In biologic therapy, dose modification in carefully selected patients when psoriasis is in remission could reduce treatment costs and the risks associated with drug exposure.
Material and methodsObservational, descriptive, cross-sectional study, performed in January 2014, of 112 patients with moderate to severe psoriasis who had been on biologic therapy for at least 6 months. The therapeutic objective in all cases was to achieve and maintain a 75% reduction in Psoriasis Area and Severity Index (PASI 75). All the patients had started treatment with the standard regimen. During treatment, the dose had been reduced in patients who achieved the therapeutic objective and escalated in those who failed to respond adequately to standard doses.
ResultsAt the time of the study, 42.9% of the patients were receiving the standard dose, 50% were on a reduced dose, and 7.1% were on an escalated regimen. The agent with which the dose was most often reduced was adalimumab (57.7%), and the agents with which therapy was most often escalated were ustekinumab (17.9%) and infliximab (12.5%). Patients who received reduced doses had significantly longer-standing disease (P=.049) and longer treatment duration with the same biologic agent (P=.009). In the group that did not fulfill the criteria for dose reduction, the proportion of patients with psoriatic arthritis was significantly higher (P=.023). Cost savings were as follows: 21.5% with adalimumab, 13.8% with etanercept, 0.9% with ustekinumab, and 0.55% with infliximab.
ConclusionsPatients with longer-standing disease and longer treatment duration with the same biologic agent were significantly more likely to be candidates for dose reduction. The proportion of patients with psoriatic arthritis was greater in the group of patients who did not fulfill the conditions for dose reduction. The overall cost saving achieved using the dose modification algorithm described in this study was 13%. Controlled studies are needed to define the profile of the patients best suited for dose reduction strategies without loss of treatment efficacy.
La modificación de dosis de biológicos en pacientes con psoriasis en remisión adecuadamente seleccionados podría reducir el riesgo de exposición al fármaco y su carga económica.
Material y métodosEstudio observacional, descriptivo y transversal en 112 pacientes con psoriasis moderada-grave tratados con biológicos durante ≥6 meses en enero de 2014. El objetivo consistió en alcanzar y mantener una respuesta PASI 75. Los pacientes iniciaron el tratamiento con la pauta estándar; en aquellos que cumplieron el objetivo se redujo la dosis, y cuando no alcanzaron la respuesta con la pauta estándar esta se intensificó.
ResultadosUn 42,9% siguió la pauta estándar, un 50% la reducida y un 7,1% la intensificada. El fármaco con el que más se redujo la dosis fue adalimumab (57,7%) y los que más se intensificaron fueron ustekinumab e infliximab (17,9% y 12,5%). Los pacientes que recibieron dosis reducidas presentaron una psoriasis de más evolución (p=0,049) y llevaban más tiempo en tratamiento con el mismo biológico (p=0,009) (diferencias significativas). Hubo una proporción significativamente superior de pacientes con artritis psoriásica entre los no aptos a reducir dosis (p=0,023). El ahorro del gasto fue del 21,5% con adalimumab, 13,8% con etanercept, 0,9% con ustekinumab y 0,55% con infliximab.
ConclusionesPresentaron una probabilidad de reducción de dosis significativamente mayor aquellos pacientes con más tiempo de evolución y más tiempo bajo el mismo tratamiento biológico. Entre los pacientes sin reducción de dosis hubo mayor proporción con artritis psoriásica. El ahorro global con este algoritmo de modificación de dosis fue del 13%. Se requieren estudios controlados que ayuden a definir el perfil de paciente más adecuado para reducir la dosis sin pérdida de eficacia del tratamiento.
