Epidermal nevi are hamartomatous lesions derived from the epidermis and/or adnexal structures of the skin; they have traditionally been classified according to their morphology. New variants have been described in recent years and advances in genetics have contributed to better characterization of these lesions and an improved understanding of their relationship with certain extracutaneous manifestations. In the second part of this review article, we will look at nevi derived from the adnexal structures of the skin and associated syndromes.
Los nevus epidérmicos son hamartomas originados en la epidermis y/o en las estructuras anexiales de la piel que se han clasificado clásicamente partiendo de la morfología. En los últimos años se han descrito variantes nuevas y se han producido avances en el campo de la genética que han permitido caracterizar mejor estas lesiones y comprender su relación con algunas de las manifestaciones extracutáneas a las que se han asociado. En esta segunda parte revisaremos los nevus derivados de estructuras anexiales de la piel y los síndromes que se asocian.
In this second part, we will review hamartomas derived from sebaceous glands, hair follicles, and apoccrine and eccrine sweat glands. Finally, we will discuss Becker nevus. Table 1 summarizes the best characterized syndromes to date associated with these adnexal nevi.
Some of the Best Characterized Syndromes Associated with Nevi Derived From Adnexal Structures (Organoid).
| Syndrome | Type of Nevus | Other Manifestations | Gene Involved (Transmission) | Reference |
|---|---|---|---|---|
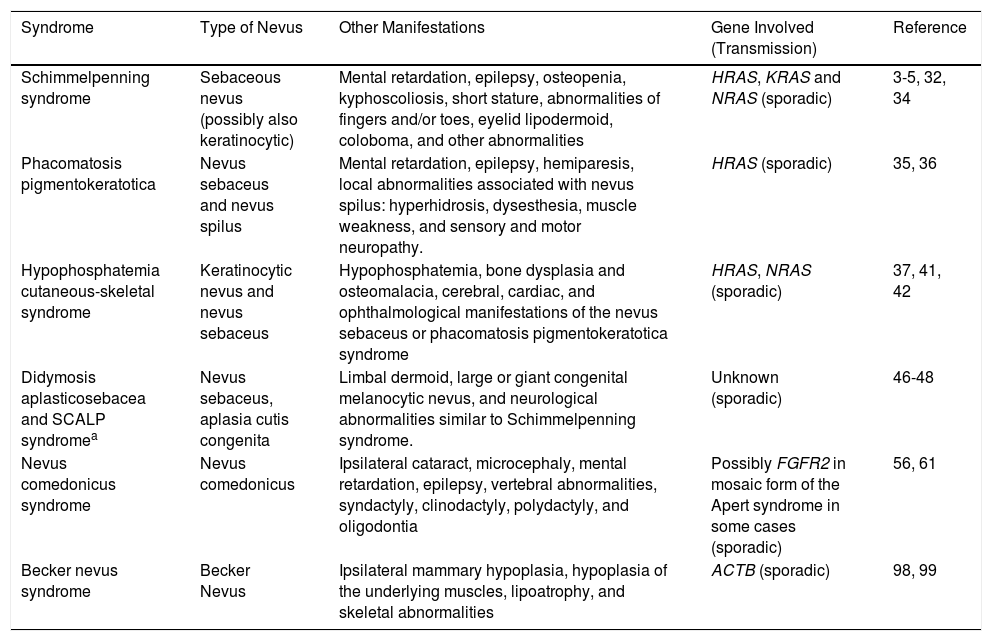
| Schimmelpenning syndrome | Sebaceous nevus (possibly also keratinocytic) | Mental retardation, epilepsy, osteopenia, kyphoscoliosis, short stature, abnormalities of fingers and/or toes, eyelid lipodermoid, coloboma, and other abnormalities | HRAS, KRAS and NRAS (sporadic) | 3-5, 32, 34 |
| Phacomatosis pigmentokeratotica | Nevus sebaceus and nevus spilus | Mental retardation, epilepsy, hemiparesis, local abnormalities associated with nevus spilus: hyperhidrosis, dysesthesia, muscle weakness, and sensory and motor neuropathy. | HRAS (sporadic) | 35, 36 |
| Hypophosphatemia cutaneous-skeletal syndrome | Keratinocytic nevus and nevus sebaceus | Hypophosphatemia, bone dysplasia and osteomalacia, cerebral, cardiac, and ophthalmological manifestations of the nevus sebaceus or phacomatosis pigmentokeratotica syndrome | HRAS, NRAS (sporadic) | 37, 41, 42 |
| Didymosis aplasticosebacea and SCALP syndromea | Nevus sebaceus, aplasia cutis congenita | Limbal dermoid, large or giant congenital melanocytic nevus, and neurological abnormalities similar to Schimmelpenning syndrome. | Unknown (sporadic) | 46-48 |
