With a lifetime incidence of approximately 10% in the general population, cutaneous squamous cell carcinoma (CSCC) is the second most common type of nonmelanoma skin cancer.
Most CSCCs are benign and can be completely eradicated by surgery or other dermatological procedures. There is, however, a subgroup associated with an increased likelihood of lymph node metastases and, therefore, with high morbidity and mortality.
This article analyzes the various factors that define aggressive CSCC. We propose a method for defining high-risk SCC on the basis of a series of major and minor criteria. This method will allow better prognostic evaluation and enable personalized management of patients with high-risk SCC, possibly leading to improved overall survival.
El carcinoma epidermoide cutáneo, con una incidencia en la población general de aproximadamente un 10% a lo largo de la vida, es la segunda neoplasia más frecuente dentro del grupo del cáncer cutáneo no melanoma.
La mayoría de los carcinomas epidermoides cutáneos muestran un comportamiento benigno y pueden ser completamente erradicados mediante cirugía y otros procedimientos dermatológicos. Sin embargo, existe un subgrupo de esta entidad que se asocia con una mayor capacidad de desarrollar metástasis nodal y, por tanto, con una elevada morbimortalidad.
En el presente artículo se analizan los diferentes factores que definen al carcinoma epidermoide cutáneo de comportamiento agresivo. Proponemos un método de definición del carcinoma epidermoide de alto riesgo basado en el establecimiento de una serie de criterios mayores y menores. Este hecho supondrá una mejor evaluación pronóstica y un manejo personalizado de este grupo de enfermos, que puede resultar en un aumento de la supervivencia global.
Cutaneous squamous cell carcinoma (CSCC) has a lifetime incidence of between 7% and 11%. It accounts for 20% to 25% of all nonmelanoma skin cancers, and is second only to basal cell carcinoma in terms of prevalence.1–5
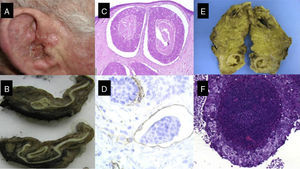
Most CSCCs are benign and can be completely eradicated by surgery and other dermatological procedures. Accordingly, 5-year survival rates after surgical excision are in excess of 90%,5 and mortality is around 1%.6 Nevertheless, there is a subgroup of CSCC associated with a higher frequency of lymph node metastasis and with high morbidity and mortality (Figs. 1-6).7–10
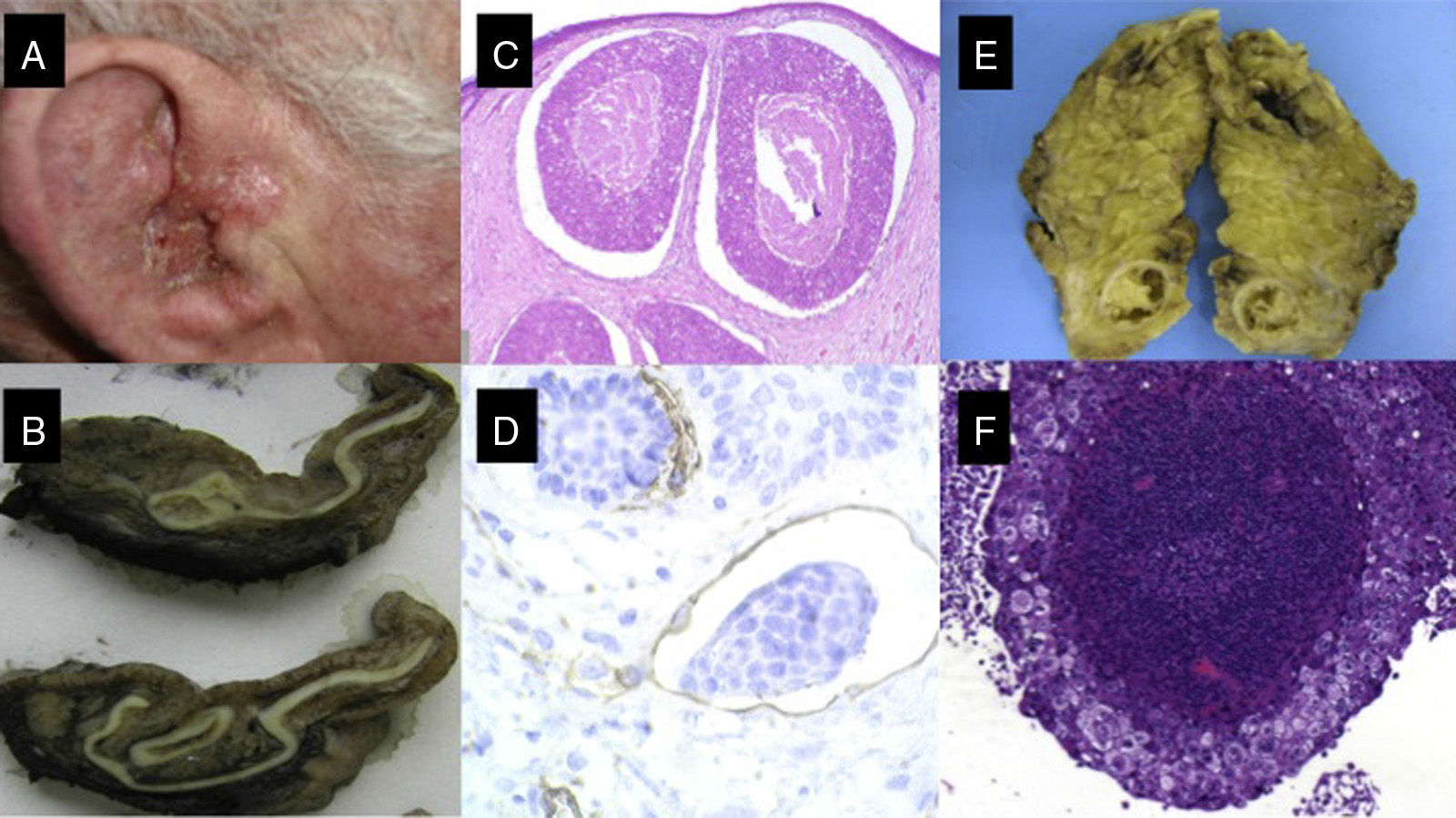
A, Cutaneous squamous cell carcinoma on the external ear of a 70-year-old patient. B, Fleshy tumor invading the external auditory canal. C and D, Histology showing a poorly differentiated squamous cell tumor with lymphovascular invasion (C, hematoxylin-eosin, original magnification x40; D, Immunostaining with CD31, original magnification x100); E, Parotid gland with nodular tumor. F, The parotid tumor is formed by a proliferation of poorly differentiated squamous cells in a pattern similar to that of the primary tumor (original magnification, hematoxylin-eosin, x40).
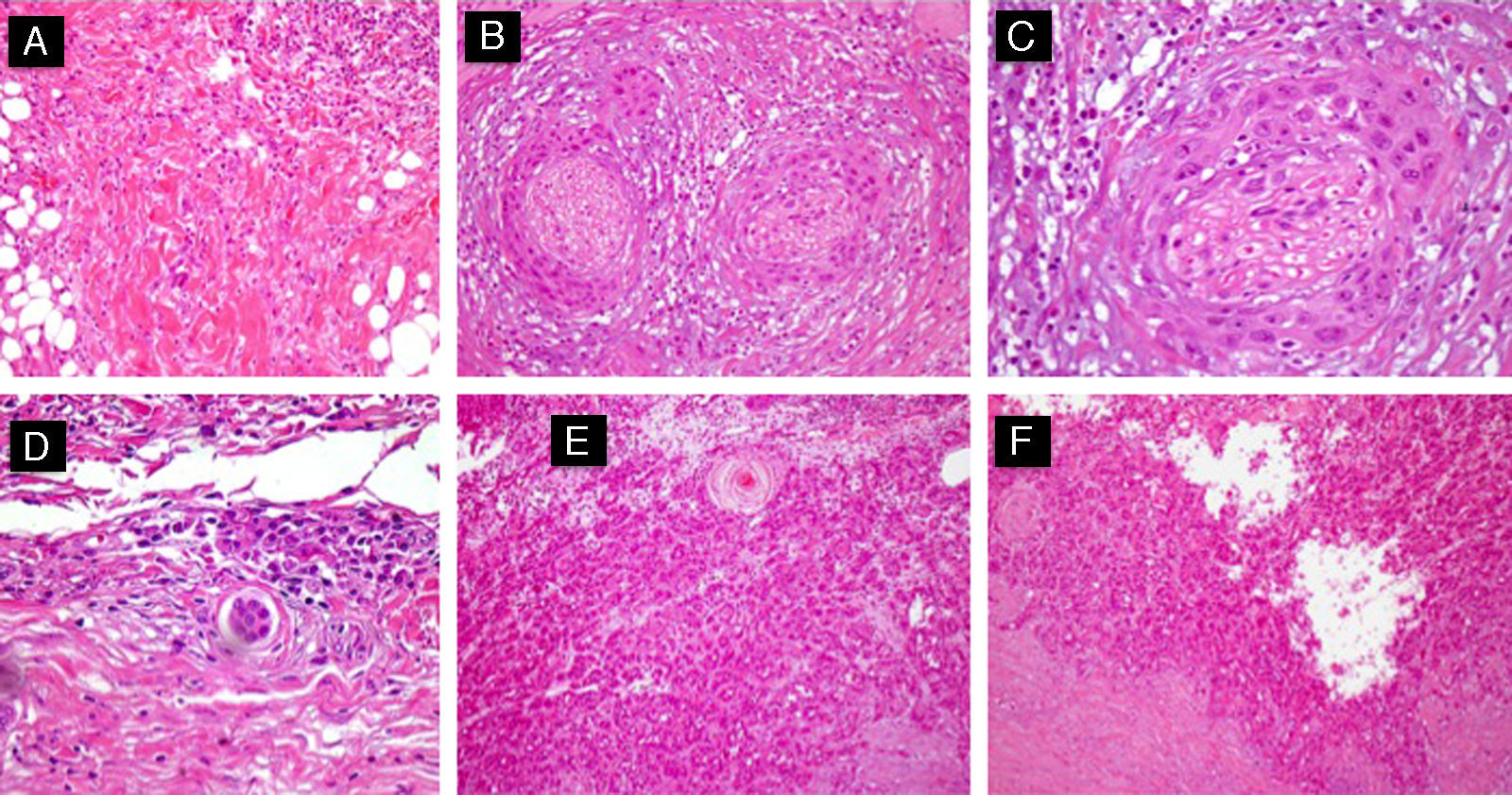
Different histologic subtypes associated with high-risk cutaneous squamous cell carcinoma. A, Isolated-cell pattern (hematoxylin-eosin, original magnification x40). B and C, Squamous cell carcinoma with perineural invasion (nerves >0.1mm) (hematoxylin-eosin, original magnification x40 [B] and x100 [C]). D, Squamous cell carcinoma with marked lymphovascular invasion (hematoxylin-eosin, original magnification x100). E, Adenoid squamous cell carcinoma (hematoxylin-eosin, original magnification x40). F, Acantholytic squamous cell carcinoma (hematoxylin-eosin, original magnification x40).
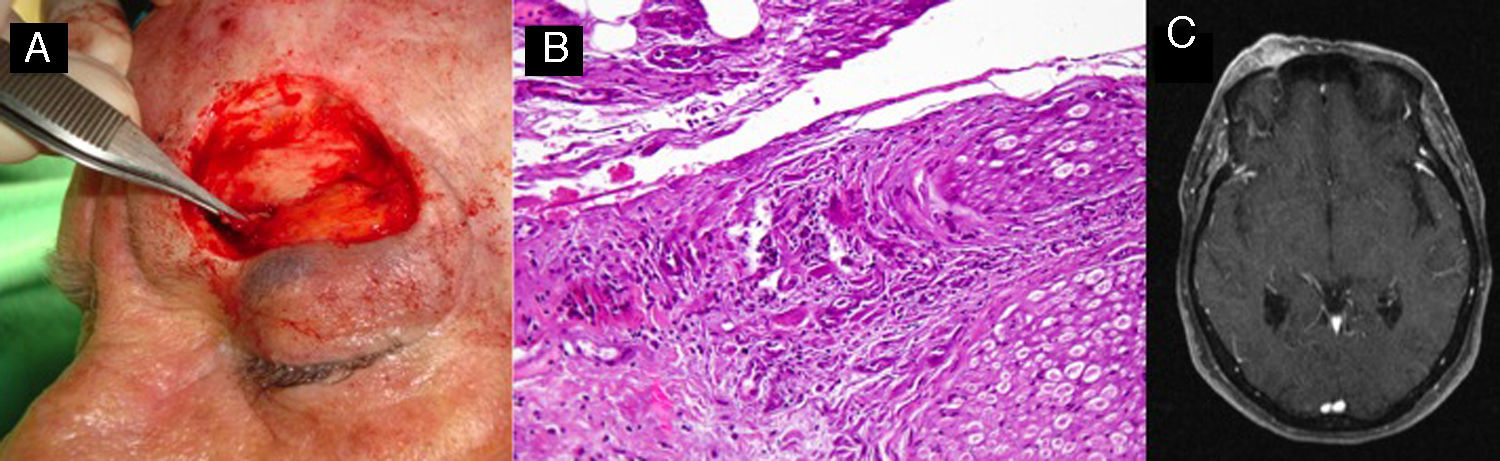
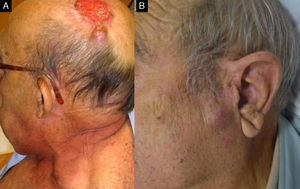
A, Perineural invasion detected incidentally during surgery. B, Histology showing invasion of the nerve trunk (diameter, >0.1mm) by atypical squamous cells (hematoxylin-eosin, original magnification x40). C, Magnetic resonance image showing intracranial invasion of the tumor along the nerve pathway.