Psoriasis is a chronic recurrent skin disease that affects 2.3% of the Spanish population1 and between 2% and 3% of the population worldwide.2 Approximately one-third of these patients have moderate to severe psoriasis, defined as disease requiring long-term systemic therapy, which is often associated with cumulative toxicity, intolerance and, in some cases, a poor response.3
The drugs best adapted to continuous treatment are the biologic agents. In most patients treated in routine practice, a reasonable therapeutic objective with the biologic agents currently available would be an improvement of more than 75% over the baseline Psoriasis Area Severity Index (PASI 75) during induction therapy, a phase that can last up to 24 weeks. The efficacy of all the biologic agents tends to reach a plateau by the end of this phase.4
Several authors have indicated that, in patients with psoriasis in remission, continuing the standard regimen of a biologic drug could result in overtreatment, and have suggested that it may be reasonable to tailor the regimen on a case-by-case basis.5,6 A number of authors have analyzed the application of dose modification strategies to biologic therapy in patients with rheumatoid arthritis in a state of remission; the results show, in the case of tumor necrosis factor (TNF) inhibitors, that efficacy is preserved in most patients.7 Modification of the dose of a biologic drug in carefully selected patients in remission could reduce both the drug-exposure risk and the economic burden on the health care system.8 This strategy has already been proposed in other chronic diseases, such as rheumatoid arthritis, as a way to tailor treatment and identify the minimum effective dosage in each case.9–12
It is particularly useful to analyze the risks and benefits associated with the use of non-standard regimens of the different biologic agents used in the treatment of psoriasis. It is also important to identify the clinical characteristics associated with the success or failure of such off-label regimens, including the risk of relapse and the likelihood that the patient will develop specific antibodies.13 The decision to use an off-label regimen should be based on the careful selection of appropriate candidates, bearing in mind that the potential risks and benefits will depend on disease severity, the patient's quality of life, and the presence of comorbidities.13
In the literature, there are very few articles and no guidelines on the modification of biologic regimens in the treatment of psoriasis.13-15 The studies that have been done provide valuable data because they include patients with recurring disease and varying comorbidities, who would probably have been excluded from clinical trials.14 In a systematic review, Brezinski et al.13 summarized the findings of 23 trials that studied non-standard dosing regimens with etanercept, adalimumab, infliximab, ustekinumab, and alefacept in moderate to severe psoriasis. In the case of treatment with TNF and IL12/23 inhibitors, they reported that continuous regimens had been shown to be more effective than intermittent therapy in maintaining control of disease and that, for most of the biologic agents, the initial response rates were not achieved with retreatment. Other authors have observed—with etanercept, adalimumab, and ustekinumab—that escalation of the dosing regimen in nonresponders generally achieved greater efficacy.16-20
In a study by Fotiadou et al.,14 the interval between adalimumab injections was increased from 2 to 3 weeks in 14 patients with moderate to severe psoriasis who achieved and sustained a PASI 100 response after the first year of treatment. None of those patients experienced a relapse and 71% of them were followed up for 2.5 years. In a study of patients with psoriatic arthritis by Cantini et al.,8 47 (88.6%) of the 53 patients who received a reduced dose of adalimumab (40 mg every 4 weeks) maintained clinical remission after a mean (SD) follow-up of 28.9 (8.4) months.
In light of these findings, the objective of the present study was to analyze the patterns of dose modification in biologic therapy in a cohort of patients with moderate to severe psoriasis in clinical practice and the repercussions of such regimens on disease control and the cost of treatment.
Patients and MethodsThis was an observational, descriptive, cross-sectional study that included all patients with moderate to severe psoriasis who had been treated with biologic agents for at least 6 months and were in the maintenance phase in January 2014. Cases in which the patient had not achieved at least a PASI 50 response by the end of the induction phase were classified as primary treatment failures and were excluded from the study. The patients were followed up by a single investigator in a specialized hospital psoriasis unit.