| Nevus comedonicus syndrome | Nevus comedonicus | Ipsilateral cataract, microcephaly, mental retardation, epilepsy, vertebral abnormalities, syndactyly, clinodactyly, polydactyly, and oligodontia | Possibly FGFR2 in mosaic form of the Apert syndrome in some cases (sporadic) | 56, 61 |
| Becker nevus syndrome | Becker Nevus | Ipsilateral mammary hypoplasia, hypoplasia of the underlying muscles, lipoatrophy, and skeletal abnormalities | ACTB (sporadic) | 98, 99 |
These are organoid lesions that contain epidermal, follicular, sebaceous, apocrine, and eccrine components. They have a prevalence of 0.3% and some familial forms exist.1,2 They arise as a result of postzygotic somatic mutations in HRAS (95%) and KRAS (5%).3,4 More recently, NRAS mutations have also been described in a case of nevus sebaceus with extracutaneous manifestations.5 Some authors refer to a group denoted mosaic RASopathies that includes sebaceous nevi and also some keratinocytic nevi, melanocytic nevi, and associated syndromic forms.6,7
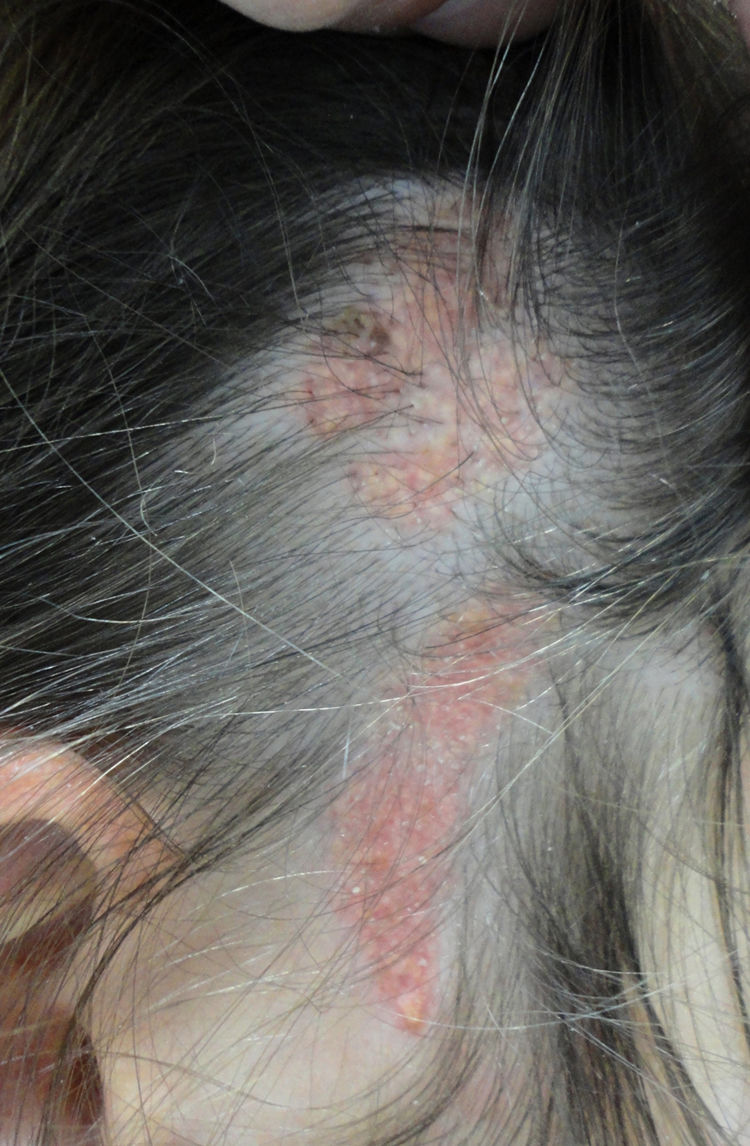
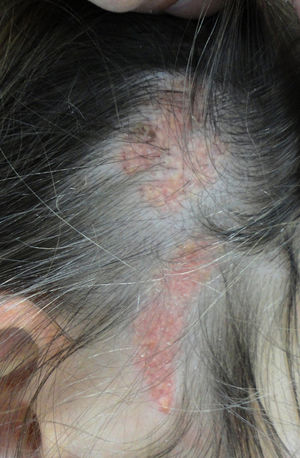
More than half the lesions are found on the scalp, a third develop on the face, while they are rarely seen on the neck and only exceptionally on the trunk or limbs.8,9 The lesions are congenital in most cases, although onset may occur in the first years of life. Characteristically, they are distributed along the Blaschko lines, adopting an ovulated or comma form. The clinical aspect varies with age, probably due to the increased number of androgen receptors in components that are stimulated during puberty.10 In children, the disease is manifest as small yellowish-orange alopecic plaques that can be mistaken for aplasia cutis congenita or a scar. From adolescence onwards, the lesion takes on a more verrucous appearance with a more yellowish color (Fig. 1).
Histological changes also vary with the age of the patient.8,9 In pediatric patients, mild epidermal acanthosis is usually observed along with presence of small poorly formed hair follicles. From puberty onwards, acanthosis becomes more evident and the number and size of the sebaceous glands increase and these are found higher in the reticular dermis or even in the papillary dermis (Fig. 2). Hair dysgenesis persists into adulthood. Generally, the presence of well-formed terminal hair marks the limits of the lesion.
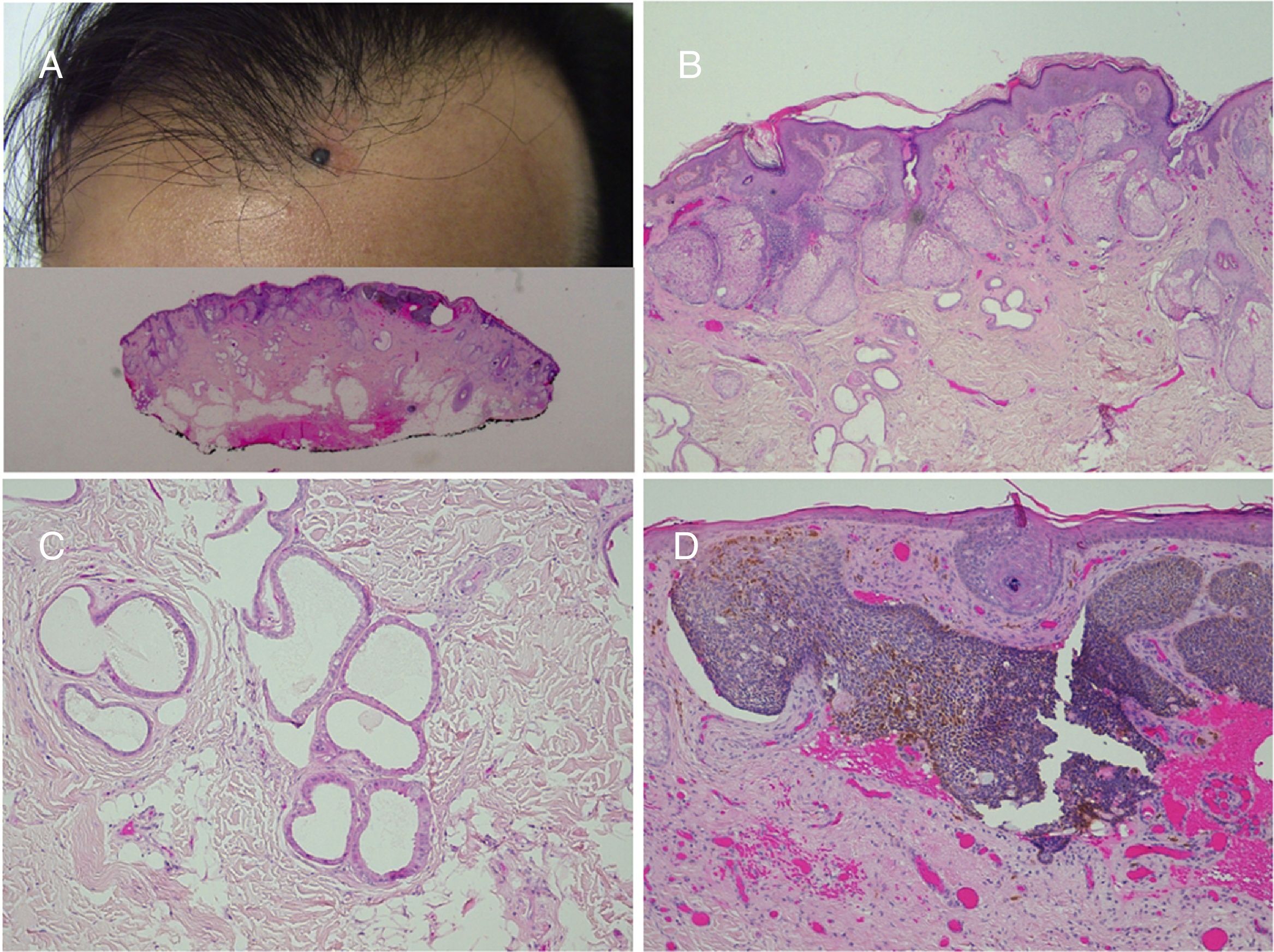
Nevus sebaceus in the left temporal region of a young patient. A,Clinical image. B,(Hematoxylin and eosin [HE], 4×): histological image showing the presence of epidermal acanthosis and multiple large sebaceous glands abnormally found high in the reticular dermis. C,(HE, 10×): detail of the sebaceous glands. D,(HE, 10×): acanthosis acquires a reticulated appearance in some areas, resembling adenoid seborrheic keratosis.
