Researchers the world over are working to find the treatments needed to reduce the negative effects of coronavirus disease 2019 (COVID-19) and improve the current prognosis of patients. Several drugs that are often used in dermatology are among the potentially useful treatments: ivermectin, antiandrogenic agents, melatonin, and the antimalarial drugs chloroquine and hydroxychloroquine. These and other agents, some of which have proven controversial, are being scrutinized by the scientific community. We briefly review the aforementioned dermatologic drugs and describe the most recent findings relevant to their use against COVID-19.
Frente a la necesidad de encontrar una alternativa terapéutica que logre disminuir el impacto negativo de la COVID-19 y mejore el pronóstico actual de los pacientes, investigadores de todo el mundo se esfuerzan por aportar información que nos acerque a esta meta. Dentro de los potenciales farmacos, existen algunos de uso frecuente en dermatología: los antipalúdicos (cloroquina e hidroxicloroquina), la ivermectina, los antiandrógenos y la melatonina. Tanto estos como otros tratamientos se encuentran en la mira de la comunidad científica, siendo algunos foco de polémica y controversia. En el presente trabajo relizamos una revisión breve de los fármacos previamente mencionados, presentando los más recientes hallazgos en relación a su uso en la COVID-19.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the agent responsible for the current coronavirus disease 2019 (COVID-19) pandemic, is an RNA virus that is closely related to severe acute respiratory syndrome coronavirus (SARS-CoV).
Although knowledge of COVID-19 increases daily, many aspects of its pathophysiology remain unknown. Based on currently available knowledge, a therapeutic plan has been proposed consisting of 3 courses of action: reduction of viral replication, hypercoagulability, and the uncontrolled inflammatory response. To this end, pending a definitive cure, the use of various drugs commonly used in dermatology, including antimalarials (AMs), antiparasitics, and antiandrogens, has been proposed, and several have been used therapeutically. In this review, we discuss the history and mechanism of action of these drugs, as well as their therapeutic potential in COVID-19 patients (Fig. 1; Table 1).
