Patients with basal cell carcinoma (BCC) have an increased risk of subsequent BCCs. It is possible that imiquimod might reduce this risk by acting on the cancerization field.
ObjectiveTo examine the ability of imiquimod to reduce subsequent BCCs.
MethodsRetrospective cohort study of patients with BCC treated at our hospital between 2003 and 2011. The patients were divided into 2 groups depending on whether they had been treated with surgery or with imiquimod. Comparing the 2 groups, we analyzed the development of new BCCs, the time that elapsed between first and subsequent tumors, and the site of occurrence of the second BCC with respect to the first one (local, same lymphatic drainage basin or anatomic region, or other). Survival methods were used to analyze the data.
ResultsWe reviewed the charts of 623 patients. Of these, 550 had been treated with surgery (88.3%) and 71 with imiquimod (11.4%). Overall, a second BCC occurred in 36.4% of patients (n=227). The rate of occurrence was 38.2% in the surgery group and 23.9% in the imiquimod group (P=.02). The hazard ratio for the occurrence of a subsequent BCC was 2.13 (95% CI, 1.28-3.53) for patients treated with surgery compared with those treated with imiquimod. Imiquimod reduced the risk of a second BCC locally, regionally, and in the lymphatic drainage area. Our findings are limited by the retrospective nature of our study and the small number of patients treated with imiquimod.
ConclusionsImiquimod may reduce the risk of subsequent BCC in patients treated for BCC and its effect could last for up to 2 years in local, regional and lymphatic cancerization fields. We believe that the cancerization field concept should be expanded to include not only the local area, but also the pertinent anatomic region and the regional lymphatic drainage area.
Los pacientes con carcinoma basocelular (CB) tienen riesgo de CB subsiguientes. El imiquimod podría reducir dicho riesgo mediante su efecto sobre el campo de cancerización.
ObjetivoExaminar la capacidad de imiquimod para reducir CB subsiguientes.
MétodosEstudio de cohorte retrospectivo de los pacientes con CB tratados en nuestro centro entre 2003 y 2011. Se establecieron 2 grupos según hubieran sido tratados con cirugía o imiquimod. Se comparó entre ambos la aparición de nuevos CB, analizando la proximidad del segundo CB respecto al primero (local, mismo territorio linfático, misma región anatómica, otro territorio) y el tiempo transcurrido entre el primer y segundo tumor. Para el análisis de los datos se emplearon estudios de supervivencia.
ResultadosSe revisaron 623 pacientes: 550 tratados con cirugía (88,3%), 2 con crioterapia y 71 con imiquimod (11,4%). Doscientos veintisiete pacientes (36,4%) presentaron un segundo CB (38,2% en el grupo cirugía, 23,9% en el grupo imiquimod, p=0,02). La función de riesgo (hazard ratio) de sufrir un segundo CB cuando fueron tratados con cirugía comparado con imiquimod fue 2,13 (1,28-3,53). El imiquimod mostró menor riesgo de segundo CB a nivel local, regional y en el territorio linfático. Limitaciones: la naturaleza retrospectiva del estudio y el número de pacientes tratados con imiquimod fue limitado.
ConclusionesEl tratamiento del CB con imiquimod podría reducir el riesgo de segundos CB. Este efecto podría permanecer hasta 2 años y se presentaría en los campos de cancerización local, regional y linfático. Creemos que el concepto de campo de cancerización debería extenderse no solo a nivel local, sino también regional y linfático.
Basal cell carcinoma is the most common tumor in humans and its incidence has risen dramatically worldwide in recent decades.1 Patients who have had BCC have a greater chance of developing subsequent BCCs, with the risk of a second BCC estimated at between 22% and 50%.2–5
Sun exposure is the main risk factor for skin cancer. The likelihood of a subsequent BCC developing at the site of a previous BCC and in the surrounding area is theoretically very similar as both areas have been subjected to a similar degree of sun damage. This concept is known as field cancerization and has been described in many epithelial tumors.6–8 Whether or not it also applies to BCC, however, is still a matter of debate.8
Surgery is the treatment of choice for BCC. Other alternatives include local immunotherapy, which can cure BCC by stimulating a local immune response. One local immune response modifier is topical imiquimod 5%, which has been licensed for the treatment of small superficial BCCs in adults and has a cure rate of between 43% and 100%.9
Imiquimod exerts its effect as a topical immunomodulator by activating both the innate and acquired immune systems.10,11 It has been shown to have an effect on the cancerization field in actinic keratosis.7
In this retrospective study we investigated the ability of imiquimod, when it is used to treat BCC, to prevent the development of second BCCs in the area treated, in the cancerization field, and beyond this field.