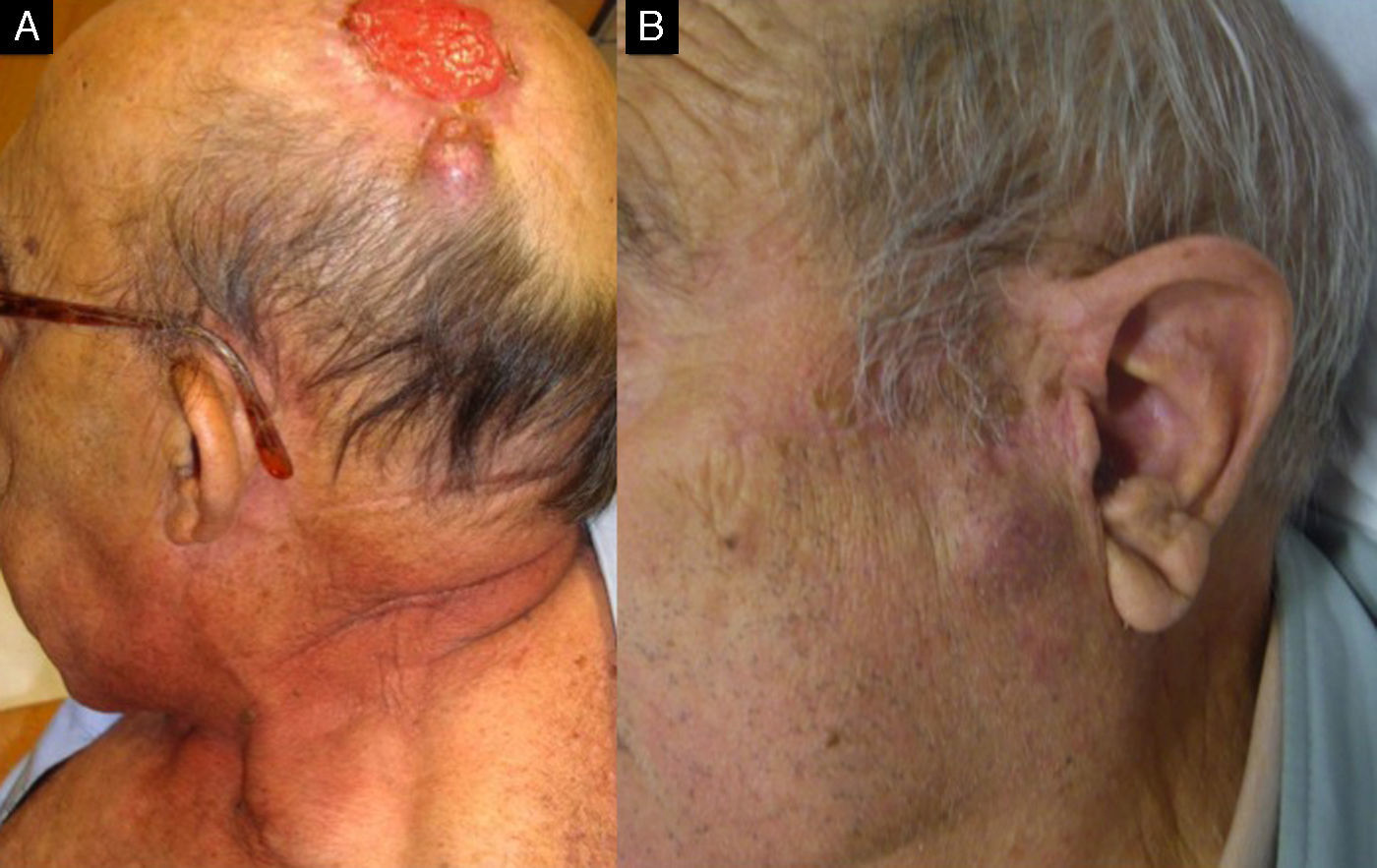