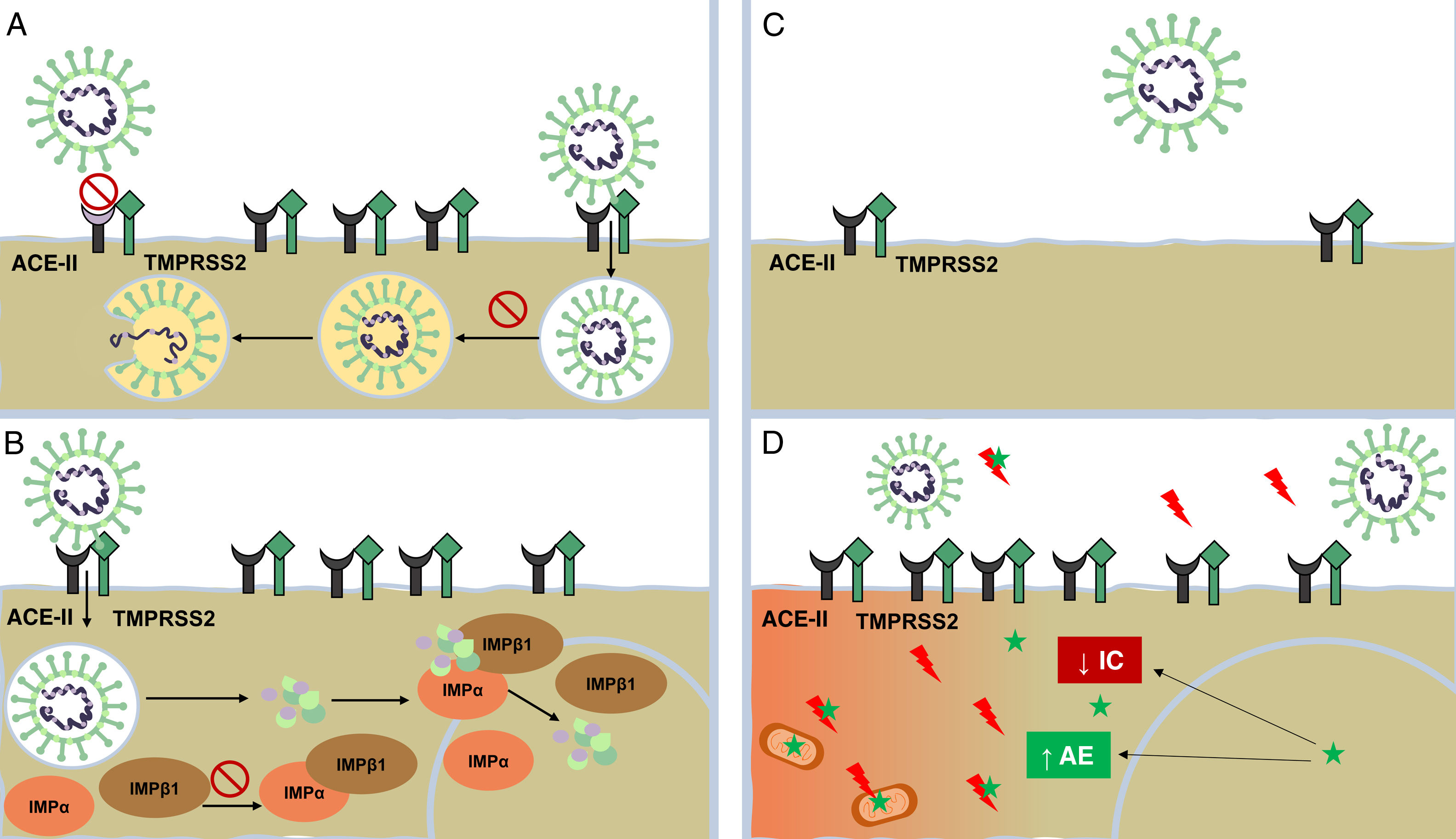
Mechanism of action of dermatologic drugs against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). A, Antimalarials (AMs) interfere with terminal glycosylation of angiotensin-converting enzyme II (ACE-II) receptor, reducing its affinity for SARS-CoV-2 and thereby hindering viral penetration. AMs also alter endocytosis and proteolysis by alkalizing the endosome and endocytic vesicles. B, Ivermectin blocks the formation of importin (IMP) α/β1, preventing transport of viral proteins to the cell nucleus. C, Antiandrogens decrease the expression of ACE-II receptor and transmembrane serine protease 2 (TMPRSS2) in the cell membrane.
D, Melatonin (green star) exerts a powerful antioxidant effect (with a marked protective effect on mitochondria and the cell nucleus) by directly blocking free radicals (red lightning bolts) and increasing the expression of antioxidant enzymes (AEs). It has an anti-inflammatory effect owing to its ability to block the action of nuclear factors capable of increasing inflammatory cytokine (IC) production.
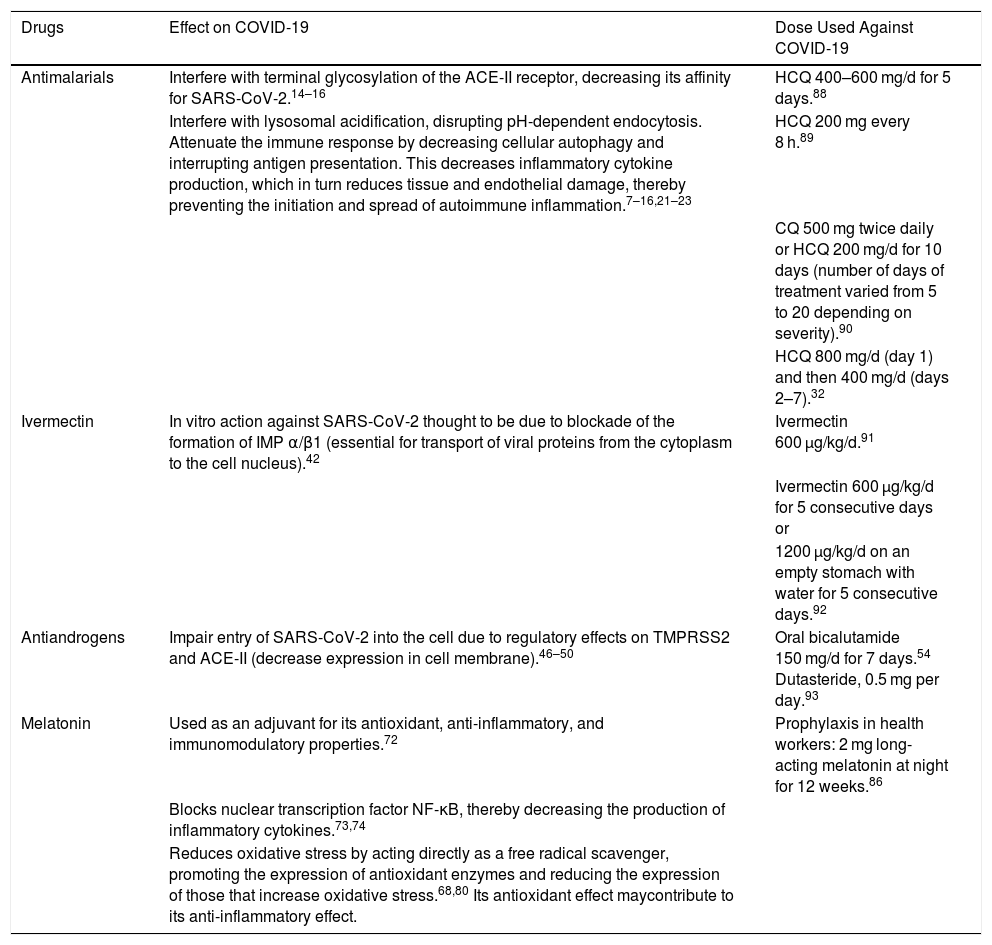
Drugs Used Against COVID-19: Mechanisms of Action and Doses Used in Studies
| Drugs | Effect on COVID-19 | Dose Used Against COVID-19 |
|---|---|---|
| Antimalarials | Interfere with terminal glycosylation of the ACE-II receptor, decreasing its affinity for SARS-CoV-2.14–16 | HCQ 400–600 mg/d for 5 days.88 |
| Interfere with lysosomal acidification, disrupting pH-dependent endocytosis. Attenuate the immune response by decreasing cellular autophagy and interrupting antigen presentation. This decreases inflammatory cytokine production, which in turn reduces tissue and endothelial damage, thereby preventing the initiation and spread of autoimmune inflammation.7–16,21–23 | HCQ 200 mg every 8 h.89 | |