Material and MethodsThe study was conducted in a secondary care level hospital serving a predominantly urban population of approximately 130 000 inhabitants.
We retrospectively compared 2 groups of patients with histologically confirmed BCC treated with either surgery or imiquimod. We reviewed the charts of patients treated for their first BCC at our hospital between January 2003 and December 2011 and recorded the date and type of treatment (surgery vs imiquimod). Due to the retrospective design of the study, we were unable to systematically record why the treating physician decided to choose one treatment over the other. The treatment regimen for imiquimod was 5 days a week for 6 weeks in all cases.
We also made note of the main variables that can influence the occurrence of subsequent BCCs based on the findings of previous studies, namely, age, sex, number of previous BCCs, and number of simultaneous BCCs. The follow-up data recorded included, where applicable, the date at which a second BCC was histologically confirmed; the proximity of the second BCC to the first one (local, same lymphatic drainage basin, same anatomic region, other); and the duration of follow-up (to diagnosis of the second BCC, discharge from care, last routine checkup, or loss to follow-up). The follow-up period analyzed was 60 months and patients followed for less than 3 months were excluded. BCCs diagnosed within 3 months of the first tumor were considered to be synchronous. Recurrent tumors were not classified as second BCCs.
Three levels of cancerization field were defined: local, lymphatic, and regional. The field was defined as local when the second tumor occurred within 5cm of the first one, lymphatic when the first and second tumors occurred in the same lymphatic drainage basin, and regional, when the 2 tumors occurred in the same anatomic region (head and neck, anterior thorax, posterior thorax, abdomen, lumbar region, upper limbs and lower limbs). Second BCCs not in the same anatomic region as the first BCC were deemed to be outside the cancerization field.
Imiquimod-induced inflammation was classified as mild (not requiring any change in treatment), moderate (requiring the addition of a corticosteroid-antibiotic cream but no change in imiquimod treatment), or intense (requiring the temporary or permanent withdrawal of treatment).
Data were analyzed using the Kaplan-Meier survival method for the estimation of risk; the event of interest was histologic confirmation of a second BCC. The Cox proportional hazards model was used to estimate the influence of variables. The χ2 test and the Fisher exact test were used to compare categorical data. Statistical significance was set at a P value of .05. Data were analyzed using the SPSS statistical package (SPSS Inc), version 19 for Windows.
ResultsWe reviewed the charts of 623 patients with histologically confirmed BCC (349 men and 274 women). The mean (SD) age was 67.8 (12.9) years.
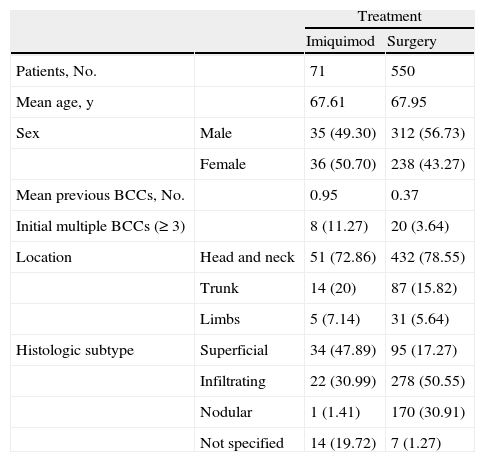
The majority of BCCs (n=484) were located on the head and neck, followed by the trunk (n=101), and the limbs (n=37). The site of 1 of the tumors was not recorded. The treatment of choice was surgery for 550 tumors (88.3%), imiquimod for 71 tumors (11.4%), and cryotherapy for 2. The baseline characteristics of the patients are shown in Table 1.
