Merkel cell carcinoma is a rare, aggressive skin cancer that is managed in a great variety of ways. However, international clinical practice guidelines give only partial coverage to issues considered major problems.The recommendations presented here aim to provide Spanish dermatologists with a guide to improving disputed aspects of diagnosis, staging, and treatment of localized Merkel cell carcinomas.
Material and methodsThe ADAPTE process was used. Members of the Spanish Group of Oncologic Dermatology and Surgery (GEDOC) with experience in treating Merkel cell carcinoma and interest in drafting these guidelines were selected. The group described the care process and listed the most important clinical questions. They then searched for guidelines and assessed them with the AGREE II (Appraisal of Guidelines for Research and Evaluation) tool. After consulting the guidelines for answers to their clinical questions, the group drafted the present statement and sent it for external review.
ResultsThe guidelines that scored highest in the AGREE II assessment step were the consensus-based interdisciplinary guideline of the European Association of Dermato-Oncology and the European Organization of Research and Treatment of Cancer, and those of the Comprehensive Cancer Network, the Alberta Health Services in Canada, the American Cancer Society, and the Cutaneous Oncology Group of the French Society of Dermatology. A total of 9 clinical questions were answered based on these guidelines.
ConclusionsThe guidelines presented here answer clinical questions that arise in routine practice. They can provide dermatologists with a starting point for decision-making, although available resources and patient preferences must always be borne in mind.
El carcinoma de células de Merkel es un tipo de cáncer de piel infrecuente y agresivo. Hay una gran variación en su manejo y las diferentes guías extranjeras que existen cubren parcialmente los problemas identificados como principales. El objetivo de la presente guía es servir de referencia a los dermatólogos españoles para mejorar aspectos controvertidos del diagnóstico, estadificación y tratamiento del carcinoma de células de Merkel.
Materiales y métodosSe empleó el método ADAPTE: se escogió a miembros del Grupo Español de Dermato-Oncología y Cirugía (GEDOC) con experiencia en el tratamiento de estos tumores y con interés en participar en la elaboración de la guía. Tras resumir el proceso de atención y elaborar las preguntas clínicas relevantes, se hizo una búsqueda de guías, que se seleccionaron según su puntuación mediante el instrumento Appraisal of Guidelines for Research and Evaluation (AGREE II). Tras la búsqueda de las respuestas en dichas guías, se elaboraron posteriormente las recomendaciones. Por último, se sometió la guía a revisión externa.
ResultadosLas guías con mejor puntuación fueron las de National Comprehensive Cancer Network, la European consensus-based interdisciplinary guideline, Alberta Healthservices Clinical practice guideline, American Cancer Society y Cutaneous Oncology Group of the French Society of Dermatology. Se obtuvieron en total 9 preguntas clínicas, contestadas a partir de estas guías.
ConclusionesEsta guía responde a preguntas habituales en la práctica clínica diaria y sirve a los dermatólogos como referencia en la toma de decisiones, siempre teniendo presentes los recursos y preferencias del paciente.
Merkel cell carcinoma (MCC) is a rare, aggressive skin tumor with an incidence of 0.28 cases per 100 000 person-years (95% CI, 0.15-0.40).1,2 Initial diagnostic and staging techniques vary and may not be available at all hospitals. In addition, the rarity of MCC means that many hospitals lack experience in the management of this disease. Although clinical practice guidelines (CPGs) exist for the management of MCC, they apply to different settings and only partly address key problems identified by dermatologists.
As part of the White Paper on Skin Cancer project led by the Healthy Skin Foundation of the Spanish Academy of Dermatology and Venereology (AEDV), it was decided to adapt existing CPGs on MCC to the Spanish context.
The aim of this new guideline is to improve quality of care for patients with MCC based on recommendations adapted to the needs of Spanish dermatologists and on the best possible evidence. The guideline reviews the main diagnostic techniques used for initial diagnosis and staging and the treatments available for localized MCC.
Material and MethodsAs CPGs for MCC already exist, we decided to adapt these following the ADAPTE process.3,4
The panel members were selected from among members of the AEDV's Group of Oncologic Dermatology and Surgery (GEDOC) according to their experience in treating MCC and interest in drafting the new guideline. All panel members signed a declaration of conflicts of interest prior to participating.
