Elastography is a recently developed ultrasound technique applicable to various medical specialties. It provides information on the physical properties of tissues in the context of physiologic and pathologic alterations. In this review we explain the physical principles of the method, the information provided by the different elastography techniques, and its new applications in clinical dermatology.
La elastografía es una técnica ecográfica de reciente desarrollo en varias especialidades médicas que aporta información sobre las propiedades físicas de los tejidos en procesos fisiológicos y patológicos. En esta revisión se explican los principios físicos de la técnica, la información que aportan las distintas modalidades de elastografía y las nuevas aplicaciones en dermatología clínica.
Since antiquity, palpation has played an important role in the general physical examination of patients because it provides information about the physical characteristics of the tissues.1 A loss of elasticity or increase in rigidity of organs or tissues has traditionally been associated with a poorer prognosis in inflammatory processes, which histologically tend to be associated with fibrosis, and in tumor processes, in which the elastic properties of healthy tissues decrease.2,3
Estimation of the elasticity or rigidity of tissues could therefore facilitate early, noninvasive monitoring and treatment of inflammatory and tumor processes.4 Elastography is a technique in which ultrasound is used to detect changes in the elasticity of tissues.5 Since the late 20th century, elastography has been used in various diseases, including tumors of the breast, thyroid, and liver, as well as in inflammatory processes in the same organs.6
The recent introduction of high-frequency linear ultrasound probes has made it possible for this technology to be applied to superficial tissues such as the bone and muscle system,6 the superficial vascular system,6 and the skin and adnexa.7
This review details the basic physical concepts of elastography and the possible utility of this technique in the assessment of the skin and adnexa.
It is important to note that most cutaneous elastography studies are small case series, a majority of which are observational and of limited scientific robustness. Nevertheless, a review that introduces elastography to dermatologists and encourages future studies of the practical utility of the technique in dermatology could still be useful.
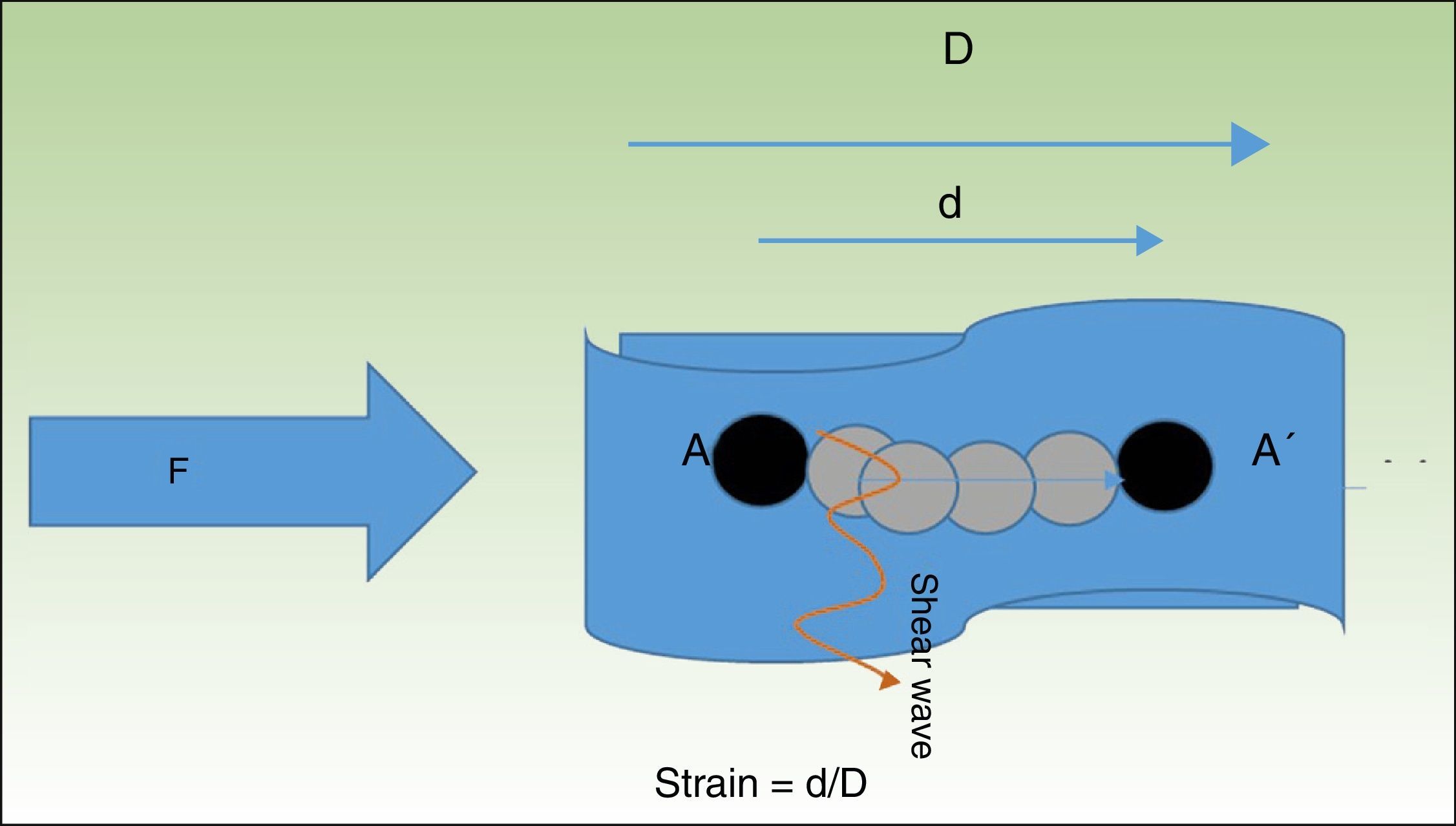
Elastography: The Physical Concepts of Strain and Shear WaveWhen a tissue is subjected to pressure, it deforms and tends to recover its initial shape (elasticity). The resistance of the tissue to deformation is called rigidity or stiffness.8
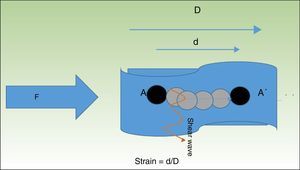
The term strain describes the change in the relative length of a structure subjected to pressure with respect to the surrounding tissue9 (Fig. 1).