Baseline Characteristics of Patients With Basal Cell Carcinoma (BCC) Treated With Surgery or Imiquimod.a
| Treatment | |||
| Imiquimod | Surgery | ||
| Patients, No. | 71 | 550 | |
| Mean age, y | 67.61 | 67.95 | |
| Sex | Male | 35 (49.30) | 312 (56.73) |
| Female | 36 (50.70) | 238 (43.27) | |
| Mean previous BCCs, No. | 0.95 | 0.37 | |
| Initial multiple BCCs (≥3) | 8 (11.27) | 20 (3.64) | |
| Location | Head and neck | 51 (72.86) | 432 (78.55) |
| Trunk | 14 (20) | 87 (15.82) | |
| Limbs | 5 (7.14) | 31 (5.64) | |
| Histologic subtype | Superficial | 34 (47.89) | 95 (17.27) |
| Infiltrating | 22 (30.99) | 278 (50.55) | |
| Nodular | 1 (1.41) | 170 (30.91) | |
| Not specified | 14 (19.72) | 7 (1.27) | |
Of the 71 BCCs treated with imiquimod, 34 were superficial, 22 were infiltrating, 1 was nodular, and 14 were of an unspecified histologic subtype. The cure rate was 85.9% (61 of 71 tumors). Ten tumors required complementary surgery. Three of these were superficial, 5 were infiltrating, and 2 were of an unknown subtype. One infiltrating BCC recurred at 3 years. The cure rate was 91% for superficial BCC (31 of 34 tumors) and 72% for infiltrating BCC (16 of 22 tumors).
In total, 227 (36.4%) of the 623 patients developed a second BCC. The rate of occurrence of a second BCC was 38.2% in the surgery group (210 of 550 patients) and 23.9% in the imiquimod group (17 of 71 patients, P=.02).
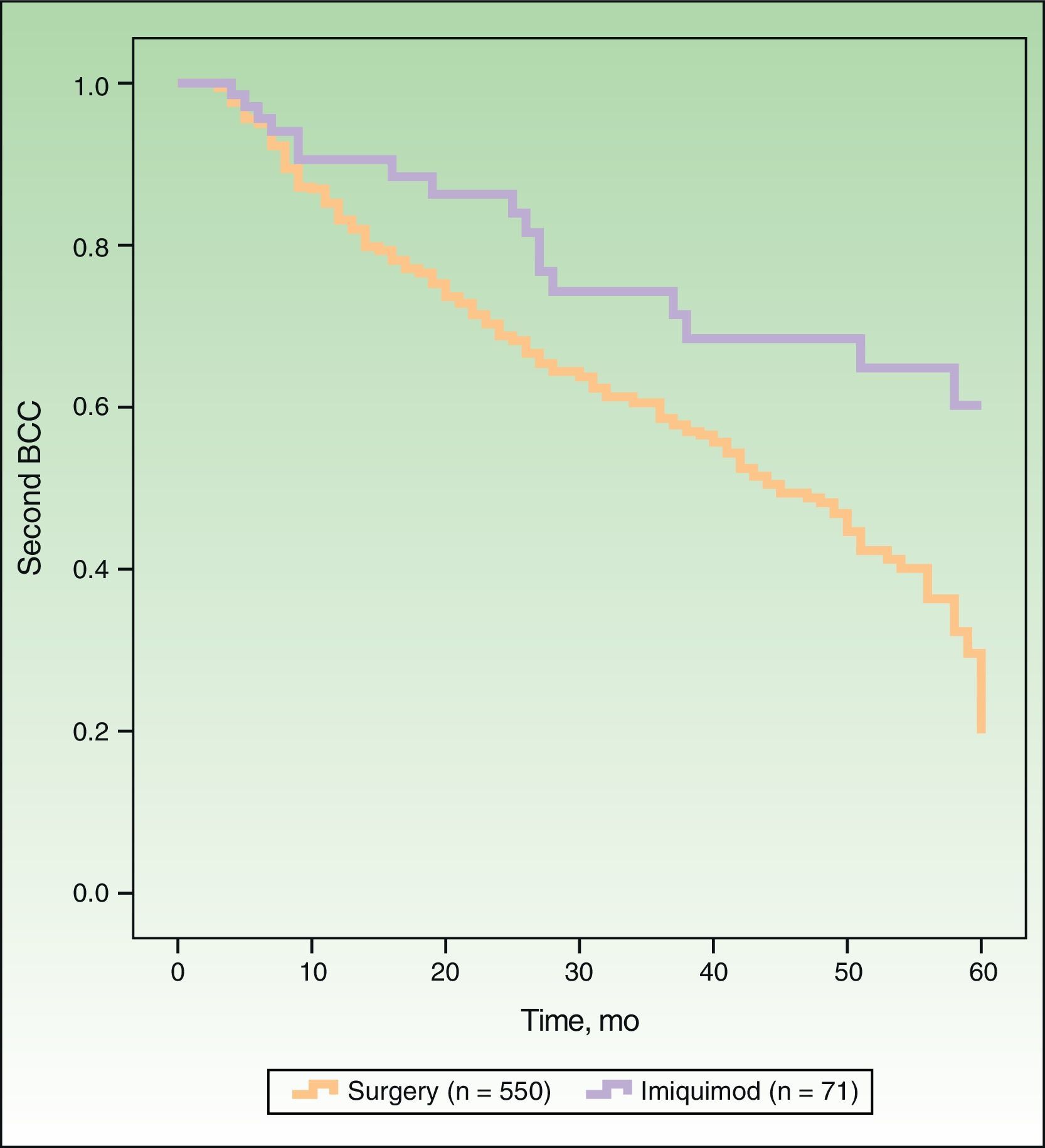
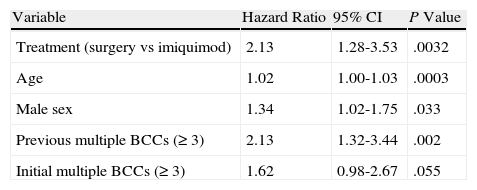
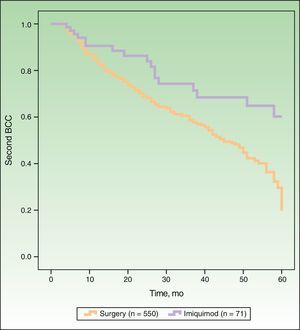
In Figure 1, the Kaplan-Meier survival curves show the risk of a second BCC according to whether patients were treated with surgery or imiquimod. Univariate analysis, with treatment type as the dependent variable, showed that surgery was associated with a significantly higher risk of a second BCC (hazard