Homeostasis, whose regulation at the molecular level is still poorly understood, is intimately related to the functions of epidermal stem cells. Five research groups have been brought together to work on new in vitro and in vivo skin models through the SkinModel-CM program, under the auspices of the Spanish Autonomous Community of Madrid. This project aims to analyze the functions of DNA methyltransferase 1, endoglin, and podoplanin in epidermal stem cell activity, homeostasis, and skin cancer. These new models include 3-dimensional organotypic cultures, immunodeficient skin-humanized mice, and genetically modified mice. Another aim of the program is to use skin-humanized mice to model dermatoses such as Gorlin syndrome and xeroderma pigmentosum in order to optimize new protocols for photodynamic therapy.
La homeostasis de la piel, cuya regulación molecular es aún bastante desconocida, está íntimamente relacionada con la función de las células madre epidérmicas. El programa SkinModel-CM, auspiciado por la Comunidad de Madrid, reúne 5 grupos de investigación con el propósito de desarrollar nuevos modelos experimentales in vitro e in vivo para analizar la función de ADN metiltransferasa 1, la endoglina y la podoplanina en la actividad de las células madre epidérmicas y en la homeostasis y el cáncer cutáneos. Estos nuevos modelos comprenden tanto cultivos organotípicos 3D, como ratones inmunodeficientes con la piel humanizada y ratones modificados genéticamente. Otro objetivo del programa es el uso de ratones con la piel humanizada como modelo para reconstruir enfermedades cutáneas, tales como el síndrome de Gorlin y el xeroderma pigmentoso, con el objeto de optimizar nuevos protocolos de intervención mediante la terapia fotodinámica.
Although the skin is the most extensive and accessible organ in our body, the biological processes that regulate its homeostasis are still not well understood. The skin is not just a simple barrier to protect us from harmful external agents and prevent water loss but rather one of the most complex and dynamic tissues in our body. It would not be an exaggeration to say that the skin is as complex as the nervous system but with one essential difference: the brain cannot replace damaged neurons whereas skin is constantly renewed and can repair a wound, repopulating skin tissue with new cells. Knowledge of the biological and molecular basis governing the architecture, differentiation, and regeneration of the skin is crucial for understanding skin diseases and developing appropriate treatments. Basic research encompassing efforts to move both from the laboratory to the clinic and from the clinic to the laboratory (what is known as translational research) have given a strong impulse to our knowledge of skin diseases. An example is the recent identification by several international research consortia of 15 new susceptibility loci for psoriasis in individuals in the European population, increasing the number of genes associated with this disease to 36.1 Many of these genes are associated with innate immunity and are shared by other autoimmune diseases, thus highlighting the importance of the skin in innate and acquired immune defense.
Molecular oncology studies have enabled the identification of key intracellular signaling pathways in skin cancer. An example is the frequent activation of the mitogen-activated protein kinases in malignant melanoma as a result of mutations in braf (the mammalian homolog of the v-raf oncogene of murine sarcoma virus identified in the brain) or nras (the mammalian homolog of rat sarcoma virus identified in neuroblastoma).2 The finding that hedgehog signaling is responsible for the formation of basal cell carcinomas (BCC) was also a major breakthrough.3 These advances have enabled the identification of new pharmacological targets and the incorporation into clinical oncology of more selective and individualized treatments for skin cancers, such as vemurafenib, a BRAFV600E mutant protein inhibitor (the braf mutation is the main one in BRAFV600E-positive cancers) in melanoma4 and vismodegib, an antagonist of the Smo (Smoothened) membrane protein implicated in the activation of the hedgehog pathway for the treatment of advanced BCC.5
Skin disease accounts for almost 20% of the caseload in primary care, and skin cancer is the most highly incident cancer in the general population. In addition to the most common noncancer diseases, such as dermatitis, acne, and psoriasis, there are more than 400 hereditary diseases of low prevalence, known as genodermatosis, which overall account for a significant percentage of the caseload in dermatology departments. The etiology of many of these diseases is well known, but in other cases, the causes are complex and we are still a long way from an in-depth understanding. Despite advances in diagnosis and improved knowledge of the molecular biology of many pathological skin processes, corresponding progress in treatment has not been made. For example, in skin cancer, surgical excision, accompanied by chemotherapy and/or radiotherapy, is still the most widespread approach. This is partly because appropriate experimental models are not available for testing of new drugs and new therapies before embarking on costly and often fruitless clinical trials.
The skin is a fascinating biological model for basic science and one that transcends the confines of dermatology. The epidermis was the first organ in which stem cells were identified in situ through a label retention technique (see below) and, along with the hematopoietic system, is the tissue where stem cells are best characterized. In addition, a close relationship has been demonstrated between stem cells and cancer, on the one hand, and between stem cells and ageing, on the other. Biomedical engineering and tissue engineering in particular have revolutionized dermatology research, providing new in vitro and in vivo experimental models for studying the majority of genodermatoses and for testing new therapies. The development of skin-humanized mouse models has enabled human skin diseases to be reproduced in mice.
SkinModel is the name given to a platform that has brought together 5 research groups in the Spanish Autonomous Community of Madrid. These groups are located in the Hospital Santiago Ramón y Cajal, the Autonomous University of Madrid, Spanish Centre for Energy, Environmental and Technological Research (abbreviated in Spanish to CIEMAT), and the Spanish National Research Council (abbreviated in Spanish to CSIC). This program consists of developing new cell-based and animal models to investigate the molecular mechanisms implicated in regulating epidermal homeostasis and to mimic certain skin diseases for testing of new therapeutic approaches.
Stem Cells and Epidermal HomeostasisIn pluricellular eukaryotes, stem cells comprise a very well-defined cell population, characterized by 2 unique properties: their capacity for differentiation into one or more cell types and their capacity for self-renewal, giving rise to cells with the same potential for differentiation. In mammals, these cell types can be of embryonic origin or adult cells. Embryonic stem cells exercise their activity over a short period of time during the development of the fertilized egg, giving rise to all the embryonic germ lines that subsequently develop into the tissues of the adult organism. Adult stem cells, in contrast, are responsible for maintaining homeostatic equilibrium in the tissues of the adult organism, that is, for maintaining the renewal rate of different cell types in each tissue and regenerating or repairing tissue after damage. These cells, which are located at specific sites known as niches, are able to differentiate into all cell types in their host tissue.6 This niche is a key element in the functional regulation of adult stem cells. The somatic cells that make up the niche of a tissue interact with resident stem cells, establishing molecular signaling that regulates their proliferative activity and capacity to differentiate.