An epidermal change that is observed fairly often in adults is follicular induction. This is a reactive phenomenon that can be seen in multiple processes and that is thought to be due to the influence of the stroma on the epithelium.11 Changes range from proliferation of basaloid cells with peripheral cobblestone appearance that should be differentiated from superficial basal cell carcinoma (Fig. 3) to anagen hair bulbs with follicular papilla present in their lower part.
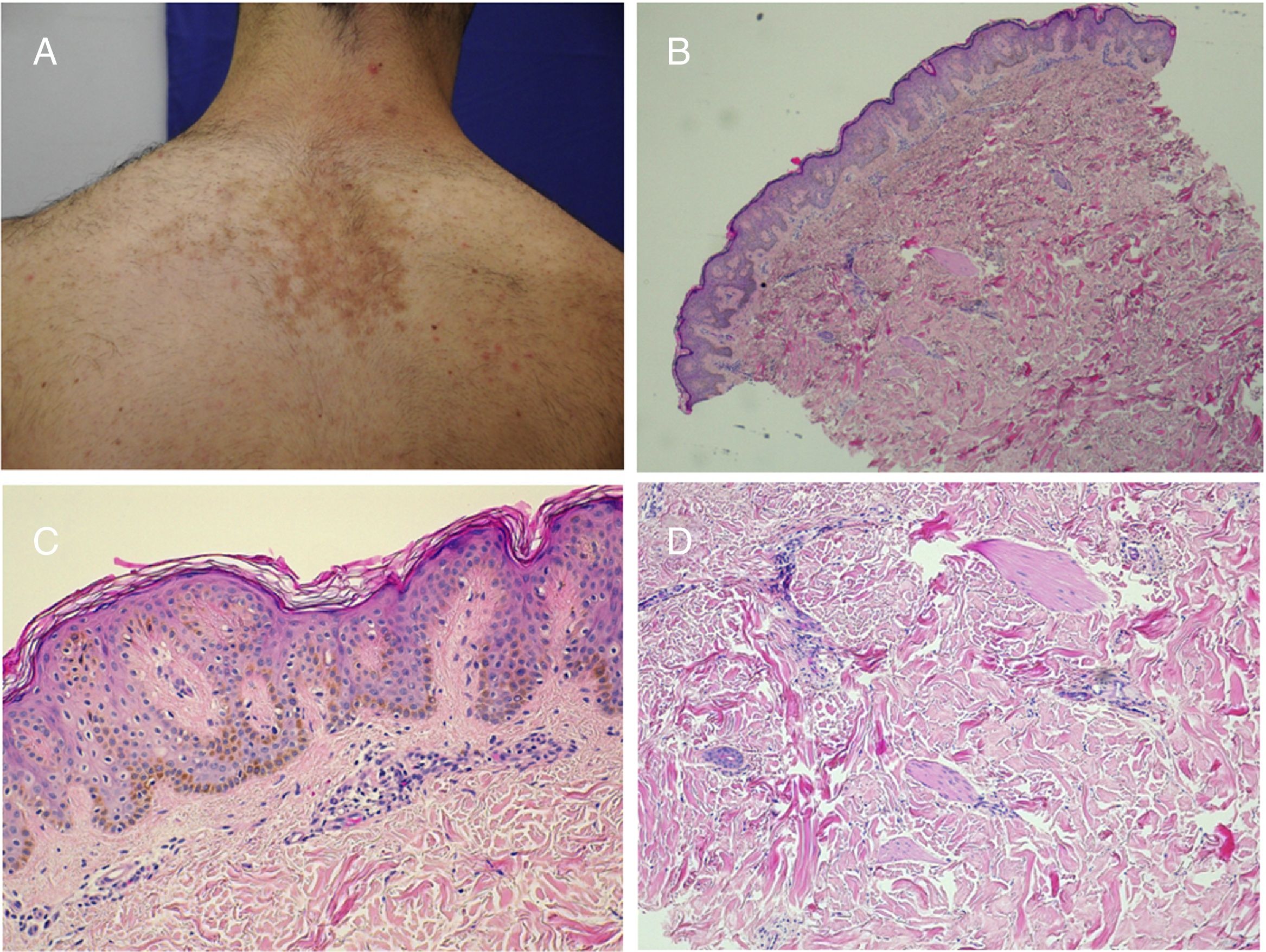
Trichoblastoma on nevus sebaceus. A,(Hematoxylin and eosin [HE] 4×): low-resolution image. B,(HE, 10×): in the periphery of the tumor, the presence of epithelial strands can be seen originating from the epidermis, with basal cobblestone appearance. These finding are suggestive of follicular induction.
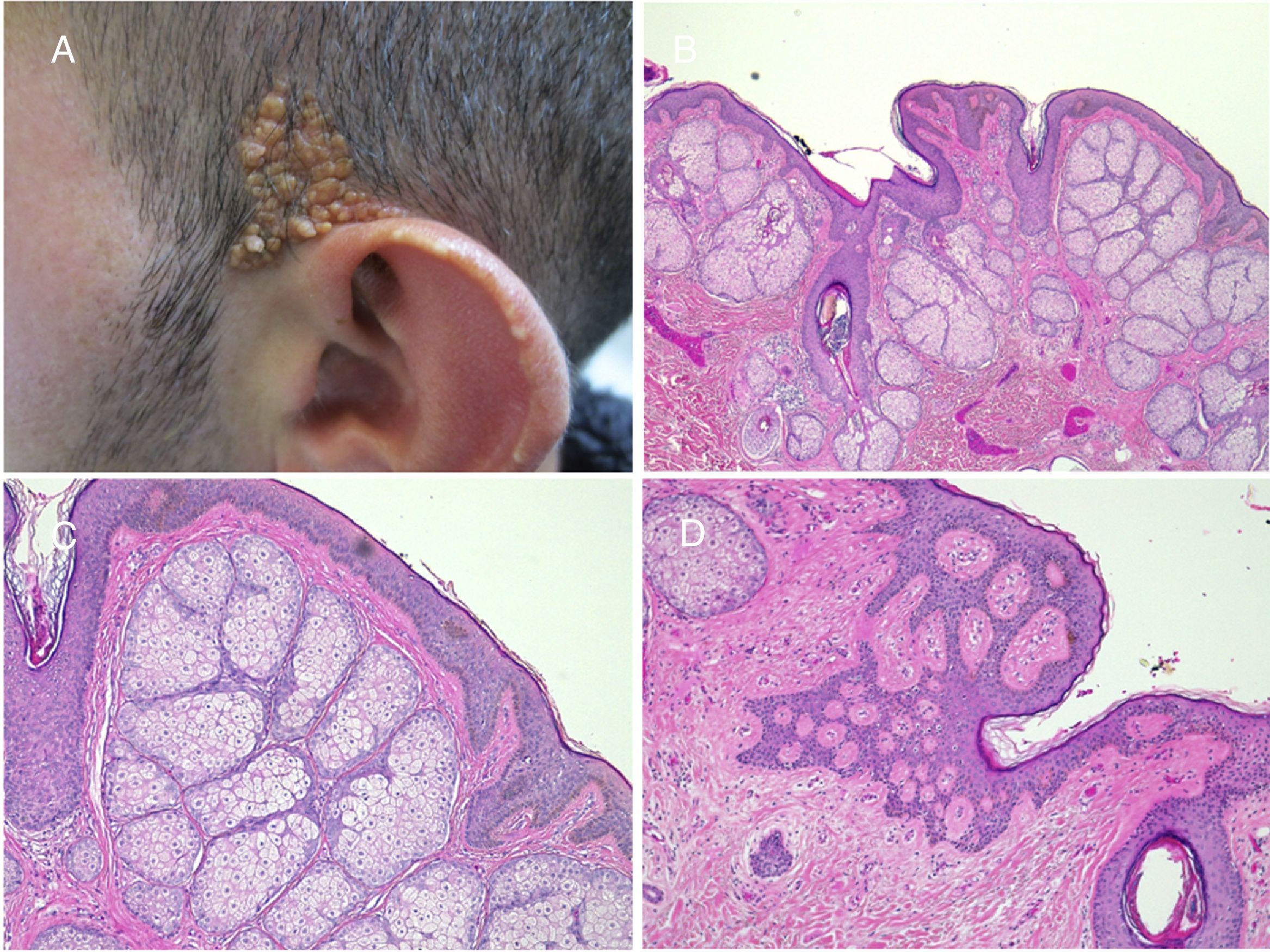
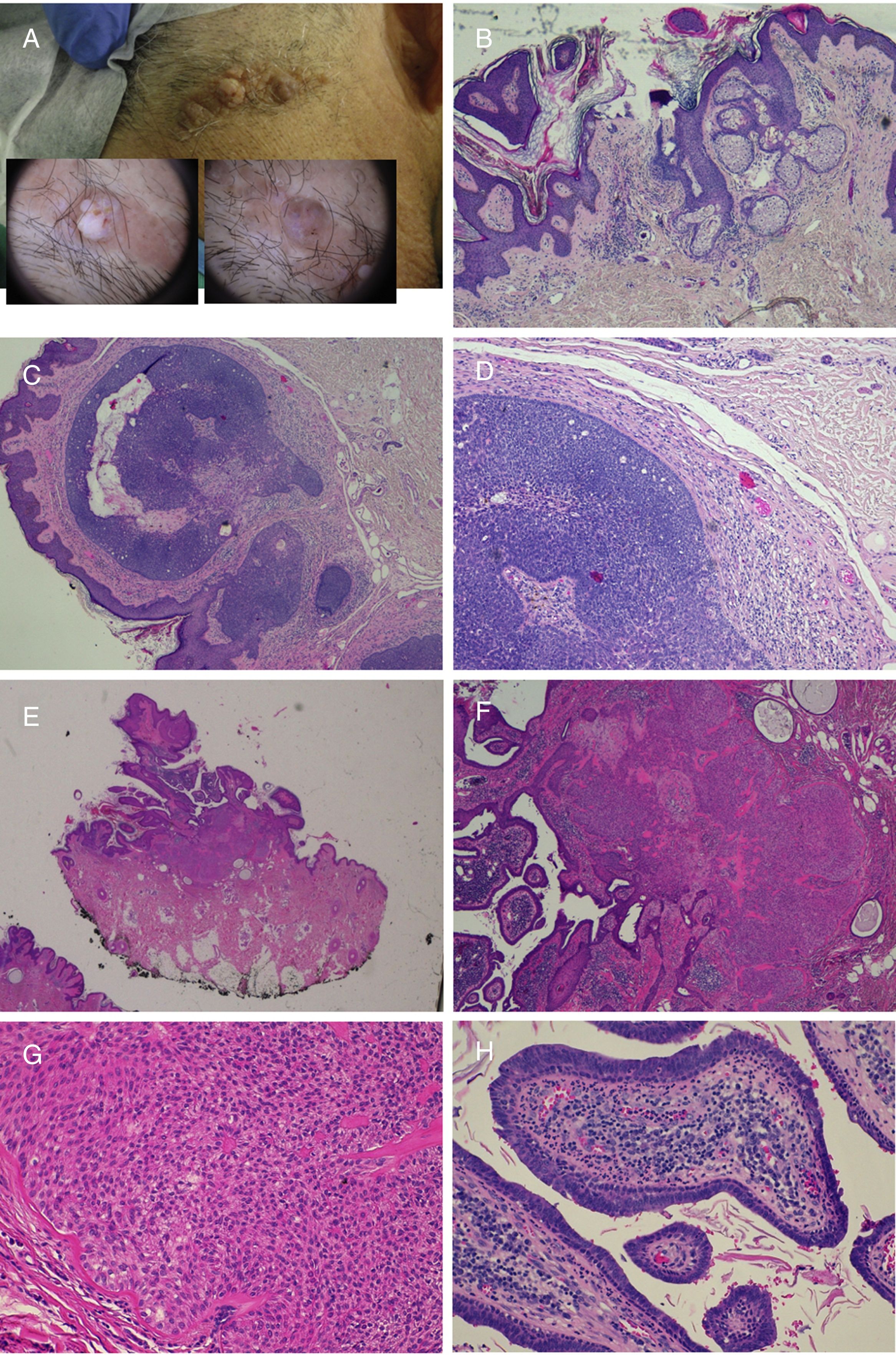
The prevalence of secondary tumors, most of which are benign, ranges from 2% to 39%.9 In the most extensive series, trichoblastoma and syringocystadenoma papilliferum predominate (Fig. 4).8,9,12–15 Other benign tumors that have been described are trichilemmoma and sebaceous, apocrine, and eccrine tumors. Leiomyomas16 and melanocytic nevi17 have also been found in association. Often, more than 1 tumor can develop on the same nevus, as can be seen in the patient in Fig. 4.18,19
Nevus sebaceus with associated trichoblastoma, syringocystadenoma papilliferum, and trichilemmoma. A,Clinical image and dermoscopy of the hamartoma and associated lesions. B,(Hematoxylin and eosin [HE], 4×): histological image of the nevus sebaceus, corresponding to the flattest part of the lesion. C,(HE, 4×): histological image of a trichoblastoma, corresponding to the darkest papular region. D,(HE, 10×): detail of the trichoblastoma. E,(HE, 2×): low-resolution histological image corresponding to the least pigmented papule, which was diagnosed as a trichilemmoma underneath syringocystadenoma papilliferum. F,(HE, 4×): detail of the trichilemmoma. G,(HE, 10×): detail of the trichilemmoma at greater magnification. H,(HE, 10×): detail of the syringocystadenoma papilliferum.