As for the scope and purpose of the new guideline, it was decided to provide recommendations about controversial aspects of diagnosis, medical and surgical treatment, and follow-up (Appendix B supplementary material 1). Aspects related to prevention and treatment of disseminated disease were excluded. The healthcare setting and context was dermatologic care in Spain and the professionals targeted were dermatologists.
Following the steps recommended by ADAPTE, we summarized the care pathway and determined the health questions for each of the stages in this pathway. The most relevant health questions were selected following the ADAPTE consensus process at a face-to-face meeting held at the headquarters of the AEDV in October 2016. A simultaneous search was conducted to identify guidelines available online and from other sources and organizations (that produce, collect, or disseminate guidelines, for example) and leading academies of dermatology and cancer (including the National Guidelines Clearinghouse, the Guidelines International Network, Guiasalud, the Institute for Clinical Systems Improvement, the UK National Institute for Health and Care Excellent (NICE), the New Zealand Guidelines Group, the Scottish Guidelines Network, the Cochrane Library, the British Academy of Dermatology, the American Academy of Dermatology, the European Academy of Dermatology, and the National Comprehensive Cancer Network [NCCN]). The documents retrieved were reviewed (not all of them were found to be CPGs) and their quality assessed using the Appraisal of Guidelines Research & Evaluation II (AGREE II) instrument.5 The CPGs with the best results were selected for consultation.
The content of the selected CPGs was then examined to create recommendation matrices that included the reference to the source guideline in each case. Data extraction and assignment of the Oxford Centre for Evidence-based Medicine levels of evidence and grades of recommendation was performed independently by pairs of panel members in all cases.6
The draft recommendations were published on the AEDV's website for external review by AEDV members (all of whom were invited to participate), GEDOC members, an oncologist, and a pathologist. Any objections raised by the reviewers were considered by the panel and, where appropriate, applied to the new guidelines.
ResultsThe guidelines that scored highest in the AGREE II quality assessment and whose aims were aligned with the scope and purpose of the adapted guidelines were the NCCN guidelines in oncology,7 the European consensus-based interdisciplinary guideline,8 the Alberta Health Services Clinical Practice Guideline,9 and the guidelines of the American Cancer Society10 and the Cutaneous Oncology Group of the French Society of Dermatology11 (Appendix B supplementary material 2).
In the following section, we describe the health questions defined in the ADAPTE process and the resulting recommendations (Table 1), adapted for publication in Actas Dermo-Sifiliográficas. The full version of the CPG, together with the issues raised for each question, is provided as supplementary material (Appendix B supplementary material 3).
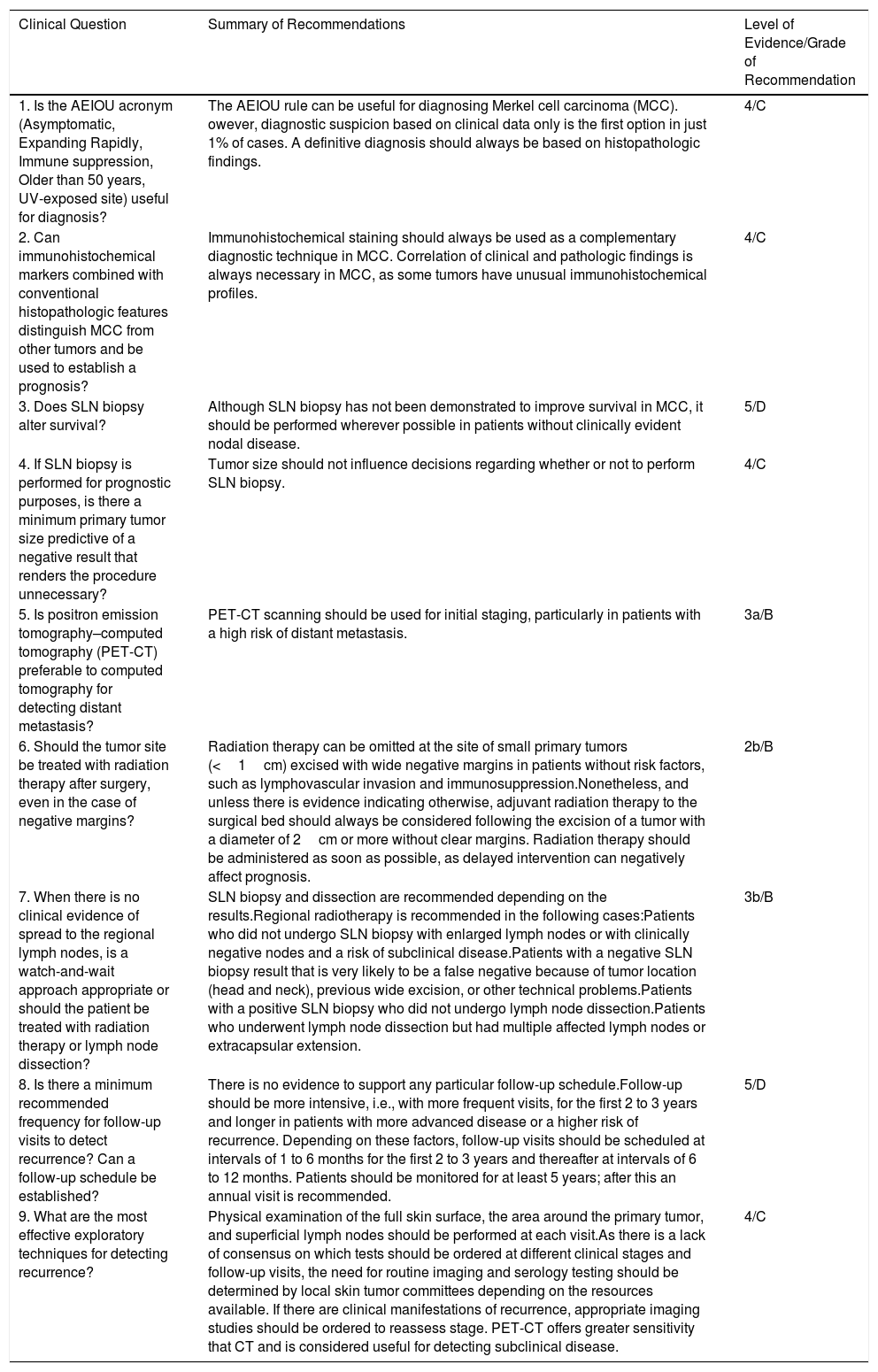
Summary of Clinical Practice Guideline Recommendations.
| Clinical Question | Summary of Recommendations | Level of Evidence/Grade of Recommendation |
|---|---|---|
| 1. Is the AEIOU acronym (Asymptomatic, Expanding Rapidly, Immune suppression, Older than 50 years, UV-exposed site) useful for diagnosis? | The AEIOU rule can be useful for diagnosing Merkel cell carcinoma (MCC). owever, diagnostic suspicion based on clinical data only is the first option in just 1% of cases. A definitive diagnosis should always be based on histopathologic findings. | 4/C |