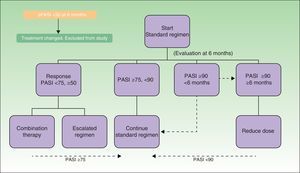
Figure 1 shows the treatment algorithm used in this study. The treatment objective was to achieve and maintain a PASI 75 response during the course of biologic treatment. All of the patients started treatment with the dosage regimen specified in the Summary of Product Characteristics (the standard regimen). In the patients who achieved remission (at least a PASI 90 response) for at least 6 months, the dose of the biologic agent was reduced by either decreasing the dose or increasing the interval between doses (reduced-dose regimen) according to a protocol agreed 2 years earlier between the hospital's dermatology department and pharmacy. When, by contrast, the standard regimen failed to produce a PASI 75 response, the regimen was escalated by reducing the interval between doses or by combining biologic therapy with a classic systemic drug. The dose reduction strategy was maintained as long as the patient continued in remission. If the patient lost the PASI 90-100 response, the standard regimen was reinstated. The escalated regimen was maintained until the therapeutic objective (at least a PASI 75 response) was achieved.
Data was collected on demographic characteristics (age, sex, weight) as well as on variables relating to the history of psoriasis (disease duration, baseline PASI, presence of psoriatic arthritis, prior systemic and/or biologic treatment) and to current biologic treatment (duration of treatment, PASI at the time of treatment modification, duration of modified regimen, and PASI at final follow-up visit).
To identify the characteristics or profiles that may make patients better candidates for biologic therapy at reduced doses, the results were analyzed to compare the patients in whom the dose was not reduced (those who received standard and escalated regimens) with those who followed a dose-reduced regimen. This analysis was performed for both continuous and categorical variables.
CostsA cost analysis was performed for the cohort as a whole and separately for each of the 3 subgroups (standard regimen, reduced-dose regimen, and escalated regimen). The cost per patient per year was calculated for each treatment regimen and drug. The unit cost of the biologic agents was calculated on the basis of the market price in Spain set by the pertinent pharmaceutical company in 2014 plus value added tax (VAT) and less the mandatory discount of 7.5%. The unit costs were €496.61 for adalimumab (40 mg syringe),21 €113.90 for etanercept (25 mg syringe), €227.80 for etanercept (50 mg syringe), €515.90 for infliximab (100 mg/20 mL vial), and €2936.27 for ustekinumab (45 mg syringe).
Statistical AnalysisA descriptive analysis of the study population was compiled from the available data. Categorical variables were described using percentages. For continuous variables, the mean was used to describe the central tendency and the standard deviation as a measure of dispersion when the distribution was normal; the median and percentiles were used in the case of non-normal distribution.
Bivariate analysis was used to analyze the association between the clinical variables and the type of regimen followed. Comparison of means was used for continuous variables and contingency tables for categorical variables. The statistical significance of the associations identified was evaluated using the statistical test appropriate to each type of variable. Statistical significance was set at a value of P less than .05. The statistical analysis was carried out using the SPSS statistical package for Windows, version 20.
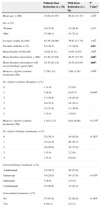
ResultsStudy PopulationThe study included 112 patients (69.6% men and 30.4% women) with the baseline patient characteristics shown in Table 1. Mean age was 49 years and mean disease duration was 23.86 years. In total, 11.6% of the patients received concomitant treatment, primarily methotrexate (5.4% of all patients studied) or acitretin (2.7%).
Demographic and Clinical Characteristics of Patients, Course of Psoriasis, and Prior Treatments.