| CQ 500 mg twice daily or HCQ 200 mg/d for 10 days (number of days of treatment varied from 5 to 20 depending on severity).90 | ||
| HCQ 800 mg/d (day 1) and then 400 mg/d (days 2–7).32 | ||
| Ivermectin | In vitro action against SARS-CoV-2 thought to be due to blockade of the formation of IMP α/β1 (essential for transport of viral proteins from the cytoplasm to the cell nucleus).42 | Ivermectin 600 µg/kg/d.91 |
| Ivermectin 600 µg/kg/d for 5 consecutive days or | ||
| 1200 µg/kg/d on an empty stomach with water for 5 consecutive days.92 | ||
| Antiandrogens | Impair entry of SARS-CoV-2 into the cell due to regulatory effects on TMPRSS2 and ACE-II (decrease expression in cell membrane).46–50 | Oral bicalutamide 150 mg/d for 7 days.54 Dutasteride, 0.5 mg per day.93 |
| Melatonin | Used as an adjuvant for its antioxidant, anti-inflammatory, and immunomodulatory properties.72 | Prophylaxis in health workers: 2 mg long-acting melatonin at night for 12 weeks.86 |
| Blocks nuclear transcription factor NF-κB, thereby decreasing the production of inflammatory cytokines.73,74 | ||
| Reduces oxidative stress by acting directly as a free radical scavenger, promoting the expression of antioxidant enzymes and reducing the expression of those that increase oxidative stress.68,80 Its antioxidant effect maycontribute to its anti-inflammatory effect. |
Abbreviations: ACE-II, angiotensin-converting enzyme II; COVID-19, coronavirus disease 2019; CQ, chloroquine; HCQ, hydroxychloroquine; IMP, importin; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; TMPRSS2, transmembrane serine protease 2.
Antimalarials are drugs traditionally used to treat malaria, and are widely used in dermatology owing to their anti-inflammatory, immunomodulatory, and photoprotective effects, as well as their efficacy as corticosteroid-sparing agents. The main dermatological conditions for which these drugs are used include cutaneous lupus, polymorphous light eruption, and porphyria cutanea tarda.