ratio [HR], 2.16; 95% CI, 1.31-3.59; P=.0026) than imiquimod. The following variables were included in the multivariate analysis: type of treatment (surgery vs imiquimod), age, sex, a past history of multiple BCCs (≥3), and current multiple BCCs (≥3). As shown in Table 2, this analysis showed significant associations between an increased risk of a second BCC and older age, male sex, previous multiple BCCs, and treatment with surgery (HR, 2.13; 95% CI, 1.28-3.53; P=.0032).
Multivariate Analysis of Risk of Second Basal Cell Carcinoma (BCC).
| Variable | Hazard Ratio | 95% CI | P Value |
| Treatment (surgery vs imiquimod) | 2.13 | 1.28-3.53 | .0032 |
| Age | 1.02 | 1.00-1.03 | .0003 |
| Male sex | 1.34 | 1.02-1.75 | .033 |
| Previous multiple BCCs (≥3) | 2.13 | 1.32-3.44 | .002 |
| Initial multiple BCCs (≥3) | 1.62 | 0.98-2.67 | .055 |
Of the 396 patients who did not experience the event (a second BCC) in our series (63.6%), 29 were event-free at the time of discharge, 300 were in follow-up at the time of the chart review, and 67 (10.7% of the whole group) had been lost to follow-up.
The analysis was repeated with a subgroup of patients who had only superficial BCC in order to create 2 comparable groups in terms of the indication of imiquimod in the summary of product characteristics (SPC). The survival curves obtained for the subgroup (data not shown) were practically identical to those found for the whole group and surgery was still associated with a higher risk of a second BCC (HR, 2.17). In this group, however, the difference was not significant (95% CI, 0.82-5.75; P=.11), probably owing to the small sample size. We did find significant differences when the imiquimod group as a whole was compared with the subgroup of patients with superficial BCC who underwent surgery (HR, 2.21; 95% CI, 1.22-3.99; P=.008).