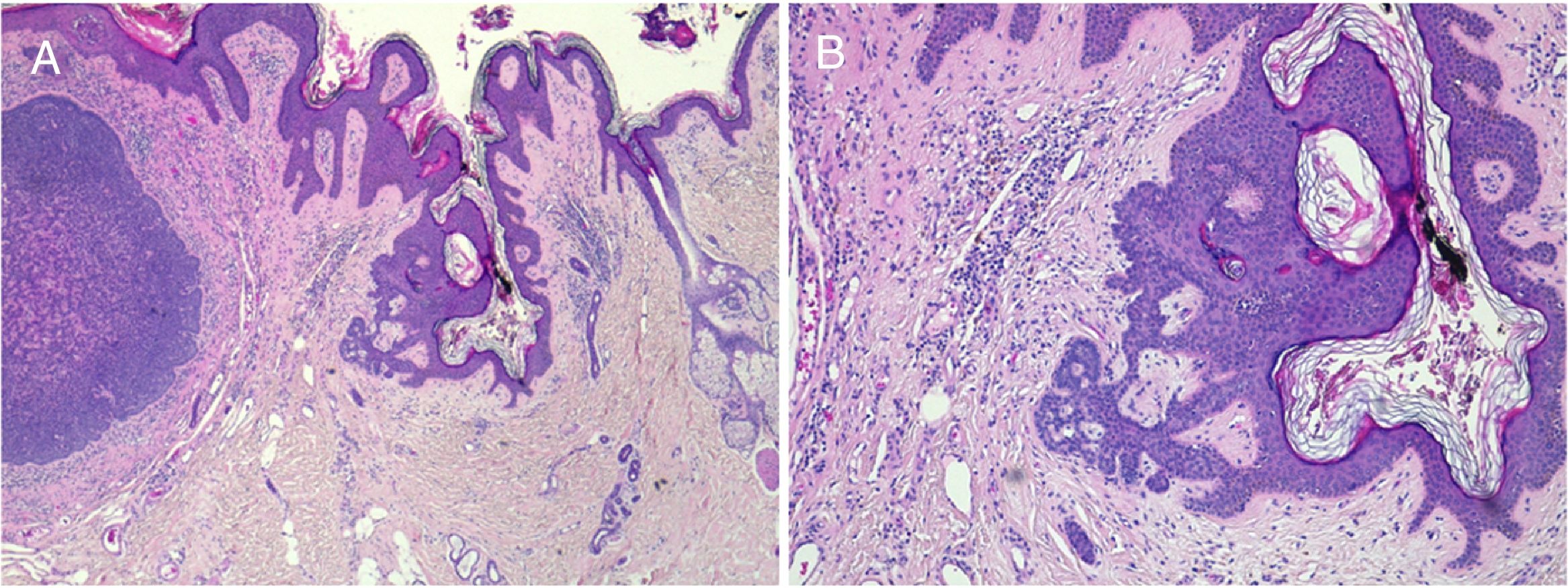
Basal cell carcinoma is the most frequently associated cancer (Fig. 5). Isolated cases of apocrine carcinoma, trichilemmal carcinoma, sebaceous carcinoma, microcystic adnexal carcinoma, porocarcinoma, and squamous cell carcinoma have been reported. There have also been reports of a case of associated leiomyosarcoma20 and another of melanoma.21 The frequent confusion between trichoblastoma and basal cell carcinoma has enhanced the potential for malignant development in sebaceous nevi in the past.22 In recent series, the incidence of basal cell carcinoma is less than 1%,12–15,23 and so many authors suggest watchful waiting rather than preemptive excision in most cases.23,24
Basal cell carcinoma over nevus sebaceus. A,Clinical image and low-resolution histological view (Hematoxylin and eosin [HE], 2×). B,(HE, 4×): histological image showing hyperplasia of the sebaceous and apocrine glands. C,(HE, 10×): Detail of decapitation secretion, characteristic of apocrine glands. D,(HE, 10×): detail of the basal cell carcinoma.
Papillomatous pedunculated nevus sebaceus. This lesion consists of erythematous-yellowish plaques with a tumor-like appearance and a papillomatous surface that present from birth. In the 7 cases described,25–27 all lesions are located on the head, neck, and upper trunk. No patient had extracutaneous manifestations. Mutations in the FGFR2 gene have been reported in 2 fetuses that had similar lesions to papillomatous pedunculated nevus sebaceus, although the clinical and histological diagnosis of these lesions has been called into question.28,29 Should this finding be confirmed, this would be a noteworthy exception, given that all sebaceous nevi analyzed to date have mutations in the RAS gene.
Cerebriform sebaceous nevus. This lesion is a rare morphological variant and few cases have been published.30,31 All cases reported occurred on the scalp. The lesions present a cerebriform appearance from birth, thus requiring differential diagnosis with other lesions such as melanocytic nevi and collagenomas.
Syndromes Associated With Nevus SebaceusSchimmelpenning syndrome, Schimmelpenning-Feuerstein-Mims syndrome, or linear nevus sebaceus syndrome (OMIM #163200) is an association of a sebaceous nevus and neurological, ocular, and/or bone disorders (Table 2). In a series of 196 patients with nevus sebaceus, 14 had neurological manifestations and 4 also had coloboma or choristoma.32 Extensive lesions and centrofacial site were correlated with the presence of extracutaneous abnormalities. The most frequently reported neurological manifestations were mental retardation (79%) and epilepsy (57%). HRAS, KRAS, and NRAS mutations have been detected in affected skin.3–5 Recently, the case has been reported of a child with nevi sebaceus, keratinocytic nevi, retarded dental development, cerebral arachnoid cyst, and optical atrophy.33 The same mutation was detected in KRAS in both nevi. The mutation appears sporadically and probably due to lethal postzygotic somatic mutations that survive in mosaic form.34
Extracutaneous Abnormalities Described in Schimmelpenning Syndrome.
| Neurological Abnormalities | Ocular Abnormalities | Bone Abnormalities | Other Abnormalities |
|---|---|---|---|
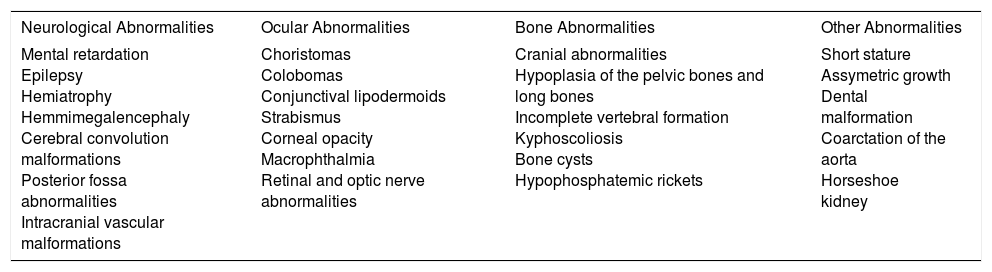
| Mental retardation Epilepsy Hemiatrophy Hemmimegalencephaly Cerebral convolution malformations Posterior fossa abnormalities Intracranial vascular malformations | Choristomas Colobomas Conjunctival lipodermoids Strabismus Corneal opacity Macrophthalmia Retinal and optic nerve abnormalities | Cranial abnormalities Hypoplasia of the pelvic bones and long bones Incomplete vertebral formation Kyphoscoliosis Bone cysts Hypophosphatemic rickets | Short stature Assymetric growth Dental malformation Coarctation of the aorta Horseshoe kidney |
Phacomatosis pigmentokeratotica is a didymosis, that is, there is concurrent presentation, in the same region, of 2 areas of mutant tissue, different from one another and the surrounding skin. It is also known as didymosis spilosebaceus and is characterized by the appearance of a sebaceous nevus and a spilus nevus in the same patient, often associated with bone abnormalities and neurological disorders such as mental retardation, epilepsy, and hemiparesis. In addition, local manifestations may be associated with nevus spilus, such as hyperhidrosis, dysesthesia, muscle weakness, and sensory and motor neuropathy. The sebaceous nevus follows the Blaschko lines, whereas the melanocytic component follows a chessboard pattern, more typical of hamartomas that are derived from this cell line.35 In 2013, Groesser et al.36 established that both cutaneous hamartomas carry the same HRAS mutation, and so they cannot be said to be a true didymosis.