HistoryCinchona is a native tree of South America, the bark of which was used by the indigenous peoples for its antipyretic properties. A well-known story tells of its use in 1630 to treat fever in the Spanish countess of Chinchón (wife of the viceroy of Peru), who in turn introduced it to Europe in 1640. However, some researchers believe that it was brought to Europe much earlier by Jesuit missionaries.1–4
At that time malaria was a common disease in Europe, and a cause of death among peasants, emperors, and kings alike. Although the cinchona preparation was therapeutically successful, its use was not initially accepted.1,3,5 However, some were convinced of its effectiveness and sought to promote its use as a treatment for malaria. One of the most fervent proponents was Robert Talbor, who used the preparation to cure King Charles II and the son of Louis XIV. This secret ingredient wasn’t revealed until after Talbor’s death.1–3,5
In 1820, Pierre Pelletier and Joseph Caventou isolated quinine, the active compound and first natural antimalarial, from the bark of the cinchona tree. The preparation was essential to prevent and treat malaria in soldiers posted to endemic areas.6,7
In 1894, Payne described lupus rash, which he believed to be a vascular disorder and treated with quinine to induce pallor, with excellent results.8 In 1940, improvements in lupus and rheumatoid arthritis were observed in soldiers treated with quinacrine, a synthetic derivative of quinine used for malaria prophylaxis during World War II.9,10 In 1951, based on these observations, Page successfully treated 18 cutaneous lupus patients with quinacrine. The work was published in The Lancet in 1951 and stimulated interest in the anti-inflammatory properties of AMs.11
Chloroquine (CQ) was synthesized in 1934 and hydroxychloroquine (HCQ) in 1955. Lupus patients were treated with quinacrine (Atabrine) in clinical trials up until 1961, after which the drug was substituted with CQ, which was better tolerated. Nowadays, CQ remains the antimalarial agent of choice, except in areas of the world where resistance has developed.12,13
Mechanism of actionA 2005 study of SARS-CoV-infected primate cells reported a marked antiviral effect of CQ against the virus.14 AMs interfere with terminal glycosylation of the angiotensin-converting enzyme II (ACE-II) receptor (used by SARS-CoV to enter the host cell), impairing receptor binding by the virus. Because they are weak bases, AMs concentrate in organelles with an acidic pH, increasing the pH and interfering with infection caused by SARS-CoV, which uses endosomes to enter the host cell.14–16 SARS-CoV-2 is also thought to use the ACE-II receptor to enter the host cell; the amino acid sequence of the receptor binding site on SARS-CoV-2 shares 74% homology with that of SARS-CoV, suggesting that the 2 viruses have similar or even identical cellular entry mechanisms.17–20 Colson and coworkers19 reported that the SARS-CoV-2 spike protein is cleaved in the autophagosome by host cell proteases such as cathepsins, which can be inhibited by increases in lysosomal pH caused by CQ accumulation.21
Increases in the levels of the cytokines interleukin (IL) 6 and IL-10 have been reported in some patients with COVID-19, in some cases progressing to create a cytokine storm followed by life-threatening multi-organ failure.22,23 HCQ and CQ have immunomodulatory effects that could suppress the uncontrolled immune response to SARS-CoV-2.24–26
Yao et al27 have proposed HCQ as the ideal drug with which to treat SARS-CoV-2, thanks to its antiviral and immunomodulatory effects. The authors recommend concomitant treatment with low-dose HCQ and an anti-inflammatory to help mitigate the cytokine storm in critically ill patients.
Studies in COVID-19Studies of AMs have mainly focused on HCQ, which is better tolerated, has a better safety profile, and shows more potent antiviral activity in vitro against SARS-CoV-2 compared with CQ.26,27
An observational study conducted between March and April 2020 in New York in COVID-19 patients treated with HCQ (loading dose 600 mg 2 times on day 1, followed by 400 mg/d for 4 d) observed no association between the use of HCQ and intubation or death in hospitalized patients.28 Mehra et al29,30 analyzed data from the national registry of patients hospitalized for COVID-19 in 671 different hospitals between December 2019 and April 2020. The authors were unable to demonstrate beneficial effects of HCQ or CQ administered alone or in combination with macrolides. The pharmacological regimens described were associated with a decrease in hospital survival and a higher frequency of ventricular arrhythmias. However, 3 of the study’s 4 authors subsequently retracted the results owing to doubts about the veracity of the data and the analyses carried out by Surgisphere Corporation.