| 2. Can immunohistochemical markers combined with conventional histopathologic features distinguish MCC from other tumors and be used to establish a prognosis? | Immunohistochemical staining should always be used as a complementary diagnostic technique in MCC. Correlation of clinical and pathologic findings is always necessary in MCC, as some tumors have unusual immunohistochemical profiles. | 4/C |
| 3. Does SLN biopsy alter survival? | Although SLN biopsy has not been demonstrated to improve survival in MCC, it should be performed wherever possible in patients without clinically evident nodal disease. | 5/D |
| 4. If SLN biopsy is performed for prognostic purposes, is there a minimum primary tumor size predictive of a negative result that renders the procedure unnecessary? | Tumor size should not influence decisions regarding whether or not to perform SLN biopsy. | 4/C |
| 5. Is positron emission tomography–computed tomography (PET-CT) preferable to computed tomography for detecting distant metastasis? | PET-CT scanning should be used for initial staging, particularly in patients with a high risk of distant metastasis. | 3a/B |
| 6. Should the tumor site be treated with radiation therapy after surgery, even in the case of negative margins? | Radiation therapy can be omitted at the site of small primary tumors (<1cm) excised with wide negative margins in patients without risk factors, such as lymphovascular invasion and immunosuppression.Nonetheless, and unless there is evidence indicating otherwise, adjuvant radiation therapy to the surgical bed should always be considered following the excision of a tumor with a diameter of 2cm or more without clear margins. Radiation therapy should be administered as soon as possible, as delayed intervention can negatively affect prognosis. | 2b/B |
| 7. When there is no clinical evidence of spread to the regional lymph nodes, is a watch-and-wait approach appropriate or should the patient be treated with radiation therapy or lymph node dissection? | SLN biopsy and dissection are recommended depending on the results.Regional radiotherapy is recommended in the following cases:Patients who did not undergo SLN biopsy with enlarged lymph nodes or with clinically negative nodes and a risk of subclinical disease.Patients with a negative SLN biopsy result that is very likely to be a false negative because of tumor location (head and neck), previous wide excision, or other technical problems.Patients with a positive SLN biopsy who did not undergo lymph node dissection.Patients who underwent lymph node dissection but had multiple affected lymph nodes or extracapsular extension. | 3b/B |
| 8. Is there a minimum recommended frequency for follow-up visits to detect recurrence? Can a follow-up schedule be established? | There is no evidence to support any particular follow-up schedule.Follow-up should be more intensive, i.e., with more frequent visits, for the first 2 to 3 years and longer in patients with more advanced disease or a higher risk of recurrence. Depending on these factors, follow-up visits should be scheduled at intervals of 1 to 6 months for the first 2 to 3 years and thereafter at intervals of 6 to 12 months. Patients should be monitored for at least 5 years; after this an annual visit is recommended. | 5/D |