The biology of stem cells has been a subject of increasing interest in biomedicine. Regenerative medicine makes clinical use of stem cells, whether of embryonic origin or adult cells, as a tool for generating new organs or tissues in vitro to replace the damaged tissue of the patient. The possibility of reverting a somatic cell of an individual into the pluripotential state of an embryonic cell (such cells are known as induced pluripotent stem cells or iPS) has attracted great interest given the potential application in regenerative medicine. This technique, however, is associated with certain problems as it requires the insertion of exogenous genes which are usually silent in the adult organism. Reexpression in tissues may therefore lead to unpredictable harmful effects, such as tumor formation.7 In addition, the use of totipotential embryonic stem cells, that is, cells with embryonic characteristics is associated with ethics controversy, given the need for human embryos and the potential for reproductive cloning to create genetically identical individuals. An interesting alternative to overcome these problems is the use of stem cells resident in adult tissues. These cells can differentiate into a variety of cell lines (including cell lines other than those that make up the original tissue) but without the possibility of generating a complete organism. A broad understanding of their biology and the biology of the surrounding tissue (their niche) is a prerequisite for appropriate use of these cells in regenerative medicine.
It has recently been reported that, for some types of cancer, only a minority of the cancer cells are able to initiate the formation of a new tumor; most tumor cells are differentiated cells that have lost their capacity for self-renewal in vivo. These observations have given rise to the concept of the cancer or tumorigenic stem cell, a new paradigm in oncology. This new conceptual framework has generated much debate, particularly when associated with the design of new therapeutic strategies8 as this subpopulation of tumor initiating cells, or cancer stem cells, appear to be resistant to conventional chemotherapy and radiotherapy.
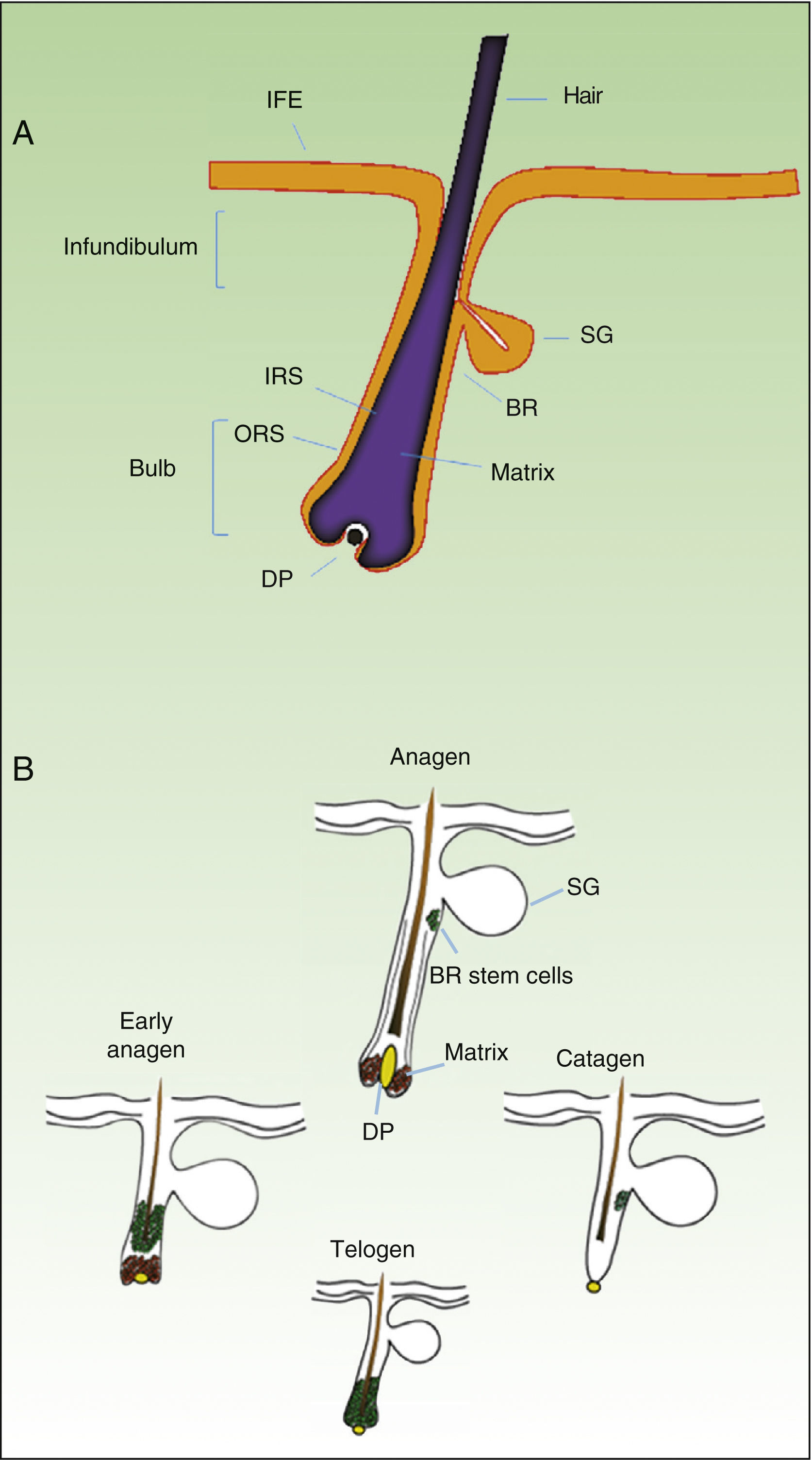
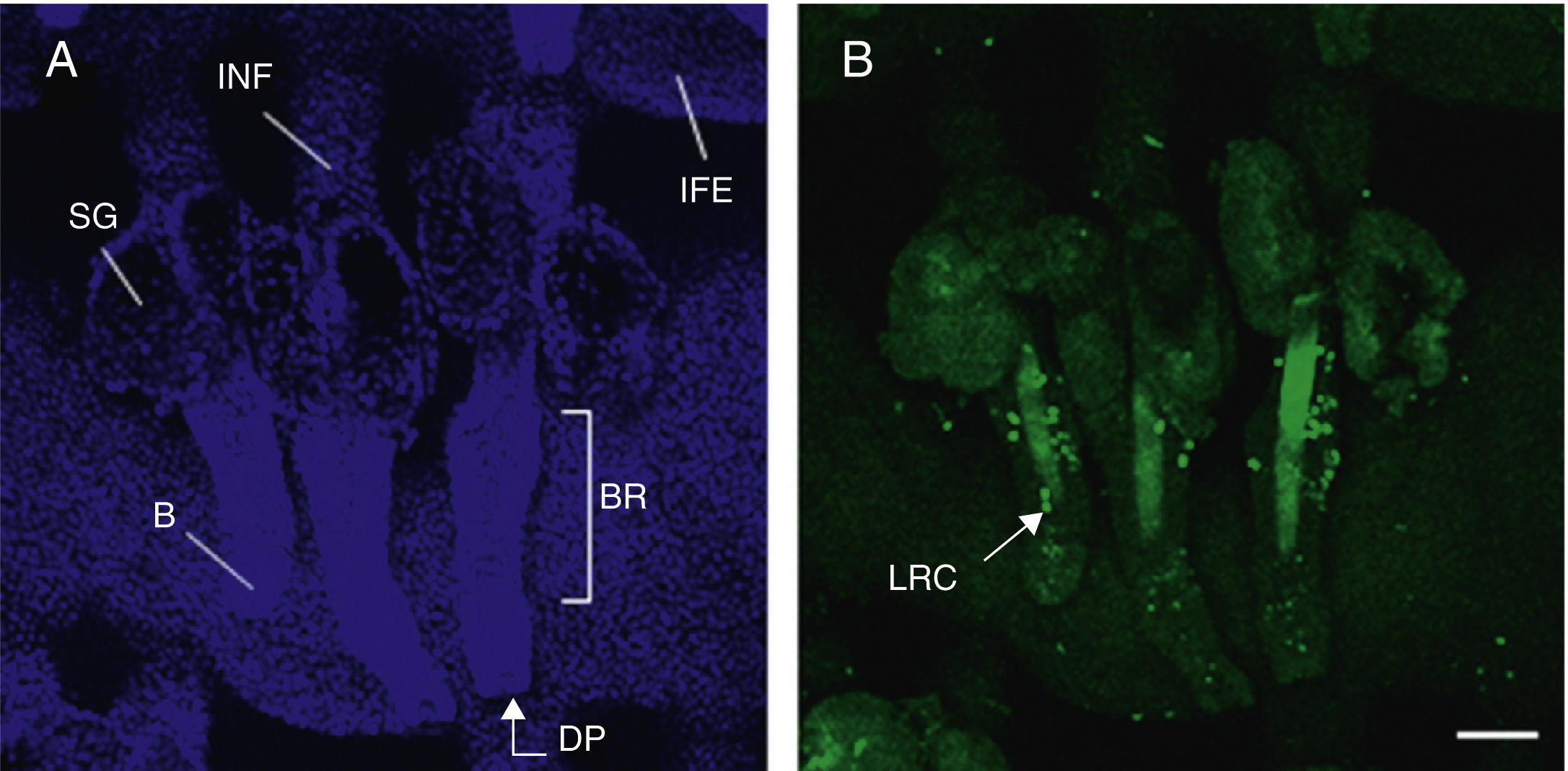
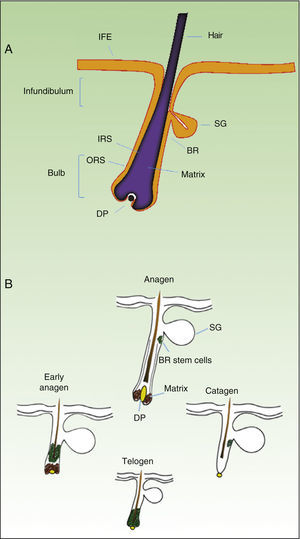
As mentioned earlier, homeostatic control of the epidermis is achieved through the activity of a small population of multipotential stem cells that rarely divide and that have a high differentiation potential. This population responds to the pathophysiologic needs of the tissue, generating numerous progenitor cells with a high replication capacity and a low differentiation capacity. These cells spread through the basal layer of the epidermis, forming a transit-amplifying (TA) cell population. Cotsarelis et al.9 were the first to demonstrate that the bulge region of the hair follicle (Fig. 1A) is a niche for epidermal stem cells. These authors used the low proliferative rate of stem cells in vivo to help in their identification. In their approach, mice were injected with tritiated thymidine, which binds binding to cell DNA during replication. After allowing sufficient time to elapse, only stem cells from the bulge region of the