporin. These 2 units provided the necessary conditions for a “natural” clinical trial. The study population consisted of 71 EN patients aged 14 years and older. The authors performed 3 types of analysis. The first focused on 42 individuals living in Madrid who were treated at either HUG (n = 23) or HULP (n = 19). Of these, 22 and 4, respectively, were treated with ciclosporin. One patient (4.3%) died at HUG and 6 (31.6%) at HULP. When hospital allocation was included in the analysis as an instrumental variable, the risk of mortality was significantly lower for ciclosporin-treated patients (0.09; 95% confidence interval [95% CI], 0.00–0.49). Next, the authors analyzed a group of 71 patients living in Madrid and elsewhere, 49 of whom were treated with ciclosporin. The mortality rate for this group was significantly lower than that expected based on the SCORTEN (0.42; 95% CI, 0.14–0.99). Finally, the authors performed a meta-analysis of 6 studies involving 134 ciclosporin-treated EN patients (including 49 from the present series). The results revealed that the risk of mortality for this group was significantly lower than that expected based on the SCORTEN (0.41; 95% CI, 0.21–0.80]). The findings indicate that 1 life could be saved by treating only 5.6 EN patients with ciclosporin. While no data on the adverse effects of ciclosporin treatment were available, none of the patients discontinued treatment.
Summary of Studies of Ciclosporin Treatment of Epidermal Necrolysis (Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis).
| Study | Type of Study | Ciclosporin Dose | No. of Patients Treated With Ciclosporin | No. of Deaths | No. of Expected Deaths Based on SCORTEN |
|---|---|---|---|---|---|
| Valeyrie-Allanore et al,4 2010 | Open prospective clinical trial (2005–2010) | 3 mg/kg/d for 10 d followed by 2 mg/kg/d for 10 d and then 1 mg/kg/d for 10 d | 29 | 0 | 2.75 |
| Singh et al,5 2013 | Open prospective clinical trial (2011–2012) | 3 mg/kg/d for 7 d followed by 2 mg/kg/d for 7 d | 11 | 0 | 1.11 |
| Kirchhof et al,6 2014 | Retrospective study(2001–2011) | 3–5 mg/kg/d for 7 d | 17 | 1 | 2.40 |
| McKenzie et al,7 2016 | Prospective study | 3–5 mg/kg/d for 4–5 d | 4 | 0 | 1.54 |
| Lee et al,8 2017 | Retrospective study(2011–2014) | 3 mg/kg/d for 10 d followed by 2 mg/kg/d for 10 d and then 1 mg/kg/d for 10 d | 24 | 3 | 7.2 |
| Gonzalez-Herrada et al,3 2017 | Retrospective(2001–2010) and prospective study(2011–2015) | 3 mg/kg/d until re-epithelialization subsequently decreasing by 10 mg/day every 48 h | 49 | 5 | 11.8 |
| Mohanty et al,9 2017 | Retrospective study (2014–2015) | 5 mg/kg/d for 10 d | 19 | 1 | 3.11 |
Abbreviation: SCORTEN, score for toxic epidermal necrolysis.
In their meta-analysis of the efficacy of various immunomodulators in the treatment of EN, Zimmermann et al2 reported beneficial effects of ciclosporin (odds ratio [OR], 0.1; 95% CI, 0.0–0.4) and a discrete benefit of corticosteroid treatment (OR, 0.7; 95% CI, 0.5–0.97). Mohanty et al9 recently published the results of a retrospective study (not included in the aforementioned meta-analysis) of 28 EN patients treated with either ciclosporin (n = 19) or with supportive treatment only (n = 9). One patient in the ciclosporin group and 5 in the supportive treatment group died (SCORTEN, 3.11 and 4.73, respectively). The risk of mortality was significantly higher in the supportive treatment group (2.13; 95% CI, 1.02–4.46]).
Early withdrawal of the causative drug and the implementation of advanced support measures are paramount in the treatment of EN. Based on the available evidence (Table 1), we believe that early ciclosporin treatment is justified. It is imperative that these interventions are carried out quickly: measures not adopted within the first 7 days will be ineffective due to the natural progression of the disease.1
Please cite this article as: Morgado-Carrasco D, Fustà-Novell X, Iranzo P. FR-Ciclosporin as a First-Line Treatment in Epidermal Necrolysis. Actas Dermosifiliogr. 2019;110:601–603.