When tissue is compressed with a force F, the tissue particles (A) undergo a displacement (A′). The quotient of the displacement (d) of the structure being examined and its total initial length (D) is known as strain. The particles are displaced in perpendicular to this pressure wave, generating waves called shear waves.
In addition to this physical phenomenon, a series of waves perpendicular to the displacement of the pressure wave—known as shear waves—are also generated in the tissue.10 It is possible to determine the velocity of the shear wave, which provides indirect quantitative information about the stiffness of the tissue (Fig. 1).
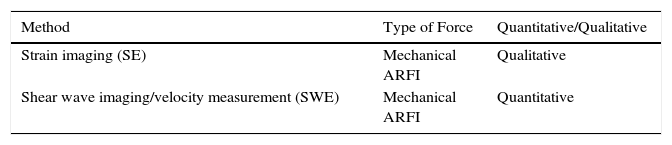
Types of Elastography and Their LimitationsAccording to the clinical guidelines on elastography published by the European Federation of Societies for Ultrasound in Medicine and Biology (EFSUMB),11 there are two basic types of elastography: strain elastography (SE), which assesses tissue deformation, and shear wave elastography (SWE), which characterizes the shear waves (Table 1). Types of elastography can also be classified according to the physical force that produces the tissue deformation. This force can be mechanical (manual or automatic) or it can be produced by an ultrasound pulse called acoustic radiation force impulse (ARFI). Each of these elastography methods offers qualitative or quantitative information about the rigidity or stiffness (terms used interchangeably in this review) of tissues.
Types of Elastography.
| Method | Type of Force | Quantitative/Qualitative |
|---|---|---|
| Strain imaging (SE) | Mechanical ARFI | Qualitative |
| Shear wave imaging/velocity measurement (SWE) | Mechanical ARFI | Quantitative |
Source: Adapted from the clinical guidelines on elastography of the European Federation of Societies for Ultrasound in Medicine and Biology (EFSUMB).
Abbreviations: ARFI, acoustic radiation force impulse; SE, strain elastography; SWE, shear wave elastography.
Semiquantitative measurement scales normally associate a number from 1 to 5 with the rigidity percentage of a structure, with 1 being softest and 5 being stiffest.11
Another way to quantify the stiffness of a structure is to express it in relation to the surrounding parenchyma. This quotient is known as the strain ratio.12
In SWE, which determines shear wave displacement velocities, measurements are quantitative and can be expressed in either kPa or m/s.13
This technique has certain limitations. Because of the great variability of the various types of elastography, studies of the technique have been isolated and there are no universal measures used by all elastography equipment.11
