Malignant melanoma accounts for 5% of all malignant skin tumors and its incidence is increasing. In the natural course of melanoma, tumors grow locally and can spread via the lymph system or the blood. Because survival is directly related to the stage of the disease at diagnosis, early detection (secondary prevention) has an impact on prognosis. Positron emission tomography (PET) is a nuclear medicine technique that generates images using molecules labeled with positron-emitting isotopes. The most widely used molecule is fluorodeoxyglucose (FDG). Because of the elevated glycolytic rate in tumor cells, which results in increased FDG uptake, greater quantities of FDG become trapped in tumor cells, enabling external detection. Today, most PET scanners are multimodal PET–computed tomography (CT) scanners, which provide more detailed information by combining morphological information with functional PET findings. The possible utility of PET-CT in patients with malignant melanoma is a subject of debate. Various questions have been raised: when the scan should be performed, whether PET-CT has advantages over conventional diagnostic methods, and whether PET-CT provides a real benefit to patients. In this review of the literature, we will analyze each of these questions.
El melanoma cutáneo supone el 5% de todas las neoplasias malignas cutáneas, con una incidencia que va en aumento. En su evolución natural el melanoma tiene un crecimiento local, posibilidad de diseminación por vía linfática y hemática. El diagnóstico precoz (prevención secundaria) determina el pronóstico de la enfermedad, ya que la supervivencia está en relación directa con el estadio al diagnóstico.
La tomografía por emisión de positrones (PET) es una técnica de medicina nuclear que utiliza moléculas marcadas con isótopos emisores de positrones para la obtención de imágenes. El más utilizado es la 18 flúor-fluorodeoxiglucosa (18F-FDG). En la célula tumoral el aumento de la tasa glucolítica principalmente determina una mayor entrada de FDG en la célula y un mayor atrapamiento, permitiendo su detección externa.
Actualmente la mayoría de equipos PET son equipos multimodalidad PET/TAC que dan una información más completa, incorporando información morfológica a los hallazgos funcionales de la PET.
La posible utilidad de la PET/TAC en pacientes con melanoma maligno es un tema controvertido que plantea varios interrogantes; en qué momento hay que realizar esta prueba, si supone una ventaja sobre los métodos de diagnóstico convencional y si proporciona un beneficio real sobre los pacientes. A través de una revisión de la literatura iremos analizando cada uno de estos aspectos.
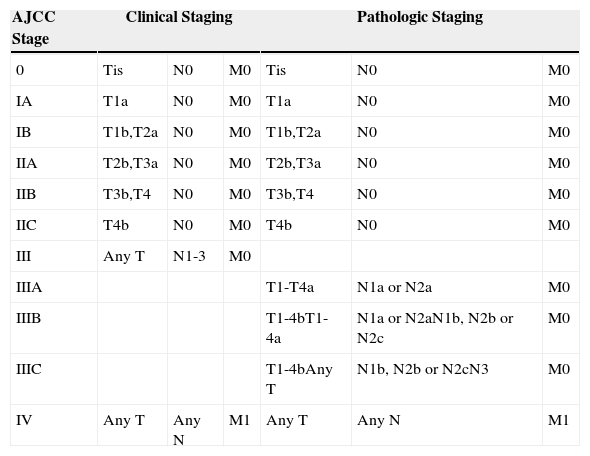
Cutaneous melanoma accounts for approximately 5% of all malignant skin cancers. It is, however, one of the cancers whose incidence has increased most in white populations around the world in recent years.1 Although it is an uncommon tumor, melanoma is considered to be very aggressive because of its ability to metastasize in early stages of the disease. Diagnosis is thus essential for adequate management. Survival is influenced by disease extent, which in turn influences both prognosis and treatment. In 2009, the American Joint Committee on Cancer (AJCC) proposed a 4-stage grouping system for cutaneous melanoma based on different risk groups (Table 1).2
American Joint Committee on Cancer (AJCC) Staging System for Cutaneous Melanoma.