In cutaneous skeletal hypophosphatemia syndrome, patients present with hypophosphatemia, bone dysplasia, and osteomalacia (sometimes described as rickets), along with cerebral, cardiac, and ophthalmologic manifestations.37–40 It has recently been shown that clinical manifestations are due to HRAS and NRAS mutations in both bone and skin lesions (multiline somatic mosaicism).37,41,42 Bone manifestations and hypophosphatemia are attributed to an increase in fibroblast growth factor23 (FGF23), a protein synthesized by osteocytes in normal conditions that induces renal phosphate excretion. The cause of this increase in FGF23 levels is not known. Cases have been published of increases in blood phosphate levels after excision of the nevus,40,43,44 but these findings are not always present and, in general, excision of skin lesions for this reason is not recommended.37
Didymosis aplasticosebacea consists of the coexistence of aplasia cutis congenita and nevus sebaceus. Some patients also present ocular manifestations characteristic of Schimmelpenning syndrome.45–47 The term sebaceous nevus, central nervous system malformations, aplasia cutis congenita, limbal dermoid, and pigmented nevus (SCALP) syndrome was coined in 2008 to describe 3 patients with didymosis aplasticosebacea who also presented a limbal dermoid tumor, large or giant congenital melanocytic nevus, and neurological disorders.47 Since then, an additional case has been reported.48
Hair Follicle NeviHair Follicle Nevus (Congenital Vellus Hamartoma)This lesion presents at birth or in the first years of life as a skin-colored papule, plaque, or nodule on the face.49 Cases of multiple lesions following the Blaschko lines have been reported.50,51 Histologically, this lesion is characterized by proliferation of small vellus hair follicles in the upper dermis with perifollicular fibrous thickening, surrounded by a highly cellular stroma. At times, the lesion can be accompanied by sebaceous or eccrine glands, or muscle fibres. There have been isolated reports of associations with ipsilateral alopecia, leptomeningeal angiomatosis, and frontonasal dysplasia.52,53
Nevus Comedonicus and Munro NevusThis is a linear plaque with multiple dilated follicular orifices following the Blaschko lines. It has a prevalence of 1/45 000 to 1/100 000.54 The lesion usually appears at birth and is located, in order of frequency, on the face, neck, arm, and trunk, including genitals. The dilated pore nevus is a variant of this lesion.55 Some texts distinguish a nonpyogenic form and another characterized by the formation of cysts, papules, pustules, and abscesses, which is known as Munro nevus. Some pyogenic forms arise from mosaic mutations of the FGFR2 gene.56,57 This is the causative gene for Apert syndrome (OMIM #101200), an autosomal dominant disease characterized by craniosynostosis, syndactyly, hydrocephalus, mental retardation, hyperhidrosis, and severe forms of acne on the face and trunk. Recently, mosaic mutations have been reported in the NEK9 gene in nevus comedonicus.58 It is not clear whether mutations in these 2 genes lead to different phenotypes.
Histological study of the lesion shows an acanthotic epidermis with presence of invaginations that contain concentric lamellae of keratin and that correspond to follicular ostia, as demonstrated by the presence of rudiments of hair follicles, as well as small sebaceous glands and, occasionally, erector muscle. In some lesions, inflammation and scarring can be observed.
The development of tumors such as basal cell carcinoma and keratoacanthoma has been reported in some isolated cases.59,60 In nevus comedonicus syndrome, ipsilateral cataracts, microcephaly, mental retardation, epilepsy, dysgenesis of the corpus callosum, vertebral abnormalities such as scoliosis, fused vertebrae, spina bifida, syndactyly, clinodactyly, polydactyly, and oligodontia may be present.61 These manifestations resemble those of Apert syndrome.
Basaloid Follicular HamartomaBasaloid follicular hamartoma is an uncommon lesion. Clinically, it is manifest as small skin-colored papules or plaques. Alopecia in the affected areas is characteristic. Five variants have been described. Generalized lesions are present in 3 of them; one acquired, associated with autoimmune diseases,62–64 another hereditary associated with other skin lesions and extracutaneous manifestations,65–68 and another hereditary form with no association.69 In the localized forms, there is a solitary form with alopecic plaques,70 and another linear form with a Blaschkoid distribution. More recently, a review has been published of this latest variant,71 in which 3 patients are reported who develop basal cell carcinoma in the hamartoma and trichoblastoma. Related to this linear form, Happle-Tinschert syndrome is characterized by basaloid follicular hamartomas with a segmental distribution, along with bone, dental, and ipsilateral cerebral abnormalities.72
The histopathological findings are independent of the clinical variant. The follicles affected are replaced by epithelial strands, 2 or 3 cells thick, which emerge radially from the follicular axis in form of an inverted chandelier. In some areas, small infundibular cysts may be observed. The stroma is limited and consists of some collagen fibers among fibrocytes surrounding the strands of basaloid cells. Differential diagnosis with trichoepithelioma and infundibular-cystic basal cell carcinoma is necessary.
The molecular abnormality responsible for the appearance of these hamartomas and the associated extracutaneous manifestations is not known. It has been shown that expression of PTCH1 is increased in basaloid follicular hamartoma, but it is lower than in basal cell carcinoma.73
Trichilemmal Cyst NevusTrichilemmal cysts are common and present as nodules in the scalp. They are usually multiple and associated with a family history. Histologically, they are characterized by a cystic formation whose wall is composed of several cell layers without a granular layer. Unlike infundibular cysts, they are not usually connected with the surface. Cells of the peripheral strand appear basaloid and they are lined with cells that become keratinized and more eosinophilic towards the cyst lumen, giving rise to a compact, orthokeratotic keratin that contains on occasions areas of calcification and cholesterol crystals.