Inter- and intraobserver variability is greater in SE (especially the manual variant) than in SWE.11
Because it uses ultrasound waves, elastography—like conventional ultrasound—generates artifacts. In areas with acoustic shadowing or cystic structures, the information provided about tissue characteristics may not reflect reality.14 However, as in the case of conventional ultrasound, these artifacts also provide information about the structure being examined.14
Elastography in Dermatology: Technique and PeculiaritiesAccording to the EFSUMB clinical guidelines on elastography,11 the following recommendations should be taken into account when elastography is performed on any organ:
- 1.
The structure should be in close proximity to the transducer (<4cm).
- 2.
The structure should be nearly homogeneous.
- 3.
When pressure is applied, there should be no slippage in the structure over deeper planes.
- 4.
Pressure should be applied by a surface larger than the structure being examined.
- 5.
No structures that damp compression—such as large blood vessels—should be present.
- 6.
The structures being examined should be completely included within the region of interest.
- 7.
The direction of the compression force should be known.
- 8.
The number of structures being examined should be limited.
When elastography is used on the skin, gel should not be used in the region of interest.11 As we can infer, the skin is an organ that adapts to the conditions in which elastography can be carried out with the appropriate technique and technology—that is, high-frequency linear probes applied to the skin and adnexa.15
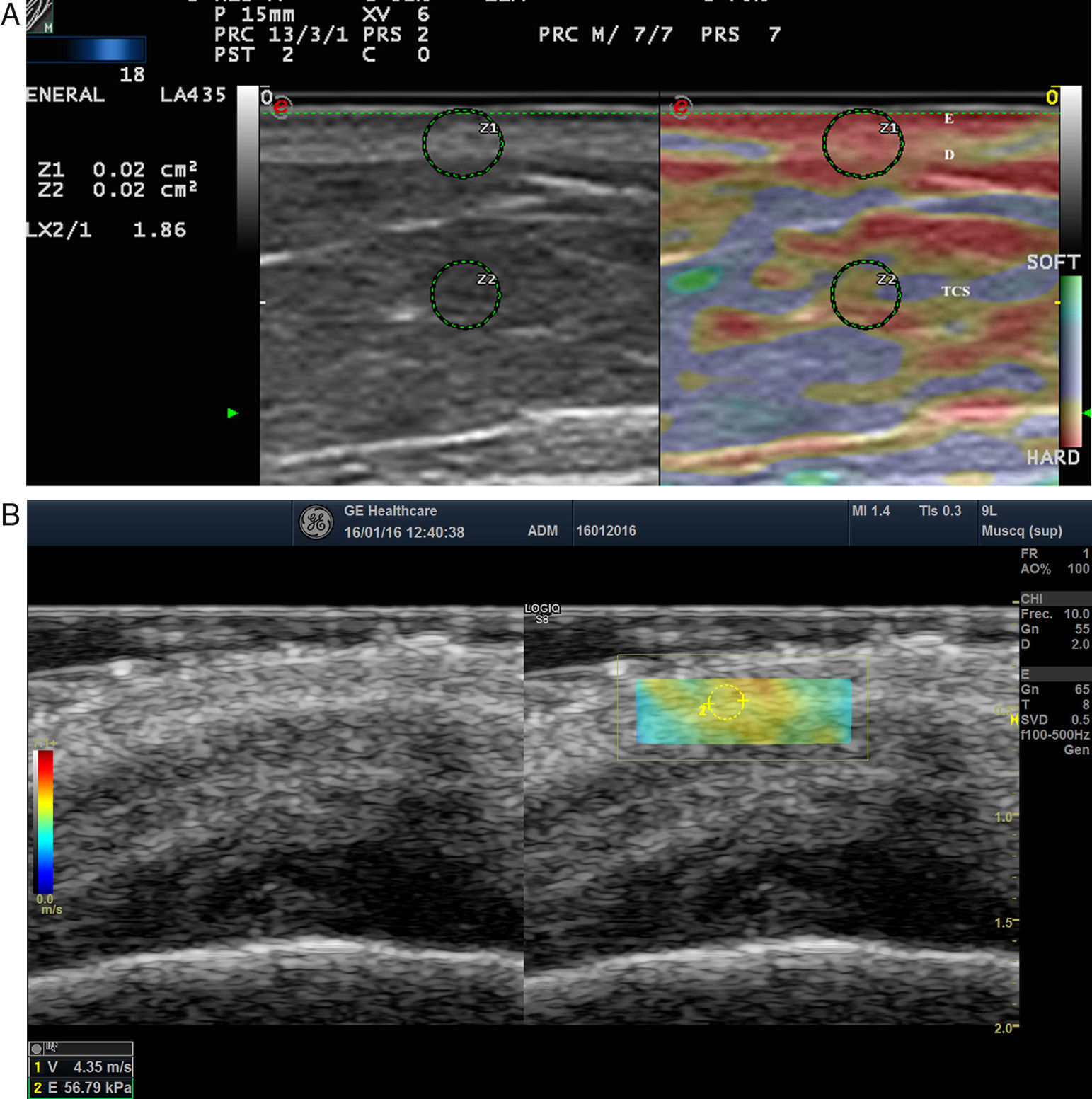
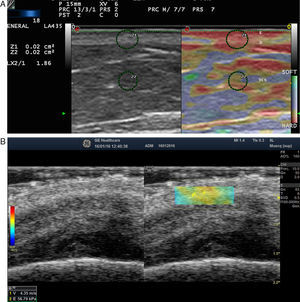
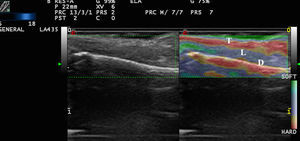
Elastography of Normal Skin and AdnexaThe stiffness of healthy skin varies according to the cutaneous layer being studied. The dermis is more rigid than the subcutaneous cellular tissue16 (Fig. 2, A, B). In the subcutaneous cellular tissue, the septa are more rigid than the fat lobules. Blood vessels, like the peripheral nerves, are not very rigid in comparison to the surrounding subcutaneous cellular tissue.16
A, Strain elastography of normal skin. Note the strain ratio of the dermis and the fat (SR=1.86), which indicates that the dermis is stiffer than the subcutaneous tissue.
E indicates epidermis; D, dermis; TCS, subcutaneous cellular tissue.
B, Shear wave elastography of the dermis of the scalp. In the lower right corner, note the parameters of velocity and pressure in the region of interest (yellowish-green rectangle).
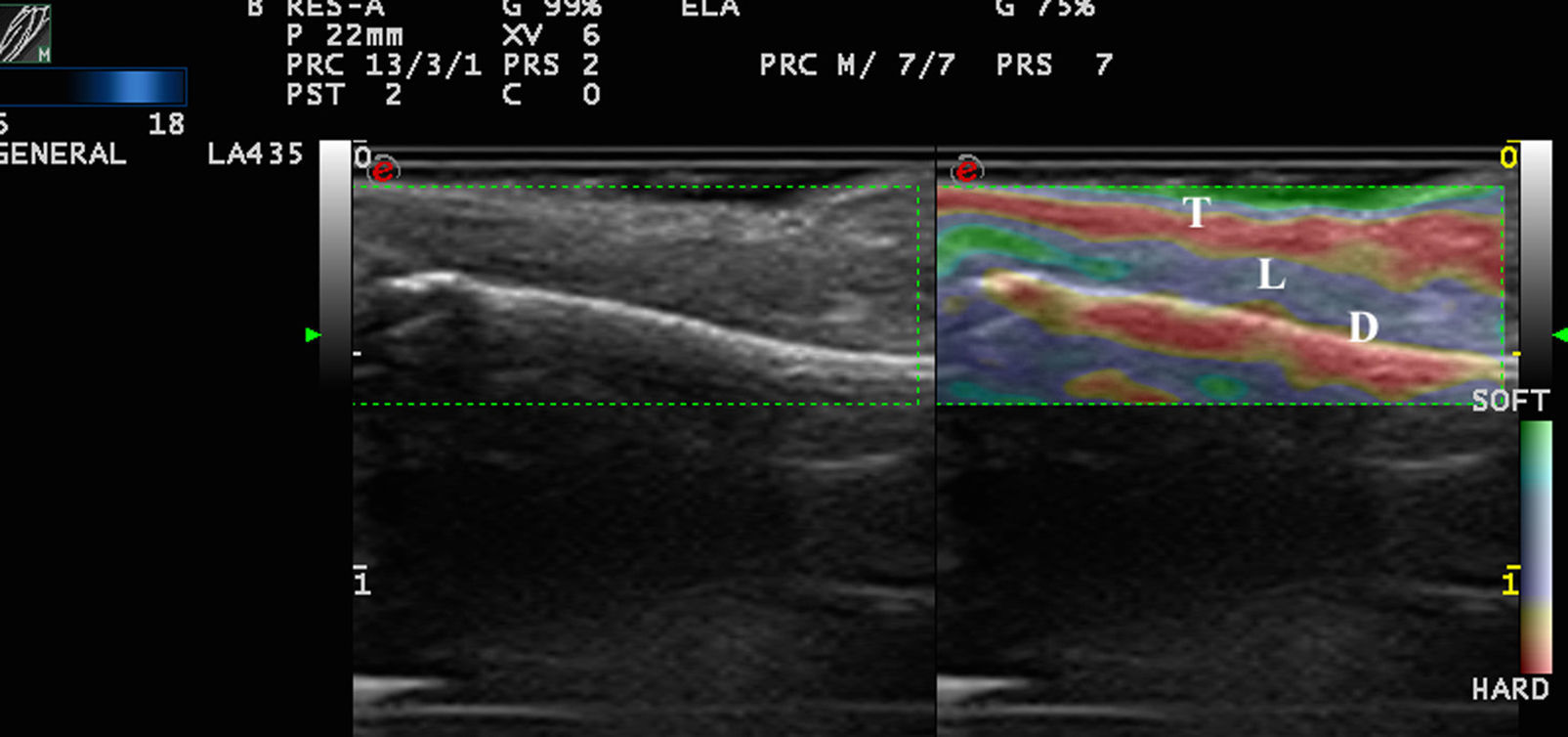
In a healthy nail, the nail plate is more rigid than the nail bed17 (Fig. 3).