| AJCC Stage | Clinical Staging | Pathologic Staging | ||||
|---|---|---|---|---|---|---|
| 0 | Tis | N0 | M0 | Tis | N0 | M0 |
| IA | T1a | N0 | M0 | T1a | N0 | M0 |
| IB | T1b,T2a | N0 | M0 | T1b,T2a | N0 | M0 |
| IIA | T2b,T3a | N0 | M0 | T2b,T3a | N0 | M0 |
| IIB | T3b,T4 | N0 | M0 | T3b,T4 | N0 | M0 |
| IIC | T4b | N0 | M0 | T4b | N0 | M0 |
| III | Any T | N1-3 | M0 | |||
| IIIA | T1-T4a | N1a or N2a | M0 | |||
| IIIB | T1-4bT1-4a | N1a or N2aN1b, N2b or N2c | M0 | |||
| IIIC | T1-4bAny T | N1b, N2b or N2cN3 | M0 | |||
| IV | Any T | Any N | M1 | Any T | Any N | M1 |
Positron emission tomography (PET) is a nuclear medicine technique that uses molecules labeled with positron-emitting isotopes to capture images. Although there are theoretically no limits on the metabolic substrates that can be used to produce radioisotopes, the most widely used radioactive tracer is fluor-18-fluorodeoxyglucose (FDG). FDG is a glucose analog that is transported into the cell in the same way as its unlabeled analog. It is not, however, a substrate for isomerase and therefore does not enter the Krebs cycle. As a consequence, it undergoes what is known as metabolic trapping. Increased intracellular uptake and trapping of FDG by tumor cells—favored by higher levels of glycolysis and an increase in specific receptors and enzymes, such as hexokinase, together with a reduction in glucose-6-phosphate (which catalyzes hexokinase)—enables external detection of this radioactive tracer.
The accumulation of FDG in tumor cells is a reflection of the increase in glucose metabolism required to sustain a high rate of growth and/or proliferation. The detection of increased FDG uptake does not necessarily indicate cancer, as accumulation of FDG is not specific to tumor tissue. Increased FDG uptake on PET scans can also indicate the presence of inflammation or infection and is probably due to the accumulation of the tracer in macrophages and granulation tissue. Such findings can give rise to false positives. False negatives, however, are also a possibility, as not all tumors are FDG-avid. Malignant lesions may not show increased FDG uptake for numerous reasons, including the presence of high levels of endogenous glucose competing with FDG for uptake, tumors that are too small to be detected (below the resolution capacity of the scanner), tumors with a mucinous, necrotic, or cystic component, well-differentiated or slow-growing tumors, and tumors in certain anatomic locations (peripheral lesions may go undetected because of the partial-volume effect and cortical lesions may display similar activity to cortical gray matter). In PET, FDG metabolism can be evaluated visually or measured semiquantitatively using the standardized uptake value, which is the ratio between the concentration of FDG (in mCi/g) in the tumor and the injected dose of radioactivity (also in mCi) divided by the patient's body weight in grams.
Most PET scanners nowadays are multimodal PET-computed tomography (CT) scanners, as by combining anatomic and functional images, they provide more detailed information. They also offer shorter scan times, which, by reducing motion due to patient discomfort, result in higher-quality, easier-to-interpret images.
The usefulness of PET-CT in melanoma is still questioned. In this review of the literature, we will look at some of the issues that remain unresolved, such as when the scan should be performed, whether PET-CT has advantages over conventional diagnostic methods, and whether it provides a real benefit to patients.
Disease StagingAJCC Stages I-IILymph Node InvolvementRegional lymph node status is one of the most important prognostic factors in early-stage cutaneous melanoma.3 Approximately 20% of patients with an intermediate Breslow thickness will have lymph node involvement at the time of melanoma diagnosis. Detection of nodal involvement is important, as lymph node dissection is only of benefit to patients with metastatic nodes. The diagnostic method of choice in patients with melanoma and nonpalpable lymph nodes is sentinel lymph node biopsy (SLNB), which has a sensitivity of between 86% and 98%. The value of PET in the detection of nodal involvement in melanoma has been analyzed in many studies, but most have reported very low sensitivity (in the range of 14% to 17%). The main limitation of PET in this regard is tumor size, as most patients with early-stage melanoma and nodal metastases have lymph nodes measuring around 2mm. Higher sensitivity rates have been reported in patients with palpable lymph nodes or lymph nodes measuring over 6 to 10mm.4,5 In the case of a patient with palpable lymph nodes, however, we would question the wisdom of using PET when a definitive diagnosis can be established through biopsy.
The combined use of PET and SLNB has, however, been shown to be of value in patients with high-risk melanoma (Breslow thickness >4mm, ulceration, high mitotic index).6
Distant InvolvementAccurate noninvasive staging is essential in melanoma, as the effectiveness of the treatments available (surgery, immunotherapy, chemotherapy, combined treatments) depends largely on the extent of disease.