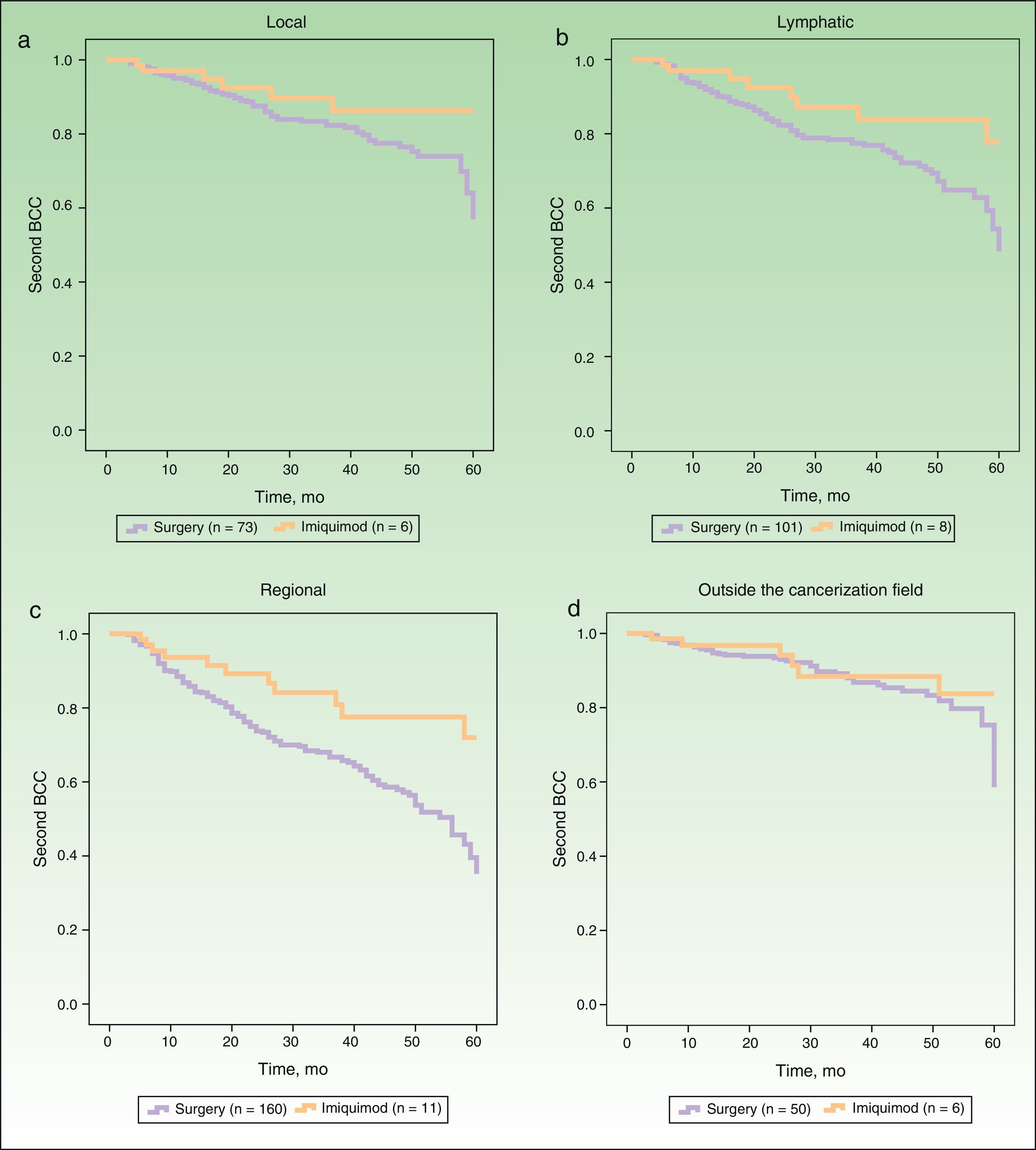
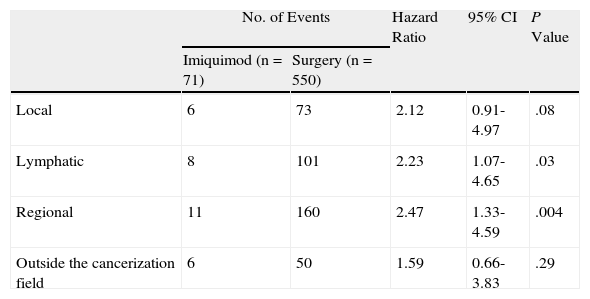
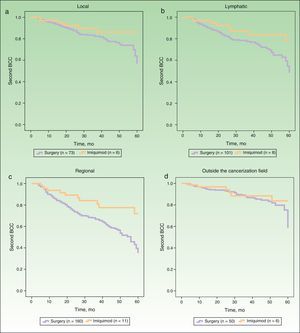
Figure 2 shows the survival curves for patients stratified by surgery and imiquimod treatment who developed a second BCC in the local, lymphatic, and regional cancerization fields (A-C) and outside the cancerization field of the first tumor (D). The univariate analysis showed significant differences in the lymphatic and regional fields, and also showed a tendency towards a protective effect of imiquimod on the local cancerization field. No significant difference in the risk of a second BCC outside the cancerization field was observed between the surgery and the imiquimod groups (Table 3).