Trichilemmal cysts with a Blaschkoid distribution have been reported. These have been denoted trichilemmal cyst nevus,74 trichilemmal cysts arising in nevus comedonicus,75 and epidermal nevus associated with trichilemmal cysts.76 An association has also been described with nevus sebaceus.77 Trichilemmal cyst nevus syndrome was described in a 31-year-old patient with linear plaques with multiple trichilemmal cysts, filiform hyperkeratosis, comedones, severe osteomalacia, and multiple fractures from 17years of age.74
Apocrine NeviApocrine Nevus (Apocrine Hamartomatous Glandular Hyperplasia)These are nodular or pedunculated lesions that occur with greatest frequency in the axilla. They have also been reported on the face, scalp, chest, and inguinal region. Most of the lesions are diagnosed in adults. In pediatric cases, lesions increase in size during puberty.78
Histologically, we see mature apocrine glands and ductal structures embedded in a fibrous stroma that extends from the upper dermis to the subcutaneous tissue. Glandular lumens are lined with an epithelium of cuboid or columnar cells surrounded by a layer of myoepithelial cells. The secreting cells show the characteristic decapitation secretion. Cases of malignant transformation have been reported.79,80
Syringocystadenoma PapilliferumThis is a solitary nodular lesion or a group of aligned papules or nodules following a Blaschkoid distribution. They are preferentially located on the head, although they can also be found on the face, neck, trunk and limbs. Half the cases are present at birth and 15% to 30% are diagnosed before the onset of adolescence. In puberty or during pregnancy, the lesion grows and the surface becomes papillomatous. The histological aspect is characteristic. Under low magnification, invaginations can be seen with papillary projections that connect with the surface of the epidermis by infundibular epithelium. The epidermis that covers the most superficial part can be seen as acanthotic, even with parakeratosis. The epithelium that surrounds the cystic cavities is formed of 2 layers: columnar cells that are oriented towards the lumen, in which we can see decapitation secretion, and cuboid basal cells in contact with a stroma rich in plasma cells.
In addition to the association with nevus sebaceus, an association has also been described with other nevi or benign apocrine tumors. Regarding its pathogenesis, PTCH and p16 deletions were detected several years ago.81 More recently, the V600E mutation in the BRAF gene has been reported in half the sporadic cases of syringocystadenoma papilliferum.82,83 Recently, the case of a boy with congenital anaplastic astrocytoma, linear syringocystadenoma papilliferum, and ocular abnormalities has been reported with detection of the mutation in both the tumor and the skin lesions but not peripheral blood or lesion-free skin. Treatment with vemurafenib was beneficial in this particular patient.84
Eccrine NeviEccrine NevusThis entity has been reported as nodular lesions, plaque lesions, or following a linear distribution. Hyperhidrosis is a characteristic. In some cases, a skin lesion cannot be detected and the only manifestation is a localized hyperhidrosis.85 Half of the lesions described are located on the forearm. Histologically, we see an increase in eccrine glands in areas of the anatomy where they should not be so numerous. Some patients present mild acanthosis and hyperkeratosis in the epidermis.86 Mucinous eccrine nevus shows a stroma rich in mucin as the only differential characteristic. In general, eccrine nevus is not associated with systemic manifestations, although there was a single report of association with other skin and ophthalmological manifestations.87
Eccrine Angiomatous HamartomaThis is a lesion characterized by hamartomatous growth of interleaved eccrine and vascular elements. It is manifest as a solitary nodular or plaque lesion with a bluish color and, occasionally, telangiectasia.88 Cases have been reported with a verrucous appearance, thus suggesting the need for differential diagnosis with verrucous hemangioma.89 The lesion is often located on the limbs of children and is often associated with pain and hyperhidrosis. Most cases present at birth or shortly afterwards, although up to a third of cases were diagnosed in adults in 1 series.90
Histologically, we observe hyperplasia of normal or dilated eccrine glands, with presence of foci of capillary angiomatosis and variable presence of follicular, lipomatous, mucinous, apocrine, and/or lymphatic structures.90 Many authors think that the lesion is primarily a vascular malformation.91 In support of this theory, several lesions have been reported accompanying vascular tumors or malformations.89,90
Porokeratotic Adnexal Ostial NevusThe term porokeratotic adnexal ostial nevus has been proposed to cover 2 entities that overlap clinically and histologically: porokeratotic eccrine ostial nevus and porokeratotic eccrine and hair follicle nevus.92 The lesions are keratotic or verrucous papules in which small cavities are observed, filled with keratin, similar to comedones. They are distributed linearly following the Blaschko lines. Bilateral and even generalized forms have been reported. The lesions tend to have an acral distribution and the nails may be involved. When extensive lesions are present, the trunk, head, and neck are affected. Anhidrosis and alopecia may also be present. Histologically, cornoid lamellae can be observed centered on the acrosyringium or in hair bulbs, which appear dilated and without a granular layer. The epidermis shows acanthosis, hyperkeratosis, and papillomatosis. Cases of squamous cell carcinoma and Bowen disease have been associated.92,93
Recently, mosaic mutations have been reported in the GJB2 gene, which encodes connexin 26 in porokeratotic eccrine nevus.94 Currently, this nevus is considered a mosaic form of KID syndrome (OMIM #148210), a dominant autosomal disease characterized by congenital erythrokeratodermia, lamellar ichthyosis, palmoplantar hyperkeratosis, hypotrichosis, hypohidrosis, repeated skin infections, and ocular and musculoskeletal abnormalities. Skin lesions share clinical and histopathological features with porokeratotic eccrine nevus. The case of a girl with KID syndrome, whose mother had porokeratotic eccrine nevus, supports this hypothesis.95
Becker NevusBecker nevus is a light brown colored spot with well-defined borders located on the trunk or shoulder that usually presents when the patient is between 10 and 20 years old. In boys, more than half the lesions develop thick dark hair from puberty onwards. The prevalence is 0.5%. Familial cases have been reported. The belief that it is more frequent in males has recently been shown to be false and the misconception is thought to be due to the lesions being less visible in females because of its androgen dependence.96
The lesion is an organoid hamartoma within the epidermal nevus group61 and has many unique features. It is distributed in an archetypal pattern in patches or chessboard pattern. Histologically, it is characterized by acanthosis with prolongation of the interpapillary crests, in which melanocytic hyperplasia has been demonstrated. In the reticular dermis, in addition to an increased number of hair follicles, an increase in smooth muscle fibers is observed, resembling a smooth muscle hamartoma, which for some authors points to a spectrum of lesions (Fig. 6).97 The recent discovery of ACTB gene mutations, lethal if they occur in the zygote and which are found exclusively in muscle fibers from Becker nevi, has given rise to the theory that their origin lies in a mutation in the mesenchymal line.98
Becker nevus. A,Clinical image. B,(Hematoxylin and eosin [HE], 4×): low resolution image showing the presence of epidermal acanthosis, hyperpigmentation of the basal layer, and presence of smooth muscle fibers in the reticular dermis. C,(HE, 10×): detail of the epidermal changes. D,(HE, 10×): detail of the presence of smooth muscle fiber in the reticular dermis.