PET has shown superior staging ability to conventional diagnostic methods, such as chest-abdomen CT and ultrasound, but the value of these imaging studies is questionable, as the likelihood of distant disease in early stages of the disease is very low. In most cases, PET scanning has not been found to lead to a change in stage or treatment plan.7 Furthermore, considering the low probability of distant disease in early stages of melanoma, the use of PET/CT has been associated with an increase in false positives, and consequently greater costs due to the need for additional diagnostic tests to follow up on the false-positive result.7,8
In a recent study, Wagner et al.9 analyzed 46 SLNB-positive patients without clinical signs of nodal involvement or distant metastasis who underwent PET 6 weeks after the biopsy. None of the patients had a positive PET scan: findings were negative in 40 patients and nonconclusive in the remaining 6. Five of the patients with a negative scan presented distant metastasis within a year. The authors concluded that PET was not capable of detecting distant disease in SLNB-positive melanoma patients, even in those who developed metastasis within 12 months. They proposed the low prevalence of macroscopic metastatic disease as a probable reason.
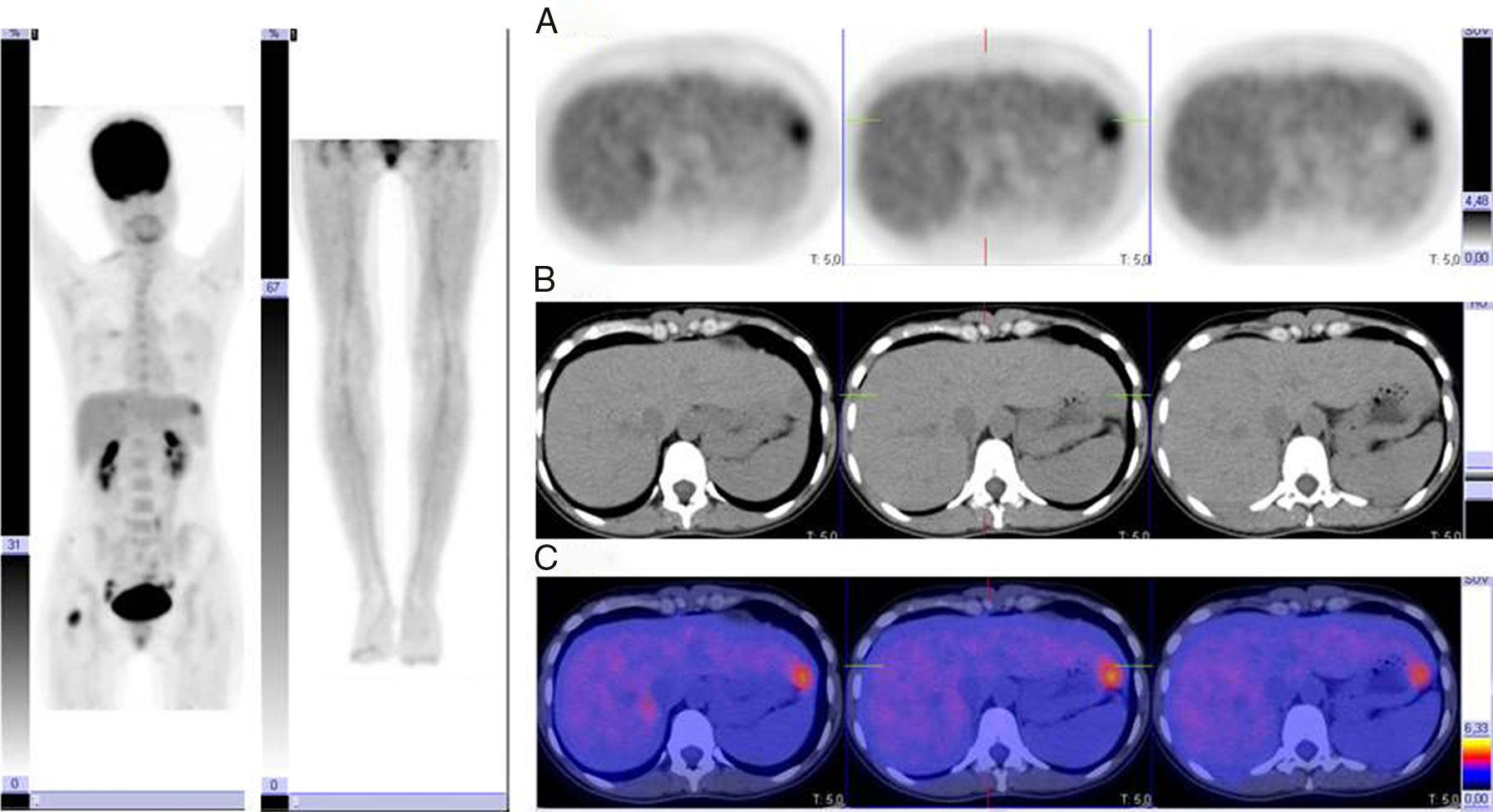
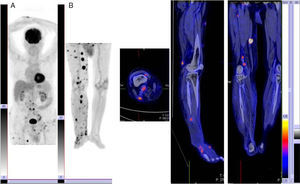
AJCC Stages III-IVPET has proven useful in the detection of distant disease in patients with stage III or IV melanoma, and performs better than conventional diagnostic techniques in this respect, with sensitivity rates of between 78% and 100%. PET can also detect disease up to 6 months earlier than conventional imaging techniques. It has been found to lead to a change in stage in 12% to 34% of cases and a change in clinical management in 6% to 8% of cases. Therefore, even though patients with stage III or IV melanoma have poor prognosis and a high rate of recurrence, staging needs to be as accurate as possible to ensure delivery of the best possible treatment. In the case of distant metastasis, PET can help to guide surgical excision by showing the number and location of lesions. It is also a helpful prognostic tool (Fig. 1).