follicle retain the radioactive label (and so are denoted label-retaining cells [LRCs]). More recent studies suggest that the hair follicle contains other stem cell populations, some of which are more active than LRCs from the bulge region.10,11 Progenitor cells have also been found in the basal layer of the interfollicular epidermis. Currently, there is still debate as to why a wide variety of epidermal stem cell populations are localized to different regions of the follicle (bulge, infundibular, and papillary dermis) and the interfollicular epidermis, where they express specific protein markers. In any case, these cells produce cell lines that form the hair follicle, the sebaceous gland, and the epidermis. Progenitor cells of the bulge region of the follicle not only contribute to hair regeneration but also participate in wound healing, as they are able to emigrate to the interfollicular epidermis and differentiate into keratinocytes.10 This population of progenitor cells is strongly multipotent and is able to differentiate in vitro not only into keratinocytes but also into neurons, glial cells, melanocytes, and mesenchymal cells.12 Moreover, in vivo, this population participates in the angiogenic processes that take place in the skin.
Hair Follicle CycleThe hair follicle is a very well characterized biological model in mice for the study of the biology of epidermal stem cells and of signals implicated in molecular dialogue between the follicular epithelium and adjacent tissue. This process is known as the epithelial-mesenchymal interaction. The growth cycle of the hair follicle in mice occurs in synchrony for a period of time after birth whereas in humans it is strictly regulated through its 3 phases: anagen (growth), catagen (cessation), and telogen (rest).13 These phases only affect the lower part of the follicle whereas the upper part of the follicle, including the bulge region, remains intact; these regions form the permanent region of the follicle (Fig. 1B). During the first telogen-anagen transition, molecular signals from the dermal papilla (of mesodermic origin) induce asymmetric division of stem cells localized to the base of the permanent zone of the follicle. In addition to the production of new stem cells, this generates an active population of TA cells that migrate and differentiate to form a bulb around the dermal papilla, which in turn advances towards the inner part of the dermis to give rise to the cyclic region of the follicle. Towards the middle of the anagen phase, the stem cells in the bulge region abandon the cycle and return to their usual quiescent state. The anagen phase ends when the proliferative potential of the TA cells is exhausted. This marks the beginning of the catagen phase, in which the bulb is degraded and the dermal papilla retracts and ascends until coming into contact with the bulge region (Figs. 1B and 2). This ascension is crucial for establishing subsequent epithelial-mesenchymal interactions between the dermal papilla and the bulge region. These interactions will trigger entry into the anagen phase.
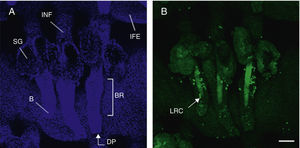
Hair follicle morphology. Confocal microscopy (maximum projections) of in toto images of the epidermis of mouse tail.