| Total no. of patients | 112 |
| Mean age, y (range) | 49.13 (23-87) |
| Sex, n (%): men/women | 78 (69.6)/34 (30.4) |
| Mean weight, kg (SD) | 81.35 (17.01) |
| Psoriatic arthritis, n (%) | 33 (29.5) |
| Mean Baseline PASI (range) | 14.44 (7.20-29-30) |
| Mean disease duration, y (range) | 23.86 (3-62) |
| Mean no. of prior systemic treatments (range) | 2.79 (0-5) |
| No. of prior systemic treatments, n (%): | |
| 0 | 3 (2.7) |
| 1 | 11(9.8) |
| 2 | 31 (27.7) |
| 3 | 36 (32.1) |
| 4 | 23 (20.5) |
| 5 | 8 (7.1) |
| Mean no. of prior biologic treatments (range) | 0.99 (0-4) |
| Number of prior biologic treatments, n (%) | |
| 0 | 46 (41.1) |
| 1 | 32 (28.6) |
| 2 | 26 (23.2) |
| 3 | 5 (4.5) |
| 4 | 3 (2.7) |
| Current biologic treatment, n (%) | |
| Adalimumab | 52 (46.4) |
| Etanercept | 24 (21.4) |
| Infliximab | 8 (7.1) |
| Ustekinumab | 28 (25) |
| Concomitant treatment, n (%) | |
| None | 99 (88.4) |
| Topical | 1 (0.9) |
| Acitretin | 2 (1-8) |
| Acitretin+UVB | 1 (0.9) |
| Methotrexate | 6 (5.4) |
| Sulfasalazine | 1 (0.9) |
| UV-B | 1 (0.9) |
At the time of starting biologic treatment, the mean PASI score was 14.44 (range 7.20-29.30); the mean number of prior systemic treatments was 2.79 and of prior biologic treatments 0.99. The mean duration of treatment with the biologic agent studied was 38.11 months. Of the 112 patients, 52 (46.4%) received adalimumab, 28 (25.0%) ustekinumab, 24 (21.4%) etanercept, and 8 (7.1%) infliximab.
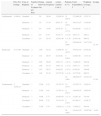
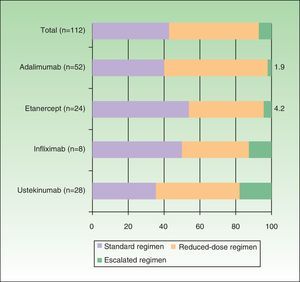
Dosage RegimensTable 2 shows the off-label regimens used in the modified dose strategies for each biologic agent. Figure 2 shows the proportion of patients following each type of regimen (standard, reduced, or escalated) for each biologic drug. Overall, 42.9% of the patients followed the standard regimen, 50% a reduced-dose regimen, and 7.1% an escalated regimen. The drug with which the dose was most often reduced was adalimumab (57.7%), and the 2 drugs with which the largest proportion of patients required an escalated regimen were ustekinumab and infliximab (17.9% and 12.5%, respectively).
Off-Label Regimens Used for Each Biologic Drug.
| Dose Reduction | Dose Escalation | |
|---|---|---|
| Adalimumab | 40mg every 3 wks | 40mg weekly |
| 40mg every 4 wks | ||
| 40mg every 6 wks | ||
| Etanercept | 25mg/wk25mg every 10 d50mg every 10 d50mg every 2 wks | 100mg/wk for a period of more than 12 wks |
| Infliximab | 5mg/kg every 9 wks5mg/kg every 11 wks | 5mg/kg every 6 wks |
| Ustekinumab | 45mg every 13 wks | 45mg every 10 wks |
| 45mg every 14 wks | 45mg every 11 wks |
Several different reduced-dose regimens were used for each biologic agent: adalimumab was administered every 3 weeks in 36.5% of all the patients receiving this biologic agent, 4 weeks (in 17.3%) and 6 weeks (3.8%); etanercept was administered at a dose of 25 mg every 10 days (8.3%), 25 mg weekly (4.2%), 50 mg every 10 days (20.8%), and 50 mg every other week (8.3%); infliximab was administered every 9 (25%) or 11 weeks (12.5%); and ustekinumab every 13 (39.3%) or 14 weeks (7.1%). In this study population, application of the dose modification algorithm based on treatment outcomes (taking into account all the patients on the standard, escalated, and reduced-dose regimens) yielded mean annual dose reductions per patient of 21% for adalimumab, 13.8% for etanercept, 0.9% for ustekinumab, and 0.55% for infliximab.