The main limitation of PET in the detection of distant metastases is that it is unable to detect small tumors due to inadequate resolution or tumors in locations such as the liver or brain due to the high level of surrounding activity. In such cases, it may give rise to false negatives, but the situation has improved greatly since the introduction of multimodal PET/CT scanners that minimize this risk. In a study published in 2007, Strobel et al.10 analyzed patients with high-risk melanoma who underwent PET/CT study, with separate analysis of findings. On the one hand, the authors analyzed the correlation between focal uptake of FDG and CT findings (thereby reducing the number of false positives due to physiological uptake or inflammation), and on the other hand, they analyzed the CT scans for morphological changes. By using this combined approach, i.e. by analyzing CT and PET images separately, they improved the sensitivity of the procedure from 85% to 98%.
Contrast-enhanced PET imaging improves the characterization of liver lesions, but magnetic resonance imaging (MRI) is mandatory when brain lesions are suspected.
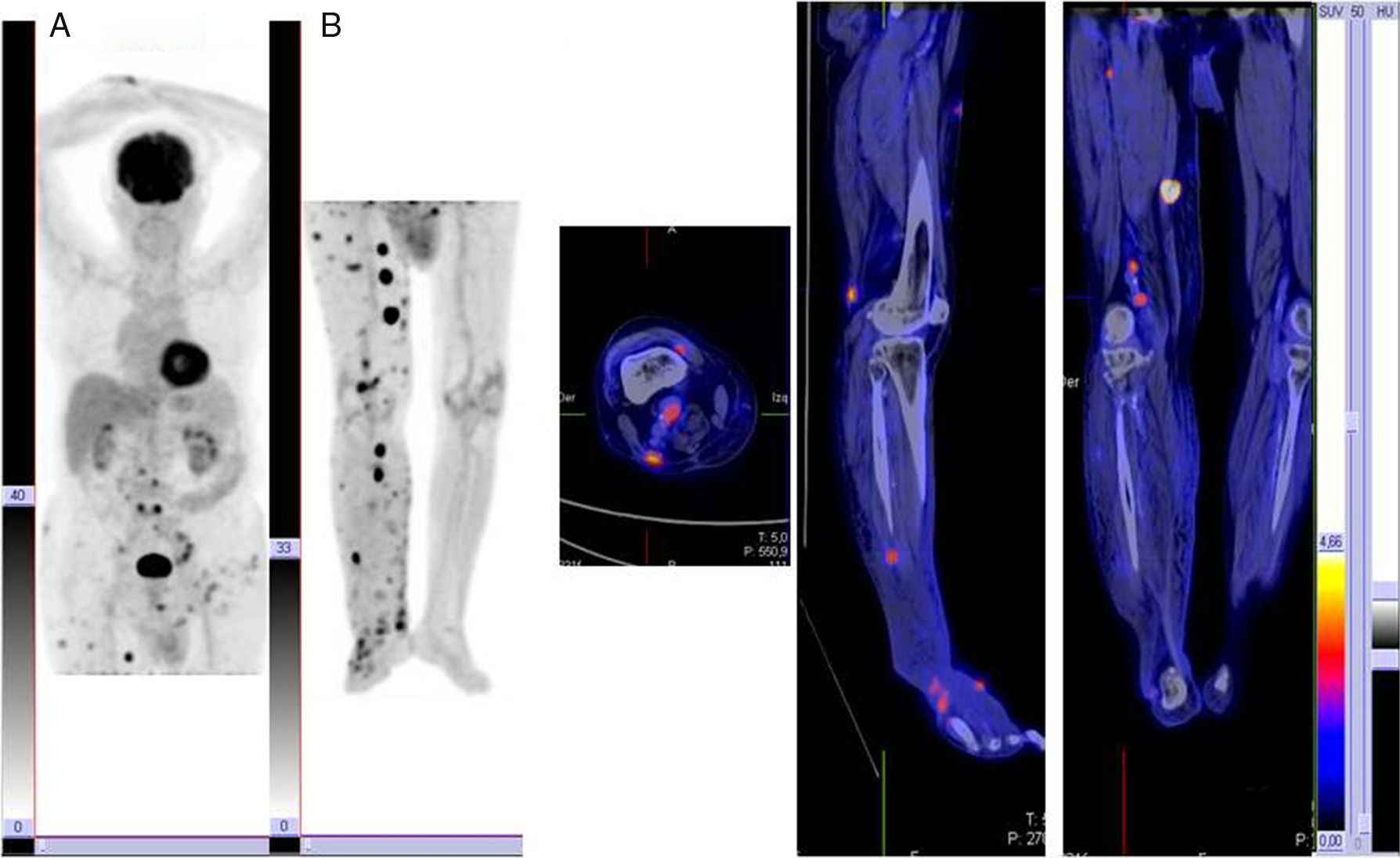
RecurrenceDetection of recurrence is one of the most widely studied and accepted applications of PET in melanoma, with sensitivity, specificity, and diagnostic accuracy rates of between 70% and 100% reported by different studies and meta-analyses. Compared with conventional diagnostic methods, PET performs better in pinpointing the location of both locoregional and distant metastases.11,12 Most lesions that go undetected by PET are, as we have already mentioned, small or located in the liver, lungs, or brain. PET thus is an essential staging tool in recurrent melanoma and should be used in combination with MRI in the case of brain metastases.
The incidental detection of second tumors (benign or malignant) during a PET study can lead to changes in treatment plans, such as cancellation of surgery or modification of surgical procedures or systemic therapy.
PET findings can lead to treatment changes in between 8% and 61% of such cases.
Assessment of Treatment ResponsePET is a highly effective tool for assessing response to chemotherapy or immunotherapy. It has been known for some years that the changes in glucose metabolism that occur after one or more treatment cycles precede changes that can be detected by conventional imaging techniques, such as CT and MRI. Few studies have analyzed the use of PET to assess response to chemotherapy in metastatic melanoma due to the poor response rates, but most of those that have agree that PET could be useful and is clearly superior to the use of the biomarker S-100B.