A. Triplet of hair follicles; nuclear staining with Höechst 33258.
B. Detection of stem cells in the bulge region of the hair follicle through immunofluorescence. After long-term marking with 5-bromo-2′-deoxyuridine (BrdU), the epidermal stem cells are identified according to their capacity for long-term retention of the marker of this nucleotide analogue by incorporating its DNA. Cells able to do this are known as label-retaining cells. Scale bar: 100¿m.
Abbreviations: B, bulb; BR, bulge region; IFE, interfollicular epithelium; INF, infundibulum; DP, dermal papilla; SG, sebaceous gland.
In the last 10 years, the molecular mechanisms that regulate the follicular activity have been rigorously characterized. Thus, it has been possible to establish that the Wnt/¿-catenin signaling pathway plays a crucial role in the hair follicle cycle, activating stem cells in the bulge region and inducing transition from the telogen phase to the anagen phase.13,14 On the other hand, the transforming growth factor (TGF)-¿/bone morphogenetic protein (BMP)/Smads pathway is also essential for appropriate regulation of the hair cycle.13–15 BMPs are secreted at the start of the telogen phase by cells in the dermis and bulge region, inducing stem cells to enter a state of quiescence and favoring the transition of the follicle to the rest phase. Similarly, different factors of the TGF-¿ family are implicated in the transition of the follicle to the telogen phase. Equilibrium is therefore reached between stimulatory and inhibitory signals, regulated sequentially during the hair cycle by these signaling pathways, which ultimately depend on strict control of the activity of stem cells in the bulge region of the follicle. However, many of the mechanisms that regulate the activity of these pathways are still unknown.
Recently, it has been shown that different DNA methyltransferase-dependent epigenetic mechanisms may play an essential part in the control of Wnt/¿-catenin-mediated signaling.16 One of the objectives of the SkinModel-CM program is to analyze the relevance of DNA methyltransferase 1 (Dnmt1) in Wnt/¿-catenin signaling and its impact on epidermal stem cell activity. Moreover, some members of the consortium have shown that endoglin, an auxiliary TGF-¿/BMP receptor, plays an important role in epidermal homeostasis and skin cancer, as it regulates signaling induced by factors of the TGF-¿ family through Smad proteins.17 For this reason, another of the lines of research of the SkinModel-CM program is to analyze the function of endoglin in the regulation of the activity of epidermal stem cells. Both targets, DnmT1 and endoglin, are implicated in the generation of new experimental models with genetically modified mice.
Experimental Models: The MouseThe mouse is the preferred animal for dermatological research. This is because the animals are inexpensive and easily handled. In addition, mouse skin is organized in a similar fashion to human skin (although with some important differences, as we will see later). Mouse models have made a decisive contribution to the progress in important areas of dermatology, such as immunology and cancer. In the case of cancer, we should mention the major contributions of mouse skin models of chemical carcinogenesis, with the formulation of concepts such as tumor initiation, promotion, and progression. This model offers an experimental system for analyzing the molecular mechanisms implicated in these stages.18,19
In the model of chemical carcinogenesis of mouse skin, cutaneous tumors are induced through the application of chemical agents to the skin of the animals; generally a single dose of a mutagenesis initiator, such as dimethyl benzanthracene, is used followed by repeated applications of tumor promoter, such as the phorbol ester 12-O-tetradecanoylphorbol-13-acetate (TPA).20 This protocol generates numerous benign papillomas, most of which are reabsorbed into the skin when the stimulus from the promoter is withdrawn. Nevertheless, a small proportion (which varies according to the strain of mice) progresses spontaneously to malignant squamous cell carcinomas (SCCs). This model was the first to demonstrate experimentally the causal implication of the Ras oncogene in carcinogenesis,19 and since then, the model has been widely used to examine the function of other genes in tumor initiation and/or progression.21 It is also an excellent system for the genetic study of cancer susceptibility or, in other words, for identifying genes associated with greater resistance or susceptibility to carcinogenesis.22
The model of mouse skin carcinogenesis was key for the studies of the function of podoplanin and endoglin in skin homeostasis performed under the auspices of the SkinModel-CM program. Podoplanin (also known as PA2.26 antigen, Aggrus, or T1¿) was identified as a surface glycoprotein of basal keratinocytes of the interfollicular epidermis and hair follicles. Expression of this molecule is induced during wound healing and skin carcinogenesis.23 Levels increase significantly in poorly-differentiated, very aggressive SCC. Subsequent studies have shown that podoplanin promotes cell migration/invasion and is able to induce epithelial-mesenchymal transition in premalignant keratinocytes and other epithelial cells.24,25 In the case of endoglin (CD105), application of a chemical carcinogenesis protocol with 7,12-dimethylbenz(a)anthracene and TPA to skin of heterozygous mice for this protein (Eng+/−) led to a singular phenotype: a decrease in the overall tumor yield and a drastic acceleration of malignant progression toward the formation of undifferentiated carcinomas.26 This phenotype is similar to the one obtained several years previously by Rosemary Akhurst's group with transgenic mice that overexpress TGF-¿1 in the epidermis.27 The results obtained by this group were used as proof of concept for establishing the double function of TGF-¿1 in carcinogenesis: as a suppressor in the early phases of tumor development and as a promoter of malignancy.28 The carcinogenic phenotype of both types of mouse—haploinsufficient for endoglin and those that overexpress TGF-¿1—is the same because endoglin acts by attenuating TGF-¿1 signaling, both in keratinocytes and in other epithelial cells.29
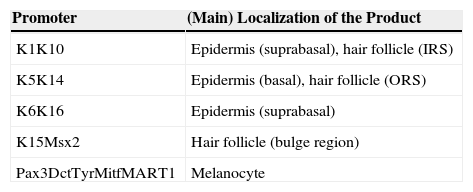
Genetic Models With Mutant Mice for Dermatological ResearchThe development of modern genetic engineering techniques led to the generation of transgenic and knockout mice30,31 and in turn to the first experimental models with genetically modified mice. With these, it was possible to achieve the expression (or lack of expression) of a certain gene in almost all tissues of the organism. Nevertheless, researchers soon became aware of the need to restrict the modification introduced in the embryo and direct the expression of the transgene to a given tissue to avoid possible nonspecific effects and, in some cases, to prevent the death of the animal. Restricted expression of the exogenous gene in the animal was achieved by using tissue-specific genetic promoters. In the case of the epidermis, the most widely used promoters are those of the genes that code for keratins K14, K5, K1, and K10. Promoters of other epidermal genes such as loricrin and involucrin have also been used successfully, as have specific melanocyte promoters (Table 1). Use of these promoters is not without difficulty, as epidermal keratins are also expressed in other stratified epithelia and, moreover, promoters of these keratins are activated during embryogenesis as soon as the epidermis is formed. This may cause anomalies in animal growth and may even lead to death if expression of the transgene is not tolerated. Investigators have attempted to overcome these difficulties through the use of inducible promoters, such as the gene that encodes keratin K6 (Table 1). This gene is normally inactive in the epidermis and is activated during wound healing or by hyperproliferative stimuli such as depilation and application of TPA.32,33 Other strategies have used tetracycline-inducible systems (Tet-On and Tet-Off).34 These models have been decisive for demonstrating the role of certain oncogenes and suppressor genes in skin cancer and inflammatory processes of the skin.35
Gene Promoters Used to Direct Transgene Expression to the Skin of Genetically Modified Mice.