Although multiple authors have ruled out the therapeutic use of AMs during this pandemic, Mitjà et al (March 2020) proposed its prophylactic use. However, they failed to demonstrate that its use was effective in preventing SARS-CoV-2 infection or disease in healthy individuals exposed to those who tested positive by polymerase chain reaction (PCR).31,32
Million et al (May 2020) conducted a meta-analysis of the effect of CQ and its derivatives in patients with COVID-19, and obtained conflicting findings: marked clinical improvement and increased mortality. The authors concluded that CQ derivatives decrease mortality in COVID-19 patients and expressed concern about the amount of misinformation circulating, warning that some studies lack basic definitions of treatment and are linked to conflicts of interest. They recommended against making hasty conclusions and urged caution when interpreting the results of the numerous studies published every day.33,34
IvermectinIvermectin is an antiparasitic widely used in humans worldwide. In dermatology it is frequently used to treat diseases such as scabies, demodicosis, and pediculosis, and it is currently part of the pharmacological arsenal used in the management of rosacea.
HistoryIn 1973, Satoshi Ōmura, a microbiologist at the Kitasato Institute in Tokyo, collected soil samples from parts of Japan in search of antibacterial compounds. He selected cultures with potential medical properties and sent them to Merck, Sharp and Dohme (MSD) laboratories in New Jersey, where his collaborator William Campbell evaluated their antiparasitic effects. In 1974, a culture from a sample collected near a golf course in Kawana (Tokyo) showed remarkable effects against helminths. The bacteria isolated in the culture was Streptomyces avermectinius. Avermectin, the active compound obtained from the culture, was chemically modified to produce ivermectin, which in turn was marketed for use in animals in 1981.35–40
When ivermectin was found to be safe and effective, MSD in conjunction with the World Health Organization, the Special Program for Research and Training in Tropical Diseases (TDR), and the Onchocerciasis Control Program (OCP) launched a study of its potential for use in humans (1982). It was approved for use in humans in 1987 and helped to successfully combat onchocerciasis in African countries.35,41
In 2015, the Nobel Prize in Physiology or Medicine was awarded to its discoverers, Ōmura and Campbell.
Mechanism of actionSeveral studies show that ivermectin possesses broad-spectrum antiviral activity. Its effect has been demonstrated in vitro against certain flaviviruses, chikungunya virus, and human immunodeficiency virus. The exact mechanism underlying this effect has not yet been elucidated, but it is thought to be mediated by action at importin (IMP) α/β1, a viral protein transporter that plays a fundamental role in some RNA viruses. Ivermectin is capable of dissociating or preventing the formation of the preformed IMP α/β1 heterodimer, thereby blocking transport of viral proteins to the host cell nucleus.42
Studies in COVID-19Patel et al (January 2020) conducted a study that included COVID-19 patients from 169 hospitals located in North America, Europe, and Asia. The study included 704 patients treated with ivermectin (150 µg/kg) and 704 controls, and found a significantly lower mortality rate in patients treated with ivermectin. The preprint of this study is not currently available, as the authors have apparently retracted their findings due to inconsistencies in the data obtained from Surgisphere.
An in vitro study by Caly et al (March 2020) reported a 99.98% reduction in SARS-CoV-2 viral RNA in Vero-hSLAM cell cultures 48 hours after treatment with 5 µM ivermectin. The mean inhibitory concentration of ivermectin was estimated at approximately 2 µM and the authors reported no toxicity at any of the concentrations evaluated.42 However, Momekov and Momekova (May 2020) warned that the in vitro inhibitory concentrations reported in this study, and especially the 5 µmol/L dose, are practically unachievable using the dosage regimens described in humans to date.43
Therefore, despite the fact that ivermectin shows important benefits, clinical trials and other studies demonstrating its effectiveness are still lacking.
AntiandrogensAndrogens play an important role in metabolic homeostasis and reproductive health in both men and women, and have important physiological effects on the skin. They can also contribute to the development of certain skin diseases, including acne, androgenetic alopecia, hirsutism, and hidradenitis suppurativa.44
Some drugs with antiandrogenic effects include cyproterone acetate, spironolactone, dutasteride, finasteride, and flutamide.