Profile of the Patients on Reduced-Dose RegimensComparison of the subgroup of patients in whom the dose of biologic drug was reduced and the rest of the patients (those on standard and escalated regimens) revealed differences, some of which were significant (Table 3). Among the continuous variables, mean duration of disease (P=.049) and total duration of treatment with the same biologic agent (P=.009) were both longer in the reduced-dose group. The comparison identified no significant differences in the following categorical variables: sex, type of drug, need for and type of concomitant treatment, or number of prior treatments (either systemic or biologic). By contrast, a higher proportion of patients in the reduced-dose group had psoriatic arthritis and this difference was statistically significant (P=.023).
Results of the Comparison of the Patients in Whom the Dose was Not Reduced (Standard or Escalated Regimens) With the Group on Reduced Dose Regimens.
| Without Dose Reduction (n=56) | With Dose Reduction (n=56) | P Valuea | |
|---|---|---|---|
| Mean age, y (SD) | 47.84 (11.97) | 50-41 (15.17) | .322b |
| Sex, n (%) | |||
| Women | 19 (33.9) | 15 (26.8) | .411c |
| Men | 37 (66.1) | 41 (73.2) | |
| Average weight, kg (SD) | 83.76 (16.06) | 78.97 (17.73) | .142b |
| Psoriatic arthritis, n (%) | 22 (39.3) | 11 (19.6) | .023c |
| Mean baseline PASI (SD) | 14.82 (5.11) | 14.07 (3.67) | .392b |
| Mean duration of psoriasis, y (SD) | 21.40 (13.04) | 26.27 (12.74) | .049b |
| Mean duration of treatment with current biologic agent (SD) | 32.70 (23.12) | 43.52 (19.91) | .009b |
| Mean no. of prior systemic treatments (SD) | 2.79(1.11) | 2.80 (1.24) | .936b |
| No. of prior systemic therapies, n (%) | |||
| 0 | 1 (1.8) | 2 (3.6) | |
| 1 | 5 (8.9) | 6 (10.7) | 0.948c |
| 2 | 17 (30.4) | 14 (25.0) | |
| 3 | 18 (32.1) | 18 (32.1) | |
| 4 | 12 (21.4) | 11 (19.6) | |
| 5 | 3 (5.4) | 5 (8.9) | |
| Mean no. of prior systemic treatments (SD) | 1.16 (1.17) | 0.82 (0.86) | 0.174d |
| No. of prior biologic treatments, n (%) | |||
| 0 | 22 (39.3) | 24 (42.9) | 0.162e |
| 1 | 12 (21.4) | 20 (35.7) | |
| 2 | 16 (28.6) | 10 (17.9) | |
| 3 | 3 (5.4) | 2 (3.6) | |
| 4 | 3 (5.4) | 0 (0.0) | |
| Current biologic treatment, n (%) | |||
| Adalimumab | 22 (39.3) | 30 (53.6) | |
| Etanercept | 14 (25.0) | 10 (17.9) | 0.478e |
| Infliximab | 5 (8.9) | 3 (5.4) | |
| Ustekinumab | 15 (26.8) | 13 (23.2) | |
| Concomitant treatment, n (%) | |||
| No | 47 (83.9) | 52 (92.9) | 0.140c |
| Yes | 9 (16.1) | 4 (7.1) | |
Notably, there was no statistically significant difference between the mean weight of the reduced-dose group and that of the rest of the patients in the study. In this study, 14 of the patients weighed over 100 kg (7 of those on the standard regimen, 2 on the escalated regimen, and 5 on the reduced-dose regimen); the 5 patients weighing over 100 kg in whom the dose was reduced were all treated with adalimumab. The dosage for the 2 patients weighing over 100 kg treated with ustekinumab was 45 mg every 12 weeks and the therapeutic objectives were achieved.