| Promoter | (Main) Localization of the Product |
|---|---|
| K1K10 | Epidermis (suprabasal), hair follicle (IRS) |
| K5K14 | Epidermis (basal), hair follicle (ORS) |
| K6K16 | Epidermis (suprabasal) |
| K15Msx2 | Hair follicle (bulge region) |
| Pax3DctTyrMitfMART1 | Melanocyte |
Abbreviations: Dct, dopachrome tautomerase; IRS, inner radial sheaf; K, keratin; MART1, melan protein 1; Mitf, microphthalmia-associated transcription factor; Msx2, homeobox 2 homolog; ORS, outer radial sheaf; Pax3, paired box 3; Tyr, tyrosine.
Adapted from Schneider.34
A more precise analysis of the function of a gene has been achieved through conditional mutagenesis with the Cre-LoxP system.36 This technology enables complete gene deactivation (leading to constitutive loss of the corresponding protein, i.e. knockout) or the replacement of the gene by the same gene sequence with a mutation whose in vivo effect is to be analyzed. It is even possible to replace a gene that has an unrelated sequence with the target gene to bring it under the control of the regulatory elements of that gene (i.e. knockin). These changes take place in a given tissue and occur in controlled fashion over time. As in models of transgenic mice, the use of promoters of keratin K14 and K5 genes as regulatory sequences for directing expression of recombinase Cre to the epidermis is a widely used technique in dermatological investigation.34 The subsequent crossing of these transgenic lines with a mouse line containing the gene of interest flanked by the loxP sites (floxed), which are recognized by the Cre enzyme, leads to gene excision and splicing of the cut DNA ends (or depending on the orientation of the loxP sites, to inversion of the sequence) in cells that express the corresponding keratin. Temporal control of recombinase expression is achieved by fusing a Cre protein with a mutated domain to which the progesterone receptor (PR) or, more frequently, the estrogen receptor (ER) binds. Thus, the Cre protein fused with ERT can only enter the nucleus in presence of the synthetic steroid tamoxifen, but not under the influence of endogenous steroids. The Cre-loxP system has also been used with a fair degree of success for identifying the cell progeny of a stem cell or progenitor (lineage tracing).37 In this case, Cre recombinase moves toward a certain cell type with the objective of activating the expression of a reporter gene that leads to permanent labeling of the progenitor cell and all its descendants. The most widely used labels are green fluorescent protein (GFP) and ¿-galactosidase. This strategy has made it possible to identify and isolate keratinocytes with stem cell characteristics from different regions of the hair follicle.6,10
In the SkinModel-CM, we are developing models with conditional (inducible) knockout mice for proteins Dnmt1, endoglin, and podoplanin, using K14.Cre-ERT2 and Krt1-15.CrePR1 mice. In the former, Cre recombinase is controlled by the promoter of keratin K14 and so expression can be induced (by tamoxifen) in keratinocytes of the basal layer of the interfollicular epidermis and the outer root sheaf of the hair follicle, leading to loss of the corresponding gene in these cells. In the latter, Cre is controlled by keratin K15, and its expression is induced (through a synthetic analogue of progesterone) in keratinocytes of the bulge region of the hair follicle.
Although the models with genetically modified mice have greatly benefited basic and translational dermatological research, even the most sophisticated genetic manipulations have failed to faithfully mimic many human skin diseases. This is largely due to anatomic and functional differences between human and mouse skin. The human epidermis is formed of 6 to 10 layers of keratinocytes and is thicker than that of the mouse (approximately 3 layers). In addition, mouse skin has abundant hair and is densely populated with hair follicles, which play a key role in the physiology of mouse skin whereas their function in humans is less apparent, except in certain anatomic regions. These differences translate, for example, into divergence in relation to the genetic changes implicated in neoplastic transformation. Thus, in the model of chemical carcinogenesis of mouse skin, carcinogenesis is driven by mutations in the H-Ras oncogene,38 and papilloma is the predominant lesion type. This exophytic lesion does not occur in human skin, except in the presence of certain types of papilloma virus. In addition, the frequency of Ras oncogene mutations in human nonmelanoma skin cancer is relatively low.39 Another example of the difference between mice and humans is that, in mice, the repair of damage to DNA caused by sunlight, through a mechanism known as global nucleotide excision repair, is less relevant than in human skin. Thus, extremely high doses of ultraviolet radiation are needed to induce xeroderma pigmentosum syndrome in mice.
Organotypic Models: Skin BioengineeringAs mentioned earlier, the skin regenerates itself naturally thanks to the existence of stem cells. It is possible to preserve this regenerating potential in vitro by using 3-dimensional (3D) skin equivalents. All that is required, basically, is a cell component, which can be simple or complex, depending on the variety of cell types that are incorporated (the basic cell component is provided by epidermal keratinocytes and dermal fibroblasts) and a matrix/scaffold that consists, generally, of a natural or synthetic biopolymer that serves as a support for the cell component and provides it with a 3D structure.
Collagen is the main component of the dermal extracellular matrix and thus collagen I, which is readily obtained, should be the ideal component for generating the dermal scaffold in skin equivalents. However, the use of collagen in organotypic cultures is restricted to the manufacture of small areas of allogeneic skin to use in certain clinical and experimental applications. In the SkinModel-CM program, we prefer to use fibrin. In 1998, some members of this consortium found that fibrin obtained from fibrinogen of a plasma cryoprecipitate could act as a support for the growth of human dermal fibroblasts and could provide the culture with a 3D structure.40 In this same study, it was demonstrated that fibrin (embedded with fibroblasts) makes an excellent substrate for the culture of human keratinocytes and could replace a base of dermal cells irradiated with a lethal dose. This procedure was the one habitually used for the in vitro culture of epidermal stem cells. It is highly likely that fibrin can be used successfully in organotypic human skin cultures because it forms, along with other plasma proteins, the temporary matrix on which the skin cells proliferate and migrate during wound healing. The use of fibrin (and other improvements in existing systems) has enabled us to produce large areas of bioengineered human skin for regeneration of skin of patients with extensive and deep burns.40,41 Using this technology, we have also created skin-humanized mouse models for the study of some types of dermatological diseases (see below).