| 9. What are the most effective exploratory techniques for detecting recurrence? | Physical examination of the full skin surface, the area around the primary tumor, and superficial lymph nodes should be performed at each visit.As there is a lack of consensus on which tests should be ordered at different clinical stages and follow-up visits, the need for routine imaging and serology testing should be determined by local skin tumor committees depending on the resources available. If there are clinical manifestations of recurrence, appropriate imaging studies should be ordered to reassess stage. PET-CT offers greater sensitivity that CT and is considered useful for detecting subclinical disease. | 4/C |
A, Asymptomatic; E, Expanding rapidly; I, immune suppression; O, older than age 50; and U, UV-exposed site.
Summary of EvidenceJust 1 article describing a retrospective, single-center cohort study of 195 patients diagnosed with MCC between 1980 and 2007 mentioned the AEIOU acronym,12 which refers to Asymptomatic, Expanding rapidly, Immune suppression, Older than age 50, and UV-exposed site. Three or more of these features were found in 89% of MCCs in the cohort.
The expert opinion is that most MCCs are diagnosed on the basis of histopathologic not clinical features13 (level of evidence 4).
AEDV RecommendationThe AEIOU rule can be useful for diagnosing MCC. However, diagnostic suspicion based on clinical data only is the first option in just 1% of cases. A definitive diagnosis should always be established according to histopathologic findings (grade of recommendation C).
Can immunohistochemical markers combined with conventional histopathologic features distinguish MCC from other tumors and be used to establish a prognosis?Summary of EvidenceImmunohistochemical findings combined with conventional histopathologic features can help distinguish MCC from other tumors and guide prognosis, but clinical and pathologic findings must always be integrated.14–16 The most specific markers for MCC are the Cam 5.2 (a low-molecular–weight cytokeratin [CK]), CK20, and neurofilaments, particularly when perinuclear dot-like positivity is observed. MCCs typically express neuroendocrine markers such as synaptophysin, chromogranin, and CD56, which help distinguish these tumors from most non-neuroendocrine tumors. Negative staining for CK7 and thyroid transcription factor 1 helps rule out metastasis from small-cell carcinoma of the lung or other organs, while negative S-100 protein and common leukocyte antigen results rule out melanoma and lymphoma, respectively. Merkel cell polyomavirus, which is normally detected using the antibody CM2B4, is present in 70% to 80% of cases, and diffuse expression confirms diagnosis and indicates a better prognosis.17,18 Variations in immunohistochemical staining patterns have been described for MCC, but they are either less specific or are supported by lower levels of evidence (level of evidence 4).
AEDV RecommendationImmunohistochemical staining should always be used as a complementary diagnostic technique in MCC. Positive staining with low-molecular–weight keratins showing a dot-like pattern is highly specific and is the minimum diagnostic requirement. The addition of CK7, CK20, and neurofilament stains to rule out metastasis from lung cancer, which is the main entity in the differential diagnosis, is highly recommendable. Expression of neuroendocrine markers is diagnostic. As some of these tumors are hybrid or may contain heterologous elements, detection of areas of squamous cell carcinoma or leiomyosarcoma cells does not rule out a diagnosis of MCC. Positive staining for Ber-EP4 is not uncommon in MCC and therefore this stain is not useful for distinguishing MCC from basal cell carcinoma. Correlation of clinical and pathologic findings is essential in MCC, as some tumors have unusual immunohistochemical profiles (grade of recommendation C).