Risk of Second Basal Cell Carcinoma Comparing Surgery and Imiquimod in Local, Lymphatic, and Regional Cancerization Fields and Outside Cancerization Field.
| No. of Events | Hazard Ratio | 95% CI | P Value | ||
| Imiquimod (n=71) | Surgery (n=550) | ||||
| Local | 6 | 73 | 2.12 | 0.91-4.97 | .08 |
| Lymphatic | 8 | 101 | 2.23 | 1.07-4.65 | .03 |
| Regional | 11 | 160 | 2.47 | 1.33-4.59 | .004 |
| Outside the cancerization field | 6 | 50 | 1.59 | 0.66-3.83 | .29 |
Most of the second BCCs (73% in the surgery group and 47% in the imiquimod group) occurred within 2 years of diagnosis of the first tumor. We examined the timing of the protective effect of imiquimod on the risk of a second BCC. We found that imiquimod tended to exert a protective effect at 12 and 24 months after the first BCC (8.5% of second BCCs vs 15.2% in the surgery group at 12 months and 2.8% vs 10% at 24 months; P=.049). At later dates, no differences were found, although the data were limited by the small number of cases.
We also analyzed whether the degree of inflammation induced by imiquimod influenced the risk of a second BCC and found no differences between patients with mild (incidence of second BCCs,27.3%), moderate (20.7%), or intense (22.2%) inflammation (P=.127).
DiscussionWe found that, compared with surgery, imiquimod exerts an effect on the cancerization field in the treatment of BCC. Patients treated surgically had a higher risk of developing a second BCC than those treated with imiquimod, with a HR of 2.16 (95% CI, 1.31-3.59) in the univariate analysis and of 2.13 (95% CI, 1.28-3.53) in the multivariate analysis.
As expected, older age, male sex, and a history of multiple BCCs, all factors identified as risk factors for subsequent BCCs in previous studies, also had a significant effect on the risk of a second BCC.
There have been reports of imiquimod exerting an effect on the cancerization field in actinic keratosis,7,8 but with the exception of 1 case report,12 we found no such reports for BCC.
Imiquimod stimulates innate and cell-mediated immunity through the toll-like receptor 7 pathway13 and induces apoptosis of neoplastic cells in BCC. It promotes the secretion of cytokines at the application site, the mobilization and activation of plasmacytoid dendritic cells, the mobilization and activation of Langerhans cells in the lymph nodes, the activation of specific cytotoxic T cells, and the accumulation of tumoricidal cells in the treated area.10
While imiquimod acts mainly at the local level, there is evidence that its effects extend beyond the application site.14 Serum concentrations of imiquimod have been detected following topical use,15 and several articles have reported systemic effects after the administration of topical imiquimod.16,17 In our practice, we have also seen some cases of flu-like symptoms in patients treated with imiquimod.
UV radiation not only acts at the level of the skin but also exerts immunosuppressive effects on the lymph nodes, impairing the antigen-presenting function of Langerhans cells.18 This could induce a cancerization field effect beyond the area directly irradiated by the UV rays. Furthermore, the mechanistic action of imiquimod on the lymph nodes would explain why its effect extends beyond the cancerization field. We hypothesize that the cancerization effect of imiquimod is not only local, but also regional and lymphatic. Akkilic-Materna et al.19 introduced the concept of lymphatic cancerization field in a report of a patient in whom topical imiquimod used to treat actinic keratosis cured a BCC in the same lymphatic region.
In our study, imiquimod had an effect not only on the local cancerization field but also on the regional and lymphatic fields. Although the differences in the local cancerization field were not significant, we did observe a clear tendency towards a protective effect of imiquimod, which would probably have reached statistical significance if more patients had been treated with this immunomodulator. It is well known that patterns of lymphatic drainage vary greatly from one patient to another, and this is a limitation for retrospective evaluation. However, the results observed for the effect of imiquimod on the lymphatic cancerization field in our series are very similar to those seen for the regional field, which is much easier to define, even retrospectively. Our findings suggest that imiquimod treatment may reduce the risk of a second BCC in the cancerization field where it is applied and in the corresponding lymphatic drainage basin and anatomic region. If our hypothesis is correct, the concept of cancerization field could be extended to include areas outside the immediate area around the primary tumor.