The most widely investigated application in elastography of skin tumors is the differentiation of benign and malignant tumors.18
In tumors, the mechanical properties of the tissue are generally altered in a way that allows the tumor to be differentiated from the adjacent healthy tissue.19,20
Benign Skin Tumors and Neck MassesAlthough benign subcutaneous tumors have a recognizable appearance in B-mode ultrasound,21 in doubtful cases elastography could play a useful role in the differential diagnosis.
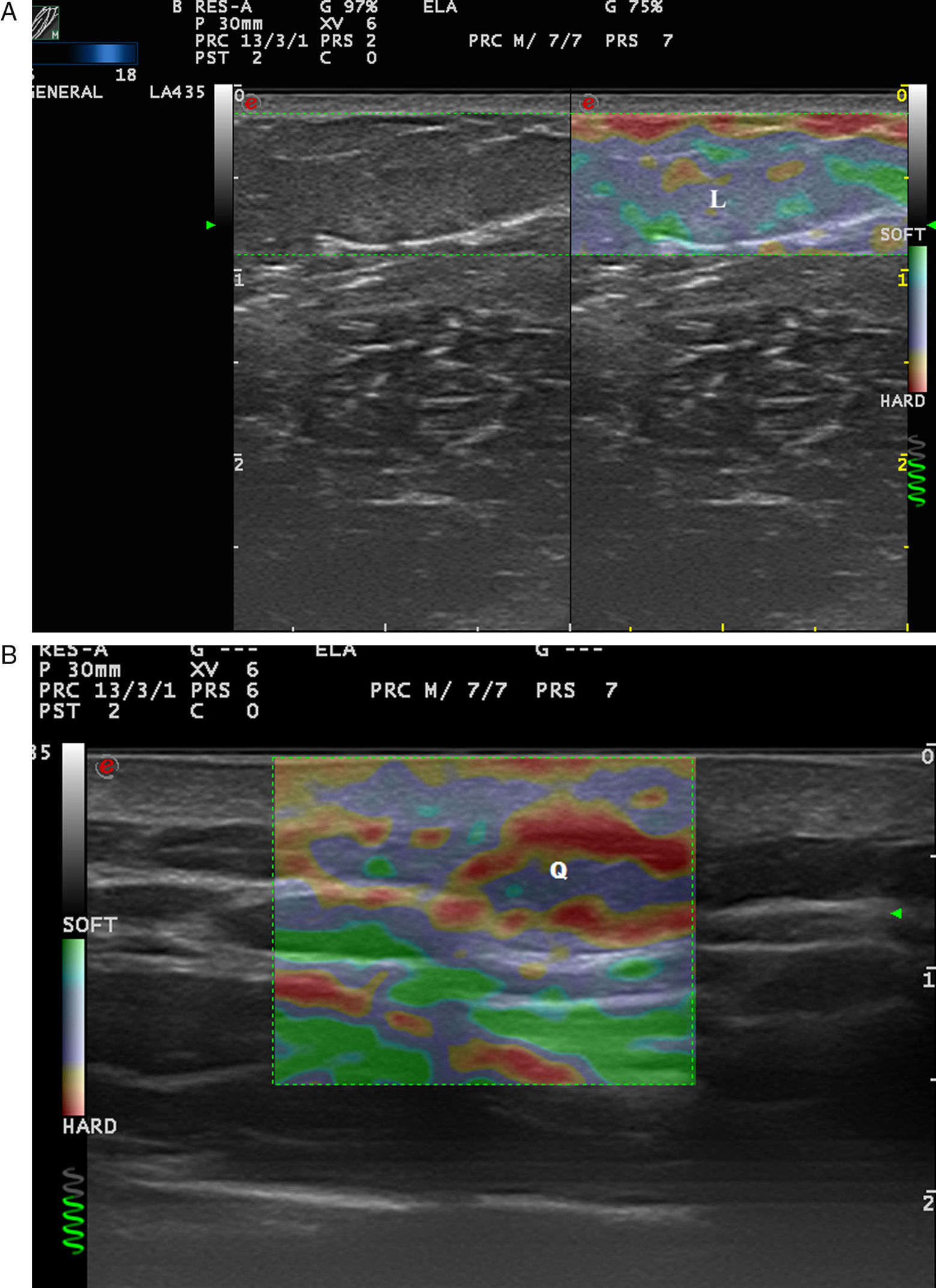
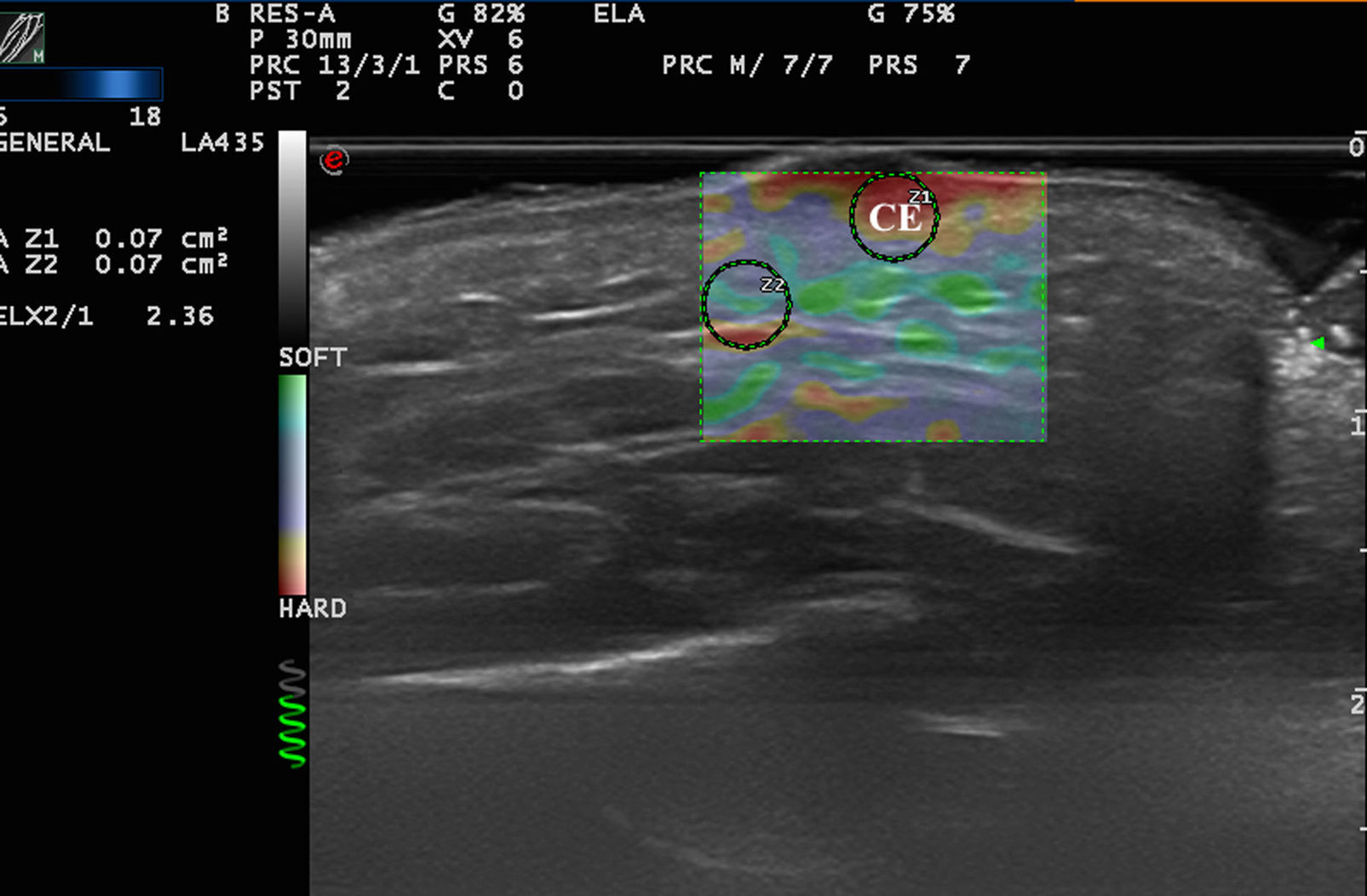
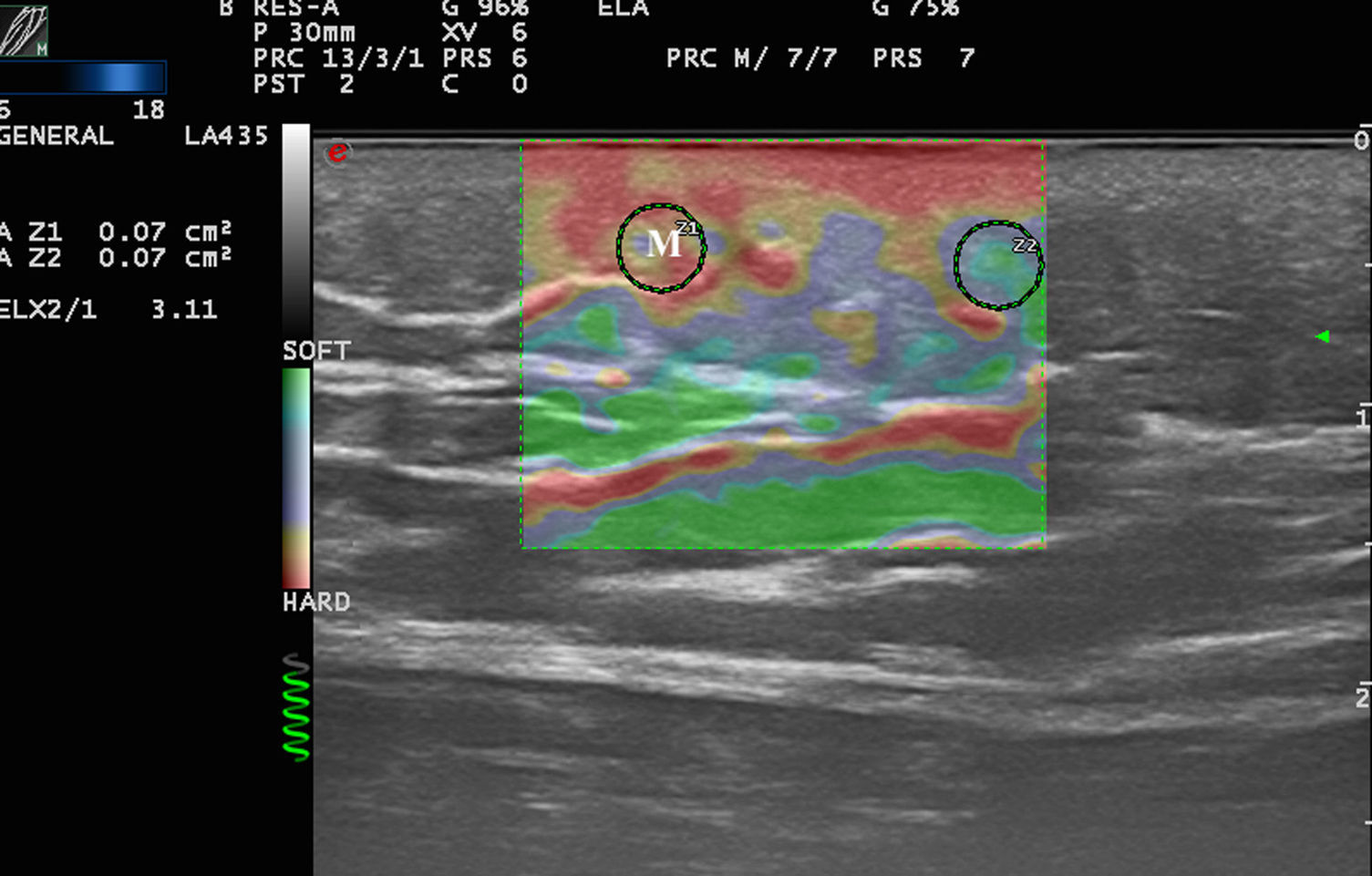
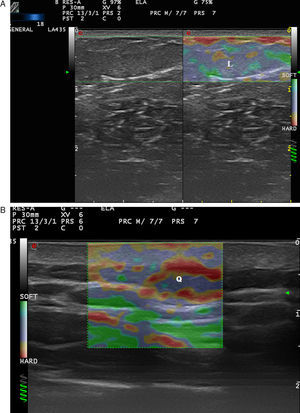
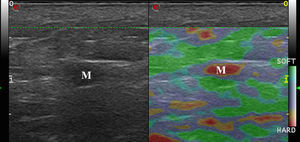
In a study by Bhatia et al.,22 52 non-nodal neck masses were evaluated using real-time qualitative ultrasound elastography. The diagnosis of the lesions was later corroborated by cytology and histology. The lesions were evaluated semiquantitatively on a scale of 0 to 3, where 0 was completely soft and 3 was completely stiff. Lipomas were less stiff than other types of lesions, most of which were cysts, malformations, and neurogenic tumors (Fig. 4). In an extension of the study, SWE was used to assess malignant and benign neck tumors.23 The mean stiffness of the malignant tumors (226.4kPa) was higher than that of the benign lesions (28.3kPa) and the difference was statistically significant. With a cut-off of 174.4kPa, sensitivity of 83.3% and specificity of 97.5% were achieved in the differentiation of benign and malignant lesions. The authors noted that all tumors were correctly diagnosed with conventional ultrasound and that elastography would not have altered the treatment, but they argued that less experienced operators could find the technique helpful in the diagnosis of neck lesions.
