Certolizumab pegol (CZP) is an anti-TNF agent that has been recently approved for the treatment of moderate to severe plaque psoriasis in adults.1 Rheumatologists, in contrast, have been using the drug in psoriatic arthritis since 2012.2 To assess the effectiveness of CZP in plaque psoriasis, a retrospective observational, multicenter study was conducted in patients treated for psoriatic arthritis who had active plaque psoriasis at the time of treatment initiation. The severity of psoriatic arthritis was assessed using the Disease Activity Score (DAS) 28 while plaque psoriasis was assessed with the 2011 modification of the Investigator Global Assessment (IGA mod 2011) scale (5-point physical global evaluation: 0, clear; 1, almost clear; 2, mild disease; 3, moderate disease; and 4, severe disease).3
The study included 22 adult patients, 16 women (73%) and 6 men (2%), from 4 hospitals in the same province of Spain. The mean duration of plaque psoriasis and psoriatic arthritis was 16 and 12 years, respectively. All patients were diagnosed with psoriatic arthritis according to the CASPAR criteria4 and plaque psoriasis. Nineteen patients had (86%) peripheral psoriatic arthritis, 15 (68%) polyarticular psoriatic arthritis, 13 (59%) dactylitis, 12 (55%) axial psoriatic arthritis, and 12 (55%) ungual psoriasis. Eighteen patients (81%) had received prior biologic therapy, and the regimen for CZP was the usual one for psoriatic arthritis in all cases: 400 mg administered subcutaneously at weeks 0, 2, and 4, followed by 200 mg every 2 weeks.
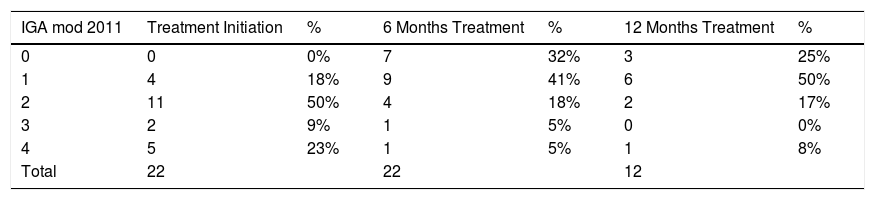
On starting treatment, mean DAS28 was 5.79 and all patients had active plaque psoriasis (IGA mod 2011 ≥ 1): almost clear in 4 (18%), mild in 11 (50%), and moderate-severe in 7 patients (32%) (Table 1).
After 6 months treatment with CZP, mean DAS28 was 3.4 and the severity of plaque psoriasis improved significantly: 16 patients (72%) were clear or almost clear of lesions, 4 (18%) had mild disease, and 2 (10%) had moderate-severe disease. After 12 months, only 12 patients had an IGA mod 2011 assessment: 9 patients (75%) were clear or almost clear of lesions, 2 (17%) had mild disease, and 1 (8%) had severe disease; and the mean DAS28 was 3.71. The statistical analysis showed significant differences (P < .05) between baseline IGA mod 2011 and that observed at 6 and 12 months, as well as a correlation between the DAS28 and IGA mod 2011 scales.
The present study has several biases and limitations, such as a small sample size and a sample comprising patients who had already received prior biologic therapy. In addition, the scale for assessing plaque psoriasis is the IGA mod 2011, given that the Psoriasis Area Severity Index is not a scale used in everyday clinical practice by rheumatologists. Despite these limitations, this study confirms a high degree of effectiveness for CZP in plaque psoriasis in patients with psoriatic arthritis, and so this agent should be considered when treating patients who present with psoriatic arthritis and moderate-severe plaque psoriasis simultaneously.
Conflicts of interestReina D and Vidal D have received speaker fees from UCB Pharma.
We would like to thank Drs. L. Mateo, A. Prior, A. Laíz, and M. Moreno for their collaboration in this study.
Please cite this article as: Reina D, Vidal D. Efectividad de certolizumab pegol en psoriasis en una cohorte de pacientes con artritis psoriásica. Actas Dermosifiliogr. 2020. https://doi.org/10.1016/j.ad.2019.01.023