Comparison between the subgroup of patients on the escalated regimen (n=8) and the rest of the population revealed statistically significant differences in the number of prior biologic treatments (2.13 vs 0.9, respectively; P<.05) and in the proportion of patients receiving concomitant treatment (50% vs 8.7%, respectively; P<.01).
CostsBy comparing the actual cost of treatment per patient per year for the cohort studied with the theoretical cost of standard treatment for the same cohort, we can calculate the percentage savings generated by this dose modification strategy (Table 4). The results for the study population as a whole indicate that the application of this dose modification algorithm in clinical practice has led to an overall saving of 13% in pharmaceutical expenditure (the cost of biologic therapy).
Comparison of Actual and Theoretical Cost per Patient per Year of Treatment. Savings Achieved Through the Use of a Dose Modification Strategy.
| Price Per Syringe | Type of Regimen | Number of Syringes per dose | Dosing Interval, wks | Annual Frequency | Actual Annual Cost, € | Patients, n (%) | Total Expenditure, € | Weighted Average, € | Savings | |
|---|---|---|---|---|---|---|---|---|---|---|
| Adalimumab | € 496.61 | Standard | 1 | 2.0 | 26.09 | 12,956.20 | 21 (40.4) | 272,080.20 | 5,232.31 | |
| Reduced | 1 | 3.0 | 17.39 | 8,637.47 | 19 (36.5) | 164,111.87 | 3,156.00 | |||
| Reduced | 1 | 4.0 | 13.04 | 6,478.10 | 9 (17.3) | 58,302.90 | 1,121.11 | |||
| Reduced | 1 | 6.0 | 8.70 | 4,318.73 | 2 (3.8) | 8,637.47 | 166.11 | |||
| Escalated | 1 | 2.0 | 52.18 | 25,912.40 | 1 (1.9) | 25,912.40 | 498.32 | |||
| 52 (100) | 529,044.84 | 10.173,94 | –21.5% | |||||||
| Etanercept | € 113.90 | Standard | 2 | 1.00 | 52.18 | 11,886.28 | 13 (54.2) | 154,521.62 | 6,438.40 | |
| Reduced | 2 | 1.43 | 36.53 | 8,320.40 | 5 (20.8) | 41,601.98 | 1,733.42 | |||
| Reduced | 2 | 2.00 | 26.09 | 5,943.14 | 2 (8.3) | 11,886.28 | 495.26 | |||
| Reduced | 1 | 1.00 | 52.18 | 5,943.14 | 1 (4.2) | 5,943.14 | 247.63 | |||
| Reduced | 1 | 1.43 | 36.53 | 4,160.20 | 2 (8.3) | 8,320.40 | 346.68 | |||
| Escalated | 4 | 1.00 | 52.18 | 23,772.56 | 1 (4.2) | 23,772.56 | 990.52 | |||
| 24 (100) | 246,045.97 | 10,251.92 | –13.8% | |||||||
| Ustekinumab | € 2,936.27 | Standard | 1 | 12.00 | 4.35 | 12,767.53 | 10 (35.7) | 127,675.31 | 4,559.83 | |
| Reduced | 1 | 13.00 | 4.01 | 11,785.41 | 11 (39.3) | 129,639.55 | 4,629.98 | |||
| Reduced | 1 | 14.00 | 3.73 | 10,943.60 | 2 (7.1) | 21,887.20 | 781.69 | |||
| Escalated | 1 | 10.00 | 5.22 | 15,321.04 | 4 (14.3) | 61,284.15 | 2,188.72 | |||
| Escalated | 1 | 11.00 | 4.74 | 13,928.22 | 1 (3.6) | 13,928.22 | 497.44 | |||
| 28 (100) | 354,414.42 | 12,657.66 | –0.9% | |||||||
| Price per 100 mg | Type of Regimen | Weight, kg | Dosing Interval, wks | Annual Frequency | Actual Annual Cost, € | Patients, n (%) | Total Expenditure, € | Weighted Average, € | Savingsa | |
|---|---|---|---|---|---|---|---|---|---|---|
| Infliximaba | € 515.90 | Standard | 97 | 8.0 | 6.52 | 16,319.60 | 1 (12.5) | 16,319.60 | 2,039.95 | |
| Standard | 89 | 8.0 | 6.52 | 14,973.65 | 1 (12.5) | 14,973.65 | 1,871.71 | |||
| Standard | 99 | 8.0 | 6.52 | 16,656.08 | 1 (12.5) | 16,656.08 | 2,082.01 | |||
| Standard | 121 | 8.0 | 6.52 | 20,357.44 | 1 (12.5) | 20,357.44 | 2,544.68 | |||
| Reduced | 84 | 9.0 | 5.80 | 12,562.17 | 1 (12.5) | 12,562.17 | 1,570.27 | |||
| Reduced | 67 | 9.0 | 5.80 | 10,019.82 | 1 (12.5) | 10,019.82 | 1,252.48 | |||
| Reduced | 78 | 11.0 | 4.74 | 9,543.98 | 1 (12.5) | 9,543.98 | 1,193.00 | |||
| Escalated | 102 | 6.0 | 8.70 | 22,881.09 | 1 (12.5) | 22,881.09 | 2,860.14 | |||
| 123,313.83 | 8 (100.0) | 123,313.83 | 15,414.23 | – 0,5% |