A clearance rate of 61% to 73% at 12 months has been reported for imiquimod in the treatment of actinic keratosis.20,21 In our series, the rate of second BCCs was lower with imiquimod than with surgery at 12 and 24 months, and the difference reached statistical significance in the second year. This finding is important, as 73% of second BCCs in the surgery group occurred within 2 years of the operation. Imiquimod could, therefore, offer an additional benefit by reducing the risk of a second BCC during the period when this risk is highest. Other studies have shown that second BCCs frequently appear within a year of diagnosis of the first tumor.2,3
According to the SPC, imiquimod is not indicated for the treatment of infiltrating BCC. In our series, however, a considerable number of infiltrating BCCs (n=22) were treated with imiquimod. The use of this drug in clinical practice may be seen as an opportunity to avoid aggressive surgery, by eliminating the tumor or at least reducing its size, regardless of whether it is superficial or infiltrating. Furthermore, not all patients who receive imiquimod undergo biopsy,22 so it is likely that some tumors thought to be superficial are actually infiltrating. In addition, depending on the area sampled, infiltrating BCCs may be diagnosed as superficial on biopsy.23
In our series, imiquimod achieved a cure rate (no recurrences or need for complementary surgery) of 84.5% (91% for superficial BCCs and 72% for infiltrating BCCs). These percentages are similar to those published elsewhere.9,11,22 The median follow-up time in our study for patients treated with imiquimod was 32.9 months, and there was just 1 recurrence, at 3 years. The finding is interesting as there is a shortage of studies of imiquimod with long follow-up periods.
Patients with BCC have a high risk of developing second and subsequent BCCs, and because nonmelanoma skin cancer is being increasingly viewed as a chronic disease,24 strategies are needed to lower the incidence of new tumors. Some authors argue that the development of subsequent BCCs is unaffected by the application of preventive measures.5,25 Patients must, therefore, be monitored over a long period, although the duration of follow-up will obviously vary from case to case.
Our results support the use of imiquimod in the treatment of superficial BCC and suggest that this drug has the added benefit of reducing the incidence of second tumors. However, it failed to achieve cure in 15% of patients. We therefore believe that surgery remains the treatment of choice and that the decision to use imiquimod should be made on a case-by-case basis. A further consideration is that treatment with imiquimod could bring additional benefit to patients with multiple BCCs.
Our study has some limitations. For instance, due to its retrospective design, we were unable to determine whether histologic subtype influenced the decision to choose surgery or imiquimod. As can be seen in Table 1, the imiquimod and surgery groups differed in several respects, the most noteworthy of which was the higher frequency of superficial BCCs in patients treated with imiquimod, which would be consistent with the indications listed in the current SPC. The proportion of patients with a history of multiple BCCs and current multiple BCCs was higher in the group of patients who received imiquimod, meaning that they had a higher initial risk of developing subsequent BCCs, an interesting observation given that the rate of second BCCs was lower in this group.
We were unable to investigate the significance of some of our findings owing to the small number of patients treated with imiquimod. The study of a larger series, ideally within the setting of a prospective, randomized trial, is needed to provide more reliable scientific evidence for these findings.
In conclusion, imiquimod may reduce the risk of second BCCs and exert an effect for up to 2 years in local, regional, and lymphatic cancerization fields. Based on our findings, we believe that the concept of cancerization field should not be restricted to the local area but extended to include both the lymphatic and regional areas.
Ethical DisclosuresProtection of humans and animals.The authors declare that no tests were carried out in humans or animals for the purpose of this study.
Confidentiality of dataThe authors declare that they have followed their hospital's protocol on the publication of data concerning patients and that all patients included in the study have received sufficient information and have given their written informed consent to participate in the study.
Right to privacy and informed consentThe authors declare that no private patient data appear in this article.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Graells J, Ojeda R, García-Cruz A. Efecto del imiquimod comparado con la cirugía sobre el campo de cancerización en el carcinoma basocelular. Actas Dermosifiliogr. 2014;105:53–59.