In fewer than 5% of cases, ipsilateral mammary hypoplasia, hypoplasia of underlying muscles, lipoatrophy, and skeletal abnormalities such as scoliosis, hemivertebrae, fused or accessory ribs, pectus excavatum or carinatum, and internal tibial torsion (Becker nevus syndrome) may be present.96,99
ConclusionThe most recent developments in the field of genetics is changing the traditional morphological interpretation of epidermal nevi. From this perspective, in the near future, we may follow a genetic-based classification of our patients with epidermal nevi and thus provide them with much more detailed information about associated extracutaneous manifestations, secondary tumor development, or even reproductive genetic counselling.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
This review was written as the final project of the I International Master in Dermatopathology and Clinical Correlation, directed by Dr. Jesús Cuevas and Dr. Pedro Jaén. We thank their supervision and encouragement for publishing the project.
We thank Dr Gabriela Corte, head of the Pathological Anatomy department of the Hospital Manacor, for her collaboration in collecting cases to illustrate this work.
Please cite this article as: Garcias-Ladaria J, Rosón MC, Pascual-López M. Nevus epidérmicos y síndromes relacionados. Parte 2: Nevus derivados de estructuras anexiales. Actas Dermosifiliogr. 2018;109:687–698.

![Nevus sebaceus in the left temporal region of a young patient. A,Clinical image. B,(Hematoxylin and eosin [HE], 4×): histological image showing the presence of epidermal acanthosis and multiple large sebaceous glands abnormally found high in the reticular dermis. C,(HE, 10×): detail of the sebaceous glands. D,(HE, 10×): acanthosis acquires a reticulated appearance in some areas, resembling adenoid seborrheic keratosis. Nevus sebaceus in the left temporal region of a young patient. A,Clinical image. B,(Hematoxylin and eosin [HE], 4×): histological image showing the presence of epidermal acanthosis and multiple large sebaceous glands abnormally found high in the reticular dermis. C,(HE, 10×): detail of the sebaceous glands. D,(HE, 10×): acanthosis acquires a reticulated appearance in some areas, resembling adenoid seborrheic keratosis.](https://static.elsevier.es/multimedia/15782190/0000010900000008/v1_201810020631/S1578219018302737/v1_201810020631/en/main.assets/thumbnail/gr2.jpeg?xkr=ue/ImdikoIMrsJoerZ+w9/t1/zx4Q/XH5Tma1a/6fSs=)
![Trichoblastoma on nevus sebaceus. A,(Hematoxylin and eosin [HE] 4×): low-resolution image. B,(HE, 10×): in the periphery of the tumor, the presence of epithelial strands can be seen originating from the epidermis, with basal cobblestone appearance. These finding are suggestive of follicular induction. Trichoblastoma on nevus sebaceus. A,(Hematoxylin and eosin [HE] 4×): low-resolution image. B,(HE, 10×): in the periphery of the tumor, the presence of epithelial strands can be seen originating from the epidermis, with basal cobblestone appearance. These finding are suggestive of follicular induction.](https://static.elsevier.es/multimedia/15782190/0000010900000008/v1_201810020631/S1578219018302737/v1_201810020631/en/main.assets/thumbnail/gr3.jpeg?xkr=ue/ImdikoIMrsJoerZ+w9/t1/zx4Q/XH5Tma1a/6fSs=)
![Nevus sebaceus with associated trichoblastoma, syringocystadenoma papilliferum, and trichilemmoma. A,Clinical image and dermoscopy of the hamartoma and associated lesions. B,(Hematoxylin and eosin [HE], 4×): histological image of the nevus sebaceus, corresponding to the flattest part of the lesion. C,(HE, 4×): histological image of a trichoblastoma, corresponding to the darkest papular region. D,(HE, 10×): detail of the trichoblastoma. E,(HE, 2×): low-resolution histological image corresponding to the least pigmented papule, which was diagnosed as a trichilemmoma underneath syringocystadenoma papilliferum. F,(HE, 4×): detail of the trichilemmoma. G,(HE, 10×): detail of the trichilemmoma at greater magnification. H,(HE, 10×): detail of the syringocystadenoma papilliferum. Nevus sebaceus with associated trichoblastoma, syringocystadenoma papilliferum, and trichilemmoma. A,Clinical image and dermoscopy of the hamartoma and associated lesions. B,(Hematoxylin and eosin [HE], 4×): histological image of the nevus sebaceus, corresponding to the flattest part of the lesion. C,(HE, 4×): histological image of a trichoblastoma, corresponding to the darkest papular region. D,(HE, 10×): detail of the trichoblastoma. E,(HE, 2×): low-resolution histological image corresponding to the least pigmented papule, which was diagnosed as a trichilemmoma underneath syringocystadenoma papilliferum. F,(HE, 4×): detail of the trichilemmoma. G,(HE, 10×): detail of the trichilemmoma at greater magnification. H,(HE, 10×): detail of the syringocystadenoma papilliferum.](https://static.elsevier.es/multimedia/15782190/0000010900000008/v1_201810020631/S1578219018302737/v1_201810020631/en/main.assets/thumbnail/gr4.jpeg?xkr=ue/ImdikoIMrsJoerZ+w9/t1/zx4Q/XH5Tma1a/6fSs=)
![Basal cell carcinoma over nevus sebaceus. A,Clinical image and low-resolution histological view (Hematoxylin and eosin [HE], 2×). B,(HE, 4×): histological image showing hyperplasia of the sebaceous and apocrine glands. C,(HE, 10×): Detail of decapitation secretion, characteristic of apocrine glands. D,(HE, 10×): detail of the basal cell carcinoma. Basal cell carcinoma over nevus sebaceus. A,Clinical image and low-resolution histological view (Hematoxylin and eosin [HE], 2×). B,(HE, 4×): histological image showing hyperplasia of the sebaceous and apocrine glands. C,(HE, 10×): Detail of decapitation secretion, characteristic of apocrine glands. D,(HE, 10×): detail of the basal cell carcinoma.](https://static.elsevier.es/multimedia/15782190/0000010900000008/v1_201810020631/S1578219018302737/v1_201810020631/en/main.assets/thumbnail/gr5.jpeg?xkr=ue/ImdikoIMrsJoerZ+w9/t1/zx4Q/XH5Tma1a/6fSs=)
![Becker nevus. A,Clinical image. B,(Hematoxylin and eosin [HE], 4×): low resolution image showing the presence of epidermal acanthosis, hyperpigmentation of the basal layer, and presence of smooth muscle fibers in the reticular dermis. C,(HE, 10×): detail of the epidermal changes. D,(HE, 10×): detail of the presence of smooth muscle fiber in the reticular dermis. Becker nevus. A,Clinical image. B,(Hematoxylin and eosin [HE], 4×): low resolution image showing the presence of epidermal acanthosis, hyperpigmentation of the basal layer, and presence of smooth muscle fibers in the reticular dermis. C,(HE, 10×): detail of the epidermal changes. D,(HE, 10×): detail of the presence of smooth muscle fiber in the reticular dermis.](https://static.elsevier.es/multimedia/15782190/0000010900000008/v1_201810020631/S1578219018302737/v1_201810020631/en/main.assets/thumbnail/gr6.jpeg?xkr=ue/ImdikoIMrsJoerZ+w9/t1/zx4Q/XH5Tma1a/6fSs=)