An important characteristic of 3D organotypic skin cultures is that the dermis forms layers when the epidermal equivalent, keratinocytes, are exposed to air (with the dermal equivalent remaining in contact with the culture medium). The strategy able to most closely mimic the natural layer formation of the epidermis in human skin in these 3D skin equivalents is the one in which cell-free human denatured dermis repopulated with living human fibroblasts is used as a scaffold. However, an acceptable epidermal layer formation is also achieved with collagen or fibrin matrices. The 3D human skin equivalents can mimic pathological phenomena such as tumor invasion and blister formation characteristic of fragile skin syndromes like epidermolysis bullosa. They can also mimic syndromes such as xeroderma pigmentosum. The main drawbacks of organotypic cultures are their short useful lifespan and the lack of many of the mesenchymal cell components present in natural skin. Although the use of hyaluronic acid as an additional component of the matrix has enabled prolongation of the useful lifespan of skin equivalents based on fibrin,42 the in vitro models of bioengineered skin are not appropriate for long-term studies or therapeutic trials, as these require continuous cell turnover through proliferation-differentiation cycles of stem cells or early epidermal progenitors. In these cases, it is essential to resort to in vivo skin-humanized mouse models.
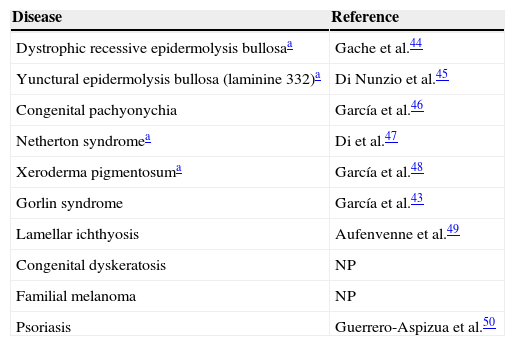
Chimeric Immunodeficient Skin-Humanized Mouse Systems as Preclinical Models of Skin DiseasesTransplants performed by investigators of the SkinModel-CM consortium (as well as those performed by other laboratories in immunodeficient mice of 3D skin equivalents generated with cells from dermatology patients) have enabled the stable modeling of numerous genetic and acquired skin diseases (Table 2).43 The simplest models are those that use cells derived from patients with monogenic diseases, such as epidermolysis bullosa. There is a group of orphan diseases, such as Gorlin syndrome and xeroderma pigmentosum, in which the genetic mutations affect the function of epidermal stem cells and lead to accelerated formation of skin neoplasms. These conditions are of particular relevance for our program, and their reconstruction in skin-humanized mouse models will be useful for testing novel therapeutic approaches such as photodynamic therapy (PDT, see below).
Diseases Modeled With Cells from Patients Through Skin Tissue Engineering and Transplant to Immunosuppressed Mice.
| Disease | Reference |
|---|---|
| Dystrophic recessive epidermolysis bullosaa | Gache et al.44 |
| Yunctural epidermolysis bullosa (laminine 332)a | Di Nunzio et al.45 |
| Congenital pachyonychia | García et al.46 |
| Netherton syndromea | Di et al.47 |
| Xeroderma pigmentosuma | García et al.48 |
| Gorlin syndrome | García et al.43 |
| Lamellar ichthyosis | Aufenvenne et al.49 |
| Congenital dyskeratosis | NP |
| Familial melanoma | NP |
| Psoriasis | Guerrero-Aspizua et al.50 |
Abbreviation: NP, not published.
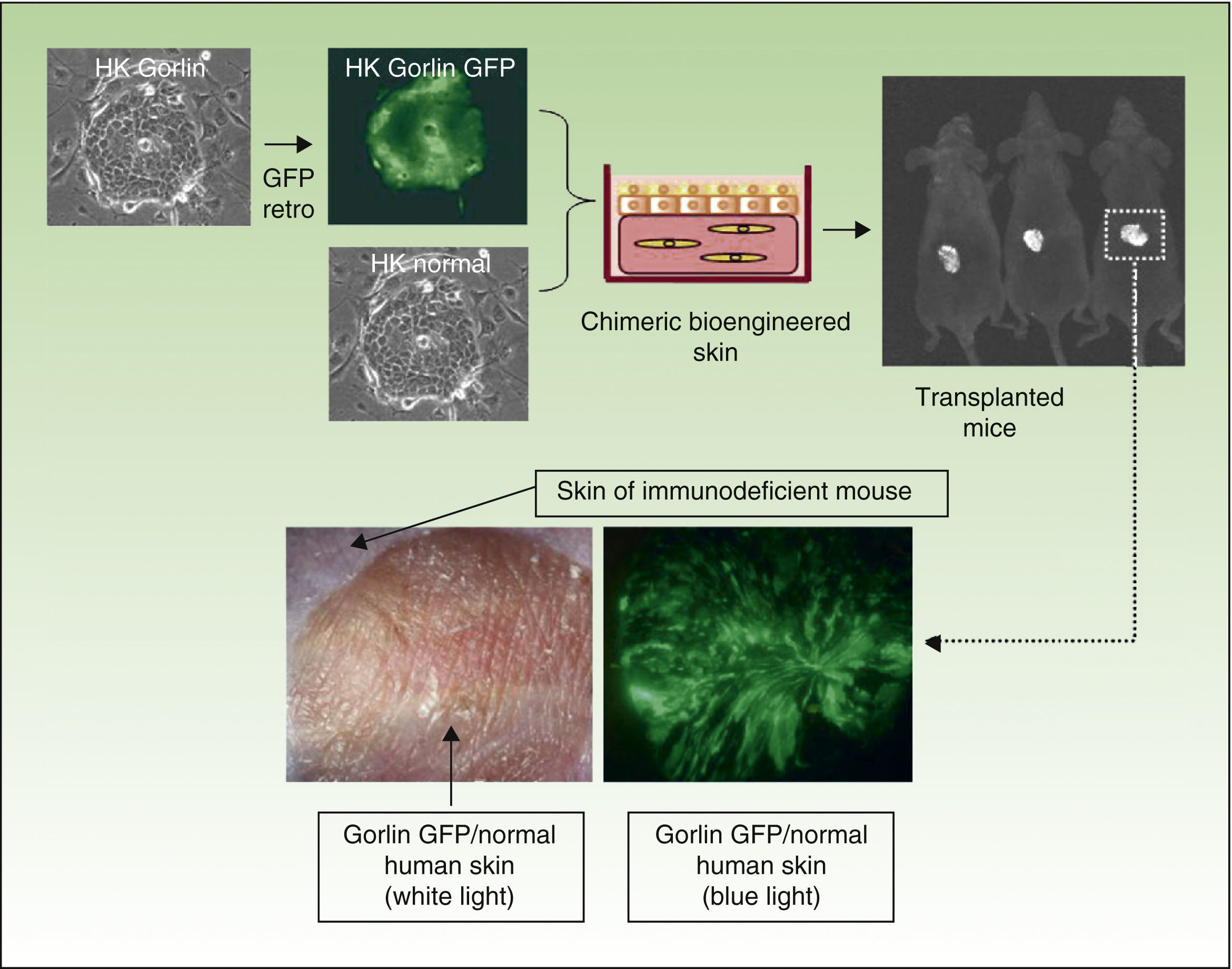
The inherent versatility of this experimental system enables regeneration of chimeric human skin, and the introduction of customized cell equivalents, for example, combinations of keratinocytes from patients and healthy donors, keratinocytes from patients and fibroblasts from healthy donors, etc. Figure 3 shows regeneration in immunodeficient mice of human skin from a 1:1 combination of keratinocytes from a patient with Gorlin syndrome, which expresses GFP, and normal unlabeled keratinocytes. These models offer the possibility of using human keratinocytes and/or fibroblasts in addition to those already introduced by genetic modification. This strategy has enabled validation of preclinical approaches of ex vivo gene therapy44,45,47 and regeneration of the epidermis from a single epidermal stem cell.51 The SkinModel-CM program is also considering development strategies for overexpression and silencing of genes that encode endoglin and podoplanin to analyze their effects on the regeneration and architecture of the skin as well as on neoplastic processes and wound healing.