When the same analysis was performed for each one of the biologic drugs, the savings were 21.5% with adalimumab, 13.8% with etanercept, 0.9% with ustekinumab, and 0.55% with infliximab.
Infliximab was the most expensive treatment option in terms of the actual cost of treatment in this cohort (€15,414/patient/year), a result was attributable to the high mean weight (92 kg) of the patients in this group.
DiscussionIn this study of routine clinical practice, we analyzed various dose modification strategies for biologic therapy in a cohort of 112 patients with moderate to severe psoriasis and the outcomes achieved in terms of disease control and cost of treatment.
Of the total of 112 patients, 52 (46.4%) received adalimumab, 28 (25%) ustekinumab, 24 (21.4%) etanercept, and 8 (7.1%) infliximab (Table 1). As this was a cross-sectional study, the uneven distribution in the cohort of the biologic agents studied may have been affected by the length of time each one of these drugs had been available on the market.
Therapeutic response was closely monitored in all the patients throughout the study and the dose modification strategy was applied according to a treatment algorithm (Figure 1). Use of this dose modification algorithm led to the following mean annual dose reductions per patient: 21% for adalimumab, 13.8% for etanercept, 0.9% for ustekinumab, and 0.55% for infliximab.
While dose modification is a common practice in dermatology departments, there are few studies in the literature providing evidence to support such strategies.13,21–23 Given that the optimization of economic resources should always be accompanied by the achievement of therapeutic objectives, more information is needed about the outcomes achieved with reduced-dose regimens, which are more common in settings where the control of expenditure is stricter.
There is currently only limited data on the safety of off-label biologic regimens. The use of such regimens should therefore be tailored to each patient, taking into account the severity of psoriasis, the patient's quality of life, and the presence of comorbidities.13 Measurement of drug levels could also facilitate the clinical decision by identifying the patients in remission who have excessively high concentrations, but most dermatology departments do not routinely monitor drug levels. Although it has been postulated that increasing the interval between doses with adalimumab may increase the risk of anti-adalimumab antibody formation,15 there is currently no evidence to confirm this hypothesis.22 With infliximab, also a TNF inhibitor, the use of lower doses or longer intervals between doses has been correlated with a higher risk of developing anti-infliximab antibodies, with a subsequent loss of treatment response.24
In our study, the comparative analysis between the reduced-dose subgroup and the rest of the patients revealed 2 significant differences: the patients on a reduced regimen had longer disease duration (P=.049) and had been on biologic therapy for longer (P=.009) (Table 3). These differences could be attributed to the fact that patients who had been in treatment for a longer period and were in remission, having achieved good disease control, might present a higher threshold for relapse so that their psoriasis could be controlled with lower doses. The differences between the groups in the PASI score were an artifact of the algorithm used, which called for a reduction of the dose when the patient was in remission. By contrast, among the patients who were not candidates for dose reduction, there was a higher proportion of patients with psoriatic arthritis (P<.05) (Table 3). It can therefore be concluded that the presence of psoriatic arthritis, a condition that influences the severity of psoriasis, could be a factor that limits a patient's suitability for a dose reduction strategy.