Park et al.24 used elastography to differentiate inflamed and unruptured epidermal cysts (Fig. 4, B), the latter being stiffer than the former.
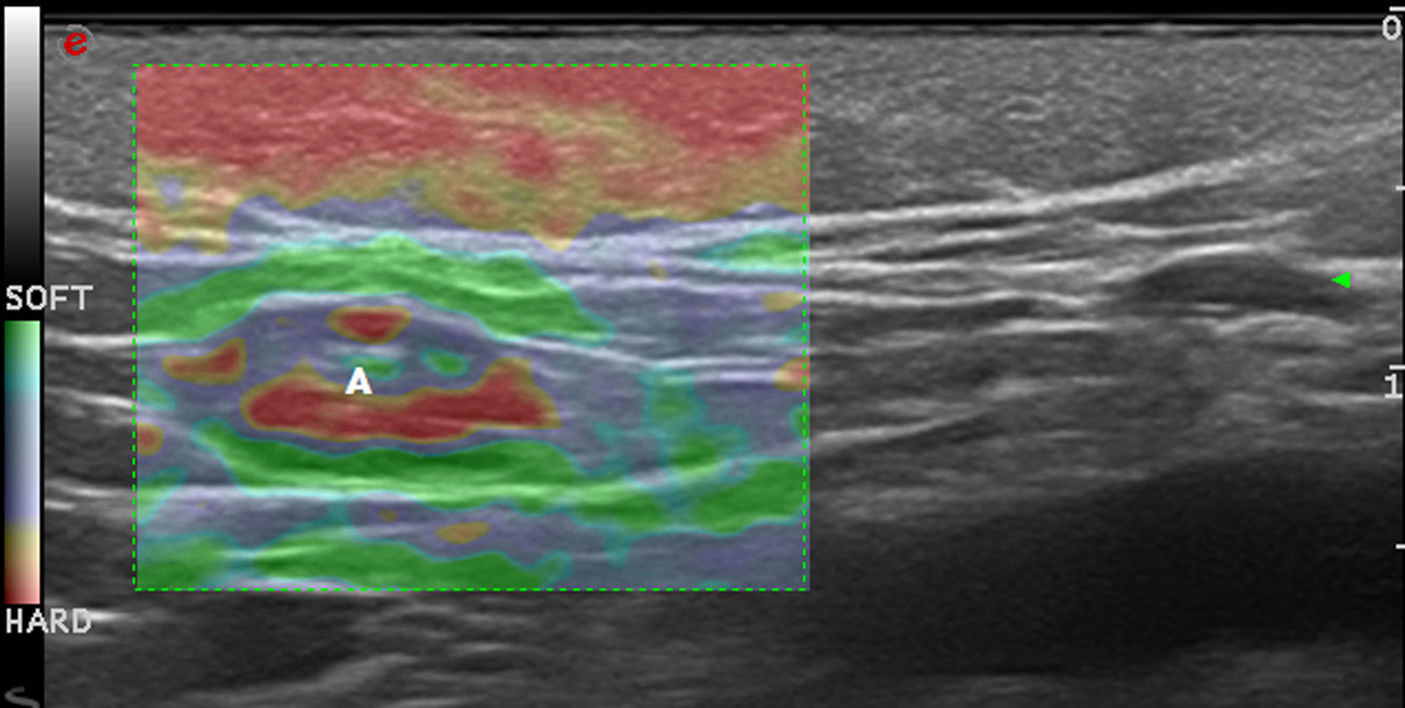
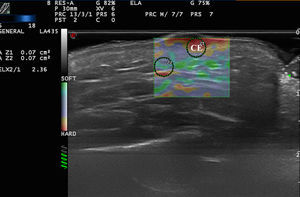
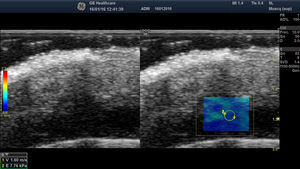
Malignant Skin TumorsElastography shows that malignant skin tumors are stiffer than the surrounding tissue18 (Fig. 5).
Dasgeb et al.25 studied 55 patients with a total of 67 epithelial tumors, of which 29 were malignant (17 basal cell carcinomas and 12 squamous cell carcinomas) and 19 were benign. In this study, the strain ratio was > 3.9 in all malignant skin tumors and < 3 in all benign skin tumors. For strain ratio values between 3.00 and 3.9, sensitivity and specificity were 100% in the diagnosis of malignant lesions.
Elastography has been used to study melanoma. In a pilot study by Botar et al.,26 42 melanomas in 39 patients were studied using SE and color Doppler ultrasound to assess vascularization.
The melanomas were hypervascularized and had multiple vascular pedicles, and SE showed that the lesions were stiffer than the adjacent skin. The lesions with the highest degree of vascularization had the greatest stiffness.