Regeneration of chimeric skin in immunosuppressed mice. Normal human keratinocytes and those of a patient with Gorlin syndrome (labeled with green fluorescent protein [GFP] by retrovirus infection) were assembled in bioengineered skin equivalents and transplanted into immunosuppressed mice. The regenerated skin (lower panels) shows the presence of fluorescent areas indicating Gorlin keratinocytes and nonfluorescent areas indicating skin regeneration at the expense of normal keratinocytes.
The experimental system of chimeric human skins regenerated in immunodeficient mice also allows the efficacy of new therapies in the treatment of skin lesions, whether tumoral or nontumoral, to be assessed. Among these treatments, the SkinModel-CM program is particularly interested in PDT, which is used in dermatology mainly in actinic keratosis and surface-spreading BCC, nonmelanocytic lesions that are very common in the world population.52,53
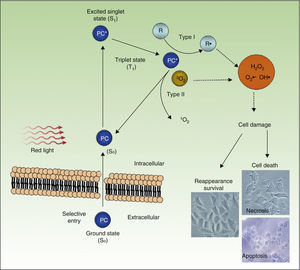
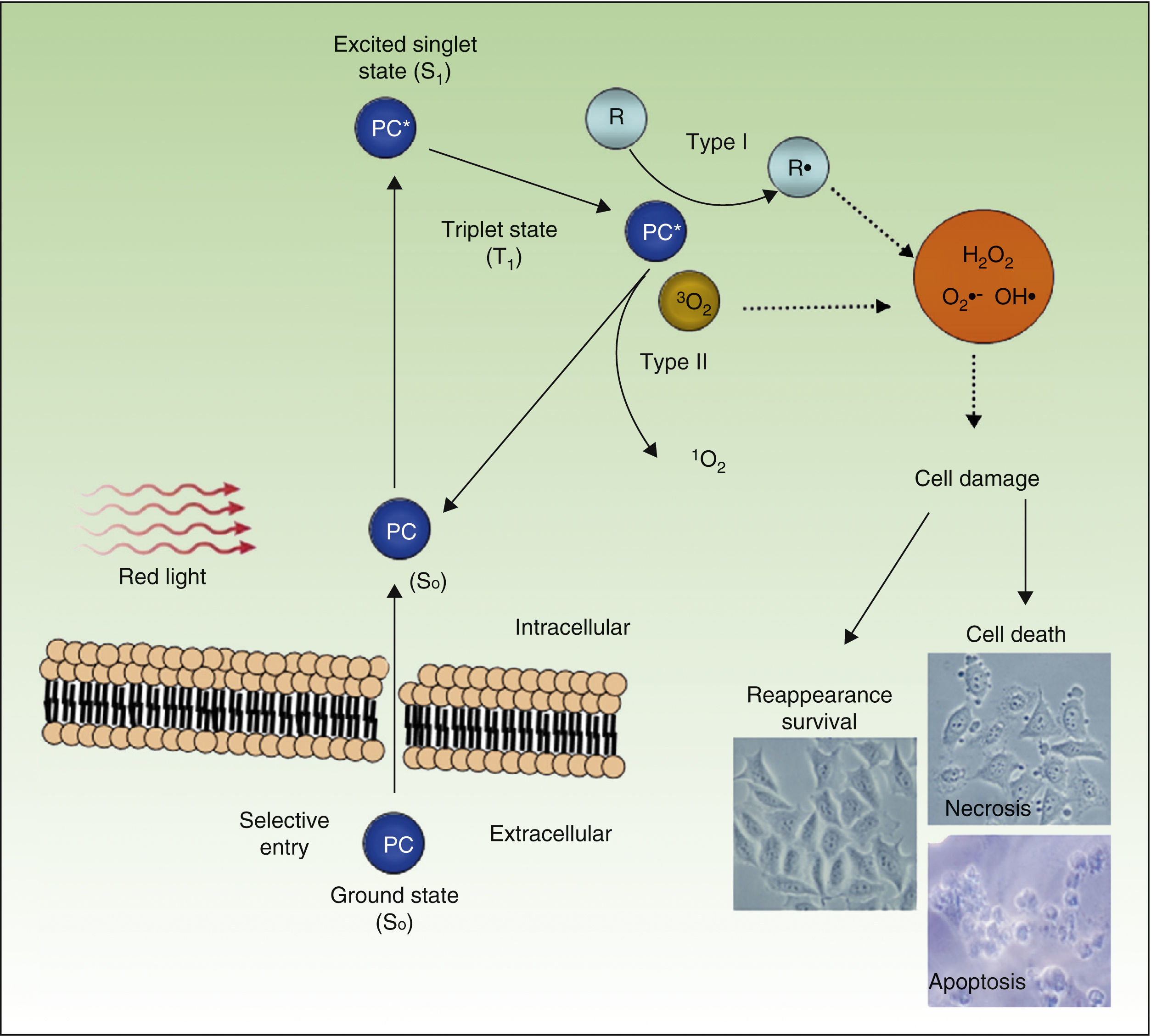
PDT uses exogenous photosensitive compounds (PCs), which preferentially accumulate in tumor cells. Subsequently, radiation with visible light of an appropriate wavelength leads to the formation of reactive oxygen species (ROS), which induce the selective death of the tumor cells. In other words, destruction of tumor tissue is caused by the combination of 3 elements that are nontoxic per se: PCs, light, and oxygen.54
Figure 4 shows the mechanism of action of PDT: PC in its ground state S0, is excited by red light of energy S1 and is promoted to the triplet T1 state. Energy transfer from the excited T1 state to molecular oxygen (O2) generates singlet oxygen (1O2) (a type 2 photoreaction). The PC in the T1 state can also transfer its energy to molecules other than oxygen (type I photochemical reaction) to form other ROS, such as the superoxide anion (•O2−), the hydroxyl radical (•OH), and hydrogen peroxide (H2O2). Most of the PCs used in PDT act through type 2 photochemical reactions and so the agent responsible for cell death is singlet oxygen 1O2.