There are very few publications on the factors that might influence the efficacy of modified biologic regimens in patients with psoriasis and such factors could serve as predictors of suitability for off-label dosing strategies in these patients. In a study of clinical practice involving 119 Spanish patients with moderate to severe psoriasis treated with adalimumab,21 multivariant analysis established that the only independent variables predicting retention of biologic treatment were a PASI 75 response at 1 year and the ability to increase the interval between injections in the patients with the best response. In that study, the likelihood of treatment retention appeared to be greater in patients who had not previously received biologic therapy and who had the best responses at various time points, an outcome that made them good candidates for an increased interval between doses.21 In our cohort, the percentage of biologic-naive patients receiving each biologic drug was variable: 77% for etanercept, 36.3% for adalimumab, 25% for infliximab, and 17.85% for ustekinumab. These variations may have had some influence on the likelihood that the dosing regimen could be modified in each one of the biologic agents.
The present study has a series of limitations that should be mentioned. In the first place, since it was an observational study of clinical practice without any predefined protocol, the decision to reduce the dose and the timing of any such decision was based on the researcher's criteria in each case. The reduced-dose regimen was empirical and applied progressively; however, the therapeutic objective was always taken into account and the standard dose was always reinstated in the case of relapse. Furthermore, given the retrospective nature of the study, not all the patients were treated for the same period of time. In addition, only patients who achieved the therapeutic objective were included, making it impossible to obtain any information concerning clinical efficacy.
In summary, we present a cohort of 112 patients treated in routine clinical practice, 50% of whom received a reduced dose after achieving a sustained response greater than PASI 90. The patients most likely to be candidates for dose reduction were those who had a longer disease duration and had been receiving biologic therapy for a longer period. Among the patients who were not candidates for dose reduction there was a higher proportion of patients with psoriatic arthritis. The application in clinical practice of a dose modification algorithm in this population yielded an overall saving of 13% in pharmaceutical expenditure. The saving varied for each one of the biologic drugs: 21.5% for adalimumab, 13.8% for etanercept, 0.9% for ustekinumab, and 0.55% for infliximab.
Prospective controlled studies are needed to further define the profile of patients best suited for dose reduction strategies. More data is also needed on the percentage of patients who have to return to the standard regimen because of a loss of response and whether in such cases the strategy is associated with a loss of long-term treatment efficacy.
Ethical DisclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of dataThe authors declare that no experiments were performed on humans or animals for this investigation.
Right to privacy and informed consentThe authors declare that no private patient data are disclosed in this article.
Conflicts of InterestOfelia Baniandrés declares that she has received remuneration from Abbvie, Pfizer, Janssen, and MSD in respect of participation in clinical trials, speakers fees, and consultancy fees. The other authors declare that they have no conflicts of interest.
The authors would like to thank Antonio Javier Blasco and Pablo Lázaro de Mercado (TAISS) for performing the statistical analysis.
Please cite this article as: Baniandrés O, Rodríguez-Soria VJ, Romero-Jiménez RM, Suárez R. Modificación de la dosis de terapias biológicas en psoriasis moderada-grave: análisis descriptivo en condiciones de práctica clínica. Actas Dermosifiliogr. 2015;106:569–577.