Does sentinel lymph node biopsy alter survival?Summary of EvidenceNo scientific studies have demonstrated that sentinel lymph node (SLN) biopsy alters survival in MCC. SLN status, however, is the strongest independent predictor of survival in this setting. Approximately one-third of patients with clinically node-negative disease have microscopic nodal disease. SLN biopsy therefore has an important role as a minimally invasive staging tool. Numerous studies have shown that pathologically negative lymph nodes (pN0) are associated with better survival compared with clinically negative lymph nodes(cN0)19–32 (level of evidence 5).
AEDV RecommendationAlthough SLN biopsy has not been demonstrated to improve survival in MCC, it should be performed wherever possible in patients without clinically evident nodal disease (grade of recommendation D).
If SLN biopsy is performed for prognostic purposes, is there a minimum primary tumor size predictive of a negative result that renders the procedure unnecessary?Summary of EvidenceMost studies recommend performing SLN biopsy whenever possible, regardless of tumor size. Unlike in melanoma, there are no clinical (size, location) or pathologic (thickness, mitotic rate, lymphovascular invasion) predictors that help determine the need for SLN biopsy in MCC33–35 (level of evidence 4).
AEDV RecommendationTumor size should not influence decisions regarding whether or not to perform SLN biopsy (grade of recommendation C).
Is positron emission tomography–computed tomography preferable to computed tomography for detecting distant metastasis?Summary of EvidenceImaging studies are recommended for the staging of MCC, but agreement is lacking on the best modalities. Some authors have recommended the use of simple chest radiography combined with other tests depending on clinical findings (chest and abdomen computed tomography [CT], brain magnetic resonance imaging, abdominal and lymph node ultrasound).36–39
Other studies have shown that positron emission tomography (PET)-CT scanning alters initial staging and treatment in 16% to 22% of cases and radiation therapy doses in 15%40–42 (level of recommendation 3a).
AEDV RecommendationPET-CT scanning should be used for initial staging, particularly in patients with a high risk of distant metastasis (grade of recommendation B).
Should the tumor site be treated with radiation therapy after surgery, even in the case of negative margins?Summary of EvidenceRetrospective observational studies have shown that radiation therapy can prevent recurrence and increase survival in MCC. In particular, a significant survival benefit has been observed for adjuvant radiation therapy in patients with stage i and ii disease.
In addition, a randomized controlled trial, which was stopped prematurely,43 and a meta-analysis44 of observational studies have shown that postoperative adjuvant radiation therapy reduces the risk of locoregional recurrence, although no evidence was found for altered survival (level of evidence 2a).
Improved overall survival has been observed in patients treated with postoperative radiation therapy, particularly in those with a primary tumor larger than 2cm.45 One retrospective study published in 2013, however, renewed doubts about the value of radiation therapy in MCC, as it found an increase in overall but not disease-specific survival46 (level of evidence 2b).
In the absence of data from large, prospective, randomized studies, it would appear reasonable to contemplate the use of radiation therapy in patients with high-risk factors for recurrence, namely, immunosuppression, large tumors, aggressive histologic patterns, and lymphovascular invasion (level of evidence 2b).
AEDV RecommendationRadiation therapy can be omitted at the site of small primary tumors (<1cm) excised with wide negative margins in patients without risk factors, such as lymphovascular invasion and immunosuppression.7
Nonetheless, and unless there is evidence indicating otherwise, adjuvant radiation therapy to the surgical bed should always be considered following the excision of a tumor with a diameter of 2cm or more without clear margins. Radiation therapy should be administered as soon as possible, as delayed intervention can negatively affect prognosis (grade of recommendation B).
When there is no clinical evidence of spread to the regional lymph nodes, is a watch-and-wait approach appropriate or should the patient be treated with radiation therapy or lymph node dissection?Summary of EvidenceJust 1 randomized clinical trial has compared observation versus adjuvant regional radiation therapy to the tumor bed following the surgical treatment of stage I MMC.43 The trial, however, was stopped prematurely due to a decrease in recruitment because of the growing use of SLN biopsy. Eighty-three patients, nonetheless, were recruited. While radiation therapy did not improve overall survival, it was associated with a significant reduction in the risk of regional recurrence (0% vs 16.7%).
All the guidelines consulted recommend SLN biopsy in this situation, followed by radiation therapy or lymph node dissection if the result is positive.