The correlation between melanoma neovascularization and prognosis is well known in the literature.27,28 Therefore, lesion stiffness could be a prognostic factor in melanoma.29
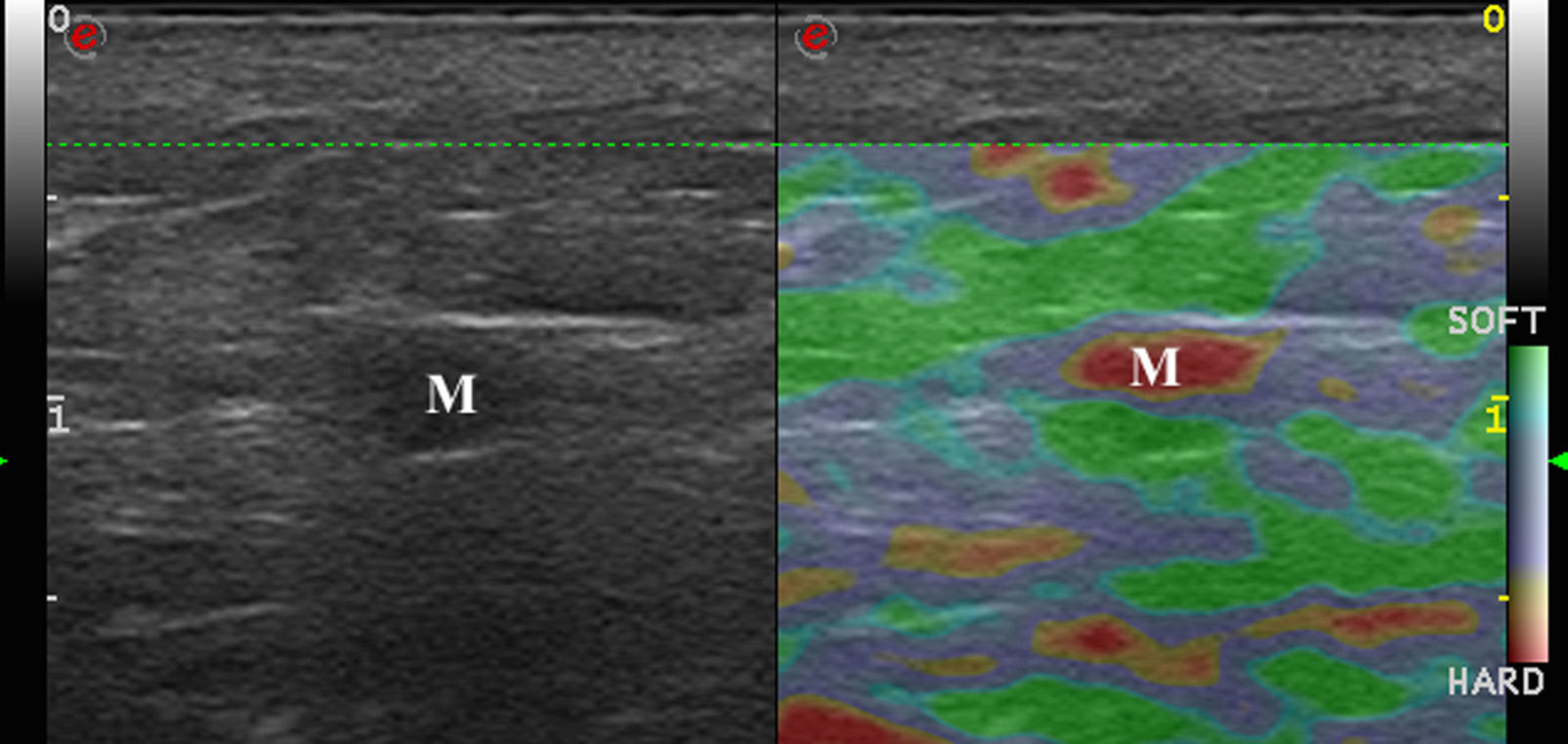
Lymph Node EnlargementThe aim of ultrasound assessment of lymph nodes is to noninvasively diagnose malignant lymph nodes in patients with clinically suspicious lesions.30
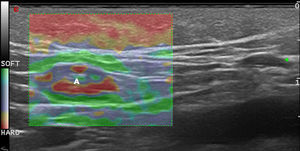
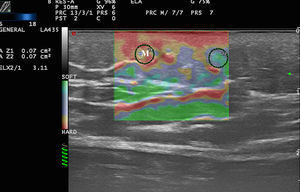
Lymph nodes have an elastic structure in which the cortex tends to be less rigid than the capsule and the hilum (Fig. 6).