Mechanisms of action of photodynamic therapy. Photodynamic therapy requires the combination of 3 elements, photosensitive compound (PC), light, and O2. PC in its ground state (S0) absorbs light and becomes excited (S1). This state transitions to the triplet state (T1). Energy transfer to O2 through type I and II reactions generates ROS, leading to oxidative stress and cell damage, in turn leading to cell death if no repair occurs.
PDT leads to cell death through a direct cytotoxic effect, affecting different organules or cell structures, such as the nucleus, mitochondria, plasma membrane, lysosomes, and cytoskeleton. The therapy also has indirect effects arising from the destruction of tumor vasculature.55,56
The most widely used PCs are compounds of the porphyrin group, such as the PC derived from hematoporphyrin used for the treatment of localized tumors accessible to light and neoplasms in the lungs, bladder, esophagus, and, in particular, the skin.54 Other new PCs approved for clinical use are the hematoporphyrin dihydrochloride and PC precursors such as 5-aminolevulinic acid (ALA) and its methylated derivative (MAL).57–59 These latter 2 are the most widely used for the treatment of skin tumors. ALA is metabolized inside the cell to form an intermediate of the heme group synthesis known as protoporphyrin IX (PpIX), a potent PC that absorbs visible light at the red end of the spectrum. ALA and MAL have been used in the treatment of benign and malignant skin lesions such as actinic keratosis, in situ carcinoma (or Bowen disease), BCC, and SCC.60 PDT is particularly recommended for treating extensive lesions, such as those that arise in immunosuppressed patients who are susceptible to developing skin tumors. Other lesions such as keratoacanthomas, actinic cheilitis, B and T lymphomas, Paget disease, and Kaposi sarcomas can also respond to this therapy.
The SkinModel-CM program aims to generate preneoplastic and neoplastic skin disease models that can be used to assess the efficacy of PDT, in particular, the Gorlin models (for the treatment of BCC) and the XP model (for the treatment of SCC). Labeling of the Gorlin keratinocytes with GFP in the skin-humanized mouse model skin shown in Figure 3 will allow macroscopic comparison of the effect of PDT on transformed keratinocytes (fluorescent) and normal keratinocytes (nonfluorescent). Similarly, we will assess the effect of PDT in XP models. Finally, the SkinModel-CM program is also considering the use of PDT in skin regeneration processes. In vitro experiments with immortalized human keratinocytes showed that PDT (using ALA as the CP) in conditions in which low ROS production is induced can promote cell proliferation, activating expression of cyclin D1 through a kinase Src-dependent mechanism.61 Likewise, the production of low levels of ROS in the skin of mice in vivo has a potent stimulatory and mobilization effect on epidermal stem cells resident in the bulge region of the hair follicle. This is manifest through effects such as activation of the hair growth cycle and acceleration of the regeneration process of the epidermis after a burn.
Conclusions and PerspectivesOur knowledge of the mechanisms that control homeostasis of the skin and the biology of epidermal stem cells has progressed notably in recent decades, thanks in part to the development of in vivo models with mice using transgenic and genetically manipulated knockout mice. However, there are still large gaps in our knowledge and many unanswered questions. For example, after division of a stem cell, which signals determine whether one or both offspring remain undifferentiated? Why are there so many subpopulations with stem cell characteristics in the epidermis and appendages? What mechanisms promote differentiation of keratinocytes of the basal layer of the epidermis to the squamous layer?
One of the main issues in dermatological clinical research is the reproduction of human skin diseases with cell-based and animal models apt for experimental manipulation and testing of new therapeutic approaches. The bioengineered human skin grafts in immunosuppressed mice have provided a robust platform to study a wide range of skin diseases, including some types of cancer. However, the efforts of investigators to develop skin equivalents that better model the complexity of human skin should continue. The SkinModel-CM Program aims to contribute to this encouraging future of dermatological research by analyzing new mechanisms and proteins potentially implicated in the activation and mobilization of epidermal stem cells. In particular, the program is developing new in vivo models with genetically modified mice and skin-humanized mice to investigate the implication of these proteins in skin homeostasis and disease and to mimic certain pathological processes and test new treatment regimens with PDT.
FundingThe SkinModel-CM consortium is funded by the Autonomous Community of Madrid (program S2010/BMD-2359).
Conflicts of InterestThe authors declare that they have no conflicts of interest.
We would like to give heartfelt thanks to the work carried out by members and collaborators of our laboratory, whose daily efforts have turned the idea of the SkinModel program into reality.
Please cite this article as: Larcher F, Espada J, Díaz-Ley B, Jaén P, Juarranz A, Quintanilla M. Nuevos modelos experimentales para el estudio de la homeostasis y la enfermedad cutánea. Actas Dermosifiliogr. 2015;106:17–28.

![Regeneration of chimeric skin in immunosuppressed mice. Normal human keratinocytes and those of a patient with Gorlin syndrome (labeled with green fluorescent protein [GFP] by retrovirus infection) were assembled in bioengineered skin equivalents and transplanted into immunosuppressed mice. The regenerated skin (lower panels) shows the presence of fluorescent areas indicating Gorlin keratinocytes and nonfluorescent areas indicating skin regeneration at the expense of normal keratinocytes. Regeneration of chimeric skin in immunosuppressed mice. Normal human keratinocytes and those of a patient with Gorlin syndrome (labeled with green fluorescent protein [GFP] by retrovirus infection) were assembled in bioengineered skin equivalents and transplanted into immunosuppressed mice. The regenerated skin (lower panels) shows the presence of fluorescent areas indicating Gorlin keratinocytes and nonfluorescent areas indicating skin regeneration at the expense of normal keratinocytes.](https://static.elsevier.es/multimedia/15782190/0000010600000001/v2_201502060417/S1578219014003114/v2_201502060417/en/main.assets/thumbnail/gr3.jpeg?xkr=ue/ImdikoIMrsJoerZ+w9/t1/zx4Q/XH5Tma1a/6fSs=)