Radiation therapy is only recommended as a stand-alone treatment for patients who are not candidates for SLN biopsy (contraindication, surgical problems, or refusal to undergo the procedure) or for lymph node regions where sentinel lymph nodes are difficult to identify (level of evidence 3b).
AEDV RecommendationIn patients with MCC and clinically negative nodes, SLN biopsy should be performed whenever possible and be followed by lymph node dissection if the result is positive.
Regional radiotherapy is recommended in the following cases:
- -
Patients who did not undergo SLN biopsy with enlarged lymph nodes or with clinically negative nodes and a risk of subclinical disease.
- -
Patients with a negative SLN biopsy result that is very likely to be a false negative because of tumor location (head and neck), previous wide excision, or other technical problems.
- -
Patients with a positive SLN biopsy who did not undergo lymph node dissection.
- -
Patients who underwent lymph node dissection but had multiple affected lymph nodes or extracapsular extension (grade of recommendation B).
No scientific studies have analyzed optimal follow-up intervals following MCC treatment.
MCC has a high risk of local and nodal recurrence and distant metastasis. Risk of recurrence is mainly determined by stage at diagnosis and is higher in the first 2 to 3 years after treatment of the primary tumor.47–50 Most guidelines therefore recommend close observation for the first 2 to 3 years. The minimum follow-up time is 5 years, but lifetime monitoring is recommended (level of evidence 5).
AEDV RecommendationThere is no evidence to support any particular follow-up schedules after treatment of a primary MCC.
Several factors should be considered when deciding on follow-up intervals and duration: risk of recurrence, disease stage, age, immunosuppression, treatment received (clear or involved margins, use of radiation therapy or not), patient anxiety levels, physician preferences, and resources available.
Follow-up should be more intensive, i.e., with more frequent visits, for the first 2 to 3 years and longer in patients with more advanced disease or a higher risk of recurrence. Depending on these factors, follow-up visits should be scheduled at intervals of 1 to 6 months for the first 2 to 3 years and thereafter at intervals of 6 to 12 months. Patients should be monitored for at least 5 years and annually thereafter (grade of recommendation D).
What are the most effective exploratory techniques for detecting recurrence?Summary of EvidenceNo studies have established the most effective exploratory techniques for monitoring patients with MCC.
All the guidelines recommend a full skin and lymph node examination at each visit accompanied by imaging studies depending on the findings of this examination and the patient's history. Some guidelines do, however, recommend routine ultrasound examination of regional lymph nodes. However, even in high-risk cases, imaging studies should be tailored to the individual, as not all patients are candidates for additional treatment and it is not clear whether early detection of metastasis improves prognosis.41,51
Recent studies have described the value of serial measurements of capsid protein and Merkel cell polyomavirus oncogene antibody titers, although these results are still controversial52–54 (level of evidence 4).
AEDV RecommendationPhysical examination of the full skin surface, the area around the primary tumor, and superficial lymph nodes should be performed at each visit.
As there is a lack of consensus on which tests should be ordered at different clinical stages and follow-up visits, the need for routine imaging and serology testing should be determined by local skin tumor committees depending on the resources available. If there are clinical manifestations of recurrence, appropriate imaging studies should be ordered to reassess stage. PET-CT offers greater sensitivity than CT and is considered useful for detecting subclinical disease (grade of recommendation, C).
DiscussionCPGs adapted from other recent guidelines help guide decision-making in the field of dermato-oncology.
The main strength of the current study is the application of a rigorous reproducible method for adapting existing guidelines to our context with the involvement of a multidisciplinary panel and a review by external experts.
This CPG should be updated within 3 years to ensure that its recommendations continue to be valid.
As with all guidelines, the recommendations in this guideline should not be construed as dictating an exclusive course of action. They should be applied flexibly, with consideration of patient preferences, physician experience, and availability of local resources.
FundingThe AEDV White Paper on Cancer was funded in full by the AEDV Healthy Skin Foundation. No pharmaceutical or other companies were involved in its production.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Doval JV, Cussac BL, Bustillo AP, Morena SPdl, González MJF, Figueras MTF, et al. Carcinoma de células de Merkel: diagnóstico y tratamiento en atención especializada dermatológica. Guía de práctica clínica de la Academia Española de Dermatología y Venerología. Actas Dermosifiliogr. 2019;110:460–468.