HistoryDrug development began in 1962 with the steroidal antiandrogens cyproterone acetate, chlormadinone acetate, megestrol acetate, and dienogest.
Flutamide was the first nonsteroidal antiandrogen approved by the Food and Drug Administration for prostate cancer. This first-in-class drug serves as the structural basis for other nonsteroidal antiandrogens such as enzalutamide and apalutamide.45,46
In 1970, Dorfman defined antiandrogens as substances that prevent the expression of androgen activity.
Mechanism of actionThe relationship between COVID-19 and androgens may be explained by the mechanism by which SARS-CoV-2 enters host cells. Androgens are implicated in the activity of transmembrane serine protease 2 (TMPRSS2) and the ACE-II receptor, both of which are critical for the entry of SARS-CoV-2 into human cells. Although other proteases are also involved, TMPRSS2 is of particular interest; it is an androgen-regulated gene, and has been previously studied for its role in prostate cancer.46–50
Studies in COVID-19Numerous studies have demonstrated more frequent complications, more severe disease courses, and higher rates of mortality caused by SARS-CoV-2 in men than women, although it is unclear whether this is due to biological factors, habits, or differences in comorbidity rates.51 These observations have prompted the scientific community to identify the factors that predispose men to a more severe clinical picture. Some researchers propose that androgens exert a crucial influence on the severity of COVID-19, as evidenced by a higher viral load, greater SARS-CoV-2 dissemination, and more severe lung involvement in male patients with a hyperandrogenic phenotype. These effects are most likely related to the aforementioned mechanism of action.48
Goren et al (March 2020) studied the prevalence of androgenetic alopecia (AA) in patients hospitalized for COVID-19 in Spain. Of 41 male patients, 29 (71%) were diagnosed with clinically significant AA.52 The authors plan to follow up on this preliminary observation and address certain limitations of their study in a clinical trial to evaluate the correlation between androgenetic alopecia and COVID-19 severity.52
Montopoli et al (April 2020) found that prostate cancer patients receiving androgen deprivation therapy (ADT) had a significantly lower risk of SARS-CoV-2 infection than patients not receiving ADT (odds ratio [OR], 4.05; 95% confidence interval [CI], 1.55–10.59). An even greater difference was observed when comparing prostate cancer patients receiving ADT with patients with any other type of cancer (OR, 4.86; 95% CI, 1.88–12.56). The authors finally concluded that ADT partially protected prostate cancer patients from SARS-CoV-2 infection.53
A study of cardiac cells derived from human embryonic stem cells highlighted the ability of 5 alpha-reductase inhibitors to reduce ACE-II levels and, therefore, decrease viral internalization.47
Clinical trials evaluating the effects of antiandrogens and hormonal therapy in COVID-19 are currently underway.54,55
MelatoninMelatonin is a derivative of the amino acid tryptophan. It is the main neuroendocrine product of the pineal gland. Until recently it was thought to exclusively regulate circadian rhythm and seasonal biorhythms. We now know that this hormonal role is merely the tip of the iceberg, and that melatonin has anti-inflammatory, antioxidant, immunomodulatory, thermoregulatory, and antitumor properties, among others, suggesting promising potential despite its current limitation to the treatment of dermatological conditions.56–58
HistoryTo understand the history of melatonin, we must discuss its discoverer, Aaron Lerner, an internationally renowned dermatologist and founder and chair of the Yale Department of Dermatology. He received numerous awards and recognitions for his dermatological research, but above all is known as the discoverer of melatonin.
Lerner was always interested in skin pigmentation and wrote an article with Thomas Fitzpatrick in which they described the biochemistry of melanin formation and pigmentation.59 The article was published in the journal Physiological Reviews and established both authors as world experts on the subject.
Lerner conducted a study in patients with Addison disease in an attempt to identify the pituitary factor responsible for the hyperpigmentation characteristic of this pathology and, with his colleagues, discovered melanocyte-stimulating hormone.