To assess the stiffness of lymph nodes, SE is used to classify nodes in 4 or 5 categories according to the proportion of stiff areas they present.31 Benign enlarged nodes generally tend to be soft, whereas malignant nodes tend to be stiffer.32 However, lymphomas are less stiff than metastatic nodes and similar in stiffness to inflamed nodes. Therefore, benign and lymphomatous nodes cannot be distinguished with elastography alone.33
In the case of melanoma (Fig. 7), Hinz et al.34 found that elastography in addition to conventional B-mode sonography combined with color Doppler sonography increased sensitivity in the detection of metastatic disease in clinically suspicious enlarged lymph nodes from 80.9% to 95.2% but found no increase in specificity (76.2%). Similar results were obtained in later studies such as that of Ogata et al.35
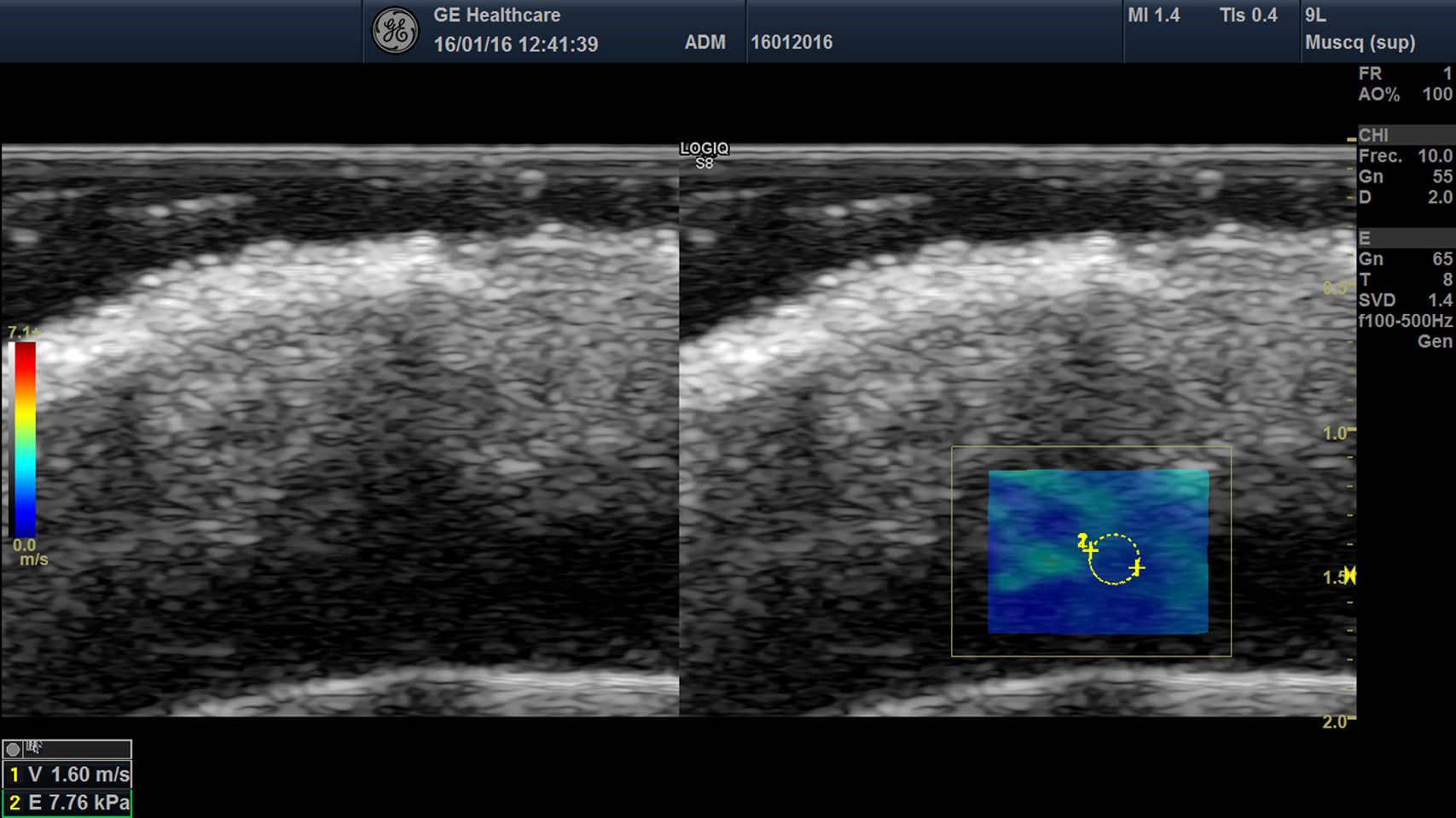
Elastography in Inflammatory Skin DiseasesNot only does inflammation cause changes in the sonographic structure of the skin and adnexa in B-mode and Doppler ultrasound,36 it also affects the stiffness of the structures7 (Fig. 8).
In a study by Gaspari et al.,37 50 patients who visited the emergency department for abscess drainage were examined using B-mode ultrasound and SE. With elastography, it was possible to observe stiff areas around the abscesses that were not visible with B-mode ultrasound.
Cucoş et al.38 measured the effects of topical corticosteroid treatment on epidermal and dermal thickness and elasticity in 16 psoriatic plaques. Epidermal thickness decreased, whereas dermal thickness increased slightly and there was no change in plaque elasticity. Despite the small number of patients in this study, the results seem to indicate that the sensitivity of SE in the treatment of psoriatic plaques is low.
Elastography has been more extensively developed in fibrotic and sclerotic processes that are primarily cutaneous or systemic (morphea/systemic sclerosis), in which clinical measurement scales have very limited sensitivity and specificity39 (Fig. 9).
Initial studies carried out with SE in systemic sclerosis, such as that of Iagnocco et al.,39 have indicated that dermal stiffness is greater in patients with systemic sclerosis than in controls. However, the reproducibility of the technique at other sites, such as the fingers, was variable, perhaps because of the proximity of the bony surface of the phalanges. Di Geso et al.40 repeated this exercise to determine the degree of correlation between measurements with SE and with B-mode ultrasound. The authors concluded that elastography reduces inter- and intraobserver variability in the assessment of dermal thickness of the fingers in patients with systemic sclerosis.
Perioral involvement is frequent in patients with systemic sclerosis.41 In a pilot study, Cannaò et al.41 described an elastographic scale based on perioral segments and showed that perioral stiffness is greater in patients with systemic sclerosis than in controls.
Elastography has also been used in other fibrotic processes, such as cutaneous processes secondary to irradiation. Using SWE to measure stiffness in the skin and subcutaneous cellular tissue in the neck, Liu et al.42 found differences between patients who had undergone radiation therapy and age-matched healthy controls (64.6±46.8kPa vs 19.9±7.8kPa). The authors correlated these changes with the degree of atrophy of the neck muscles and with the decrease in thickness of the subcutaneous cellular tissue of the neck.
Application of Elastography in Other Skin ConditionsA SE study by Suehiro et al.43 found lower strain values in patients with lipodermatosclerosis than in patients with lymphedema, regardless of the degree of lymphedema.
Elastography has also been used to assess pressure ulcers. Experimental studies in phantoms and animals have found that the stiffness of the surface skin increases quickly after sustained pressure and that this could be an early marker for the detection of areas at risk of ulceration.44
ConclusionsElastography in dermatology is an emerging technique that has great potential in the physical characterization of the tissues of the skin and adnexa. The various elastography techniques offer complementary and synergistic information in the assessment of cutaneous tissues. Dermatologists can consider scenarios in which elastography can offer complementary information that would improve patient care.
Conflicts of InterestThe author declares that he has no conflicts of interest.
The author is grateful to the dermatology department at Hospital Universitario Puerta de Hierro in Majadahonda for collaborating on the drafting of this article, and to ESAOTE Spain and General Electric Spain for collaborating with elastography equipment and software.
Please cite this article as: Alfageme Roldán F. Elastografía en dermatología. Actas Dermosifiliogr. 2016;107:652–660.