One of the first studies to investigate the value of PET in the assessment of treatment response in melanoma patients was that by Mercier et al., 13 who studied patients with locally advanced melanoma who underwent PET before and after hyperthermic isolated limb perfusion with melphalan and/or tumor necrosis factor. Pretreatment scans were performed to establish the true extent of disease and to confirm the suitability of patients for the scheduled treatment. The authors reported that PET was more sensitive than the standard protocol used at the center (88% vs 65%). In another study, Beasley et al.14 reported that while PET identified few cases of complete response after treatment, it had important prognostic value in terms of predicting survival and duration of complete response. Furthermore, protocol-based PET scanning can detect localized disease outside the limb in which surgical resection is indicated (Fig. 2).
In the case of assessing response to chemotherapy, the metabolic image captured by PET can identify nonresponders and therefore avoid unnecessary treatments and toxic effects. There also appears to be a relationship between survival and metabolic response as assessed by PET.15 Chemotherapy response by PET imaging has been found to be associated with longer overall survival, particularly in the first year, and longer disease-free survival. This association was not detected using S-100B.16
Conclusions- •
Lymph node status is the most important prognostic factor in patients with cutaneous melanoma. SLNB permits the microscopic detection of lymph node involvement, enabling accurate staging and appropriate treatment. FDG-PET is not a substitute for SLNB in such cases due to its lower sensitivity in the detection of microscopic disease.
- •
FDG-PET may be a useful initial staging tool in melanoma patients with risk factors for distant metastases.
- •
The main indication of FDG-PET in cutaneous melanoma is to detect recurrence or to restage disease following the detection of recurrence, particularly in patients who are candidates for resection with curative intent.
- •
PET is a highly effective technique for assessing response to chemotherapy or immunotherapy, as metabolic changes precede morphologic changes detected by conventional techniques such as CT and MRI. It also helps to identify nonresponders and therefore avoids unnecessary, toxic treatments.
The authors declare that they have no conflicts of interest.
Please cite this article as: Sánchez-Sánchez R, Serrano-Falcón C, Rebollo Aguirre AC. Diagnóstico por imagen en dermatología: utilidad de la tomografía por emisión de positrones-tomografía computarizada en el melanoma cutáneo. Actas Dermosifiliogr. 2015;106:29–34.