Lerner and his coworkers came to Yale in 1955. While waiting for their laboratory to be completed, a member of his team named Yoshiyata Takahashi came across a 1917 study that described how pineal extracts from cows could lighten the color of tadpoles. Lerner and his team decided to investigate whether this pineal factor was an already known molecule or something else. The study lasted 4 years and culminated with the discovery of melatonin in 1958.60–62
In 1964, Marczynski demonstrated that melatonin is a sleep promoter.63
Mechanism of action and use in COVID-19Initially, COVID-19 impairs the host’s immune response. SARS-CoV-2 manages to evade the innate immune response and induces apoptosis while decreasing T lymphocyte numbers. Later, accelerated viral replication leads to cell death and endothelial and vascular damage. This gives rise to massive release of inflammatory cytokines (the so-called cytokine storm), which recruit and activate non-infected immune cells, generating an excessive systemic inflammatory response with severe pulmonary repercussions.64
Although melatonin is not a virucidal drug, it has been postulated as a useful means of mitigating the deleterious effects of Ebola, dengue, encephalomyocarditis, and Venezuelan equine encephalitis viruses, among others.65–71
The proposed use of melatonin as an adjuvant is based mainly on its ability to reduce the negative impact of the uncontrolled immune response induced by SARS-CoV-2 and to regulate and interrupt certain signaling pathways activated by this virus. Based on the pathophysiology of the disease caused by SARS-CoV-2 and other viruses, Zhang et al (2020) proposed that melatonin may be useful for the treatment of COVID-19 owing to its antioxidant, anti-inflammatory, and immunomodulatory properties.72
A notable anti-inflammatory effect of melatonin is its ability to block nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), which promotes inflammation through the production of inflammatory cytokines that in turn exacerbate and maintain systemic inflammation in COVID-19.73,74 Melatonin reduces IL-6 levels in type 2 diabetes mellitus patients with chronic periodontitis and in patients with multiple sclerosis, among other diseases.75–77 Not only is IL-6 an important mediator of the cytokine storm, but its levels have been closely correlated with the severity and outcome of acute respiratory distress syndrome and with SARS-CoV-2 viral load in the blood, suggesting that reductions in IL-6 levels could benefit patients in the hyperimmune phase of COVID-19.78
The pro-inflammatory stage of COVID-19 is characterized by the production of an excess of reactive oxygen species (ROS) and free radicals (FR), which are harmful to the body. Melatonin interacts directly with ROS, acting as a kind of FR scavenger. In addition, it promotes the expression of enzymes with antioxidant activity and reduces the expression of those that increase oxidative stress.79,80 The antioxidant effect of melatonin may also contribute to its anti-inflammatory effect. These and other aspects of the use of melatonin in COVID-19 were further investigated by Zhang et al (March 2020) in a study that was praised by several authors who have supported the use of melatonin in COVID-19.81,82
Other authors have emphasized the importance of including antioxidant agents in COVID-19 treatments in order to reduce the number of deaths due to cardiac complications, in which oxidative stress plays a fundamental role.83,84
The use of melatonin in conjunction with vitamin D, with which it would exert a powerful synergistic effect, was recently proposed.85
Melatonin is an inexpensive and safe drug with potentially beneficial effects in COVID-19. Currently, there are 2 clinical trials underway investigating the use of melatonin to treat COVID-19. The first aims to evaluate the efficacy of melatonin for COVID-19 prophylaxis in health workers and the second is examining the efficacy and safety of intravenous melatonin in COVID-19 patients in intensive care units.86,87
ConclusionIt is important to remember that there are many other potential drug candidates to combat COVID-19. In this review we focus exclusively on those frequently used in dermatology. These drugs, which are very different from one other, with apparently unrelated mechanisms of action, could nonetheless be useful. Some (eg AMs and ivermectin) have previously been used to treat viral diseases, while others (eg antiandrogens and melatonin) have gone somewhat unnoticed by the media, given their limited use to treat specific diseases.
Given the biochemical profile of melatonin and the massive impact that the current pandemic has had on the global economy, it seems important to conduct more clinical trials to further investigate its potential for the treatment of COVID-19.
An extensive review of the literature available on COVID-19 has shown us that there is an abundance of information available. However, despite the urgency of the situation, it is essential to maintain the quality and meticulousness of scientific studies, as hasty conclusions based on poorly designed studies delay and hinder the scientific process. For their part, readers can and should critically analyze the studies that are published on a daily basis.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Ortega-Peña M, González-Cuevas R. Fármacos de uso frecuente en dermatología como terapia para COVID-19. Actas Dermosifiliogr. 2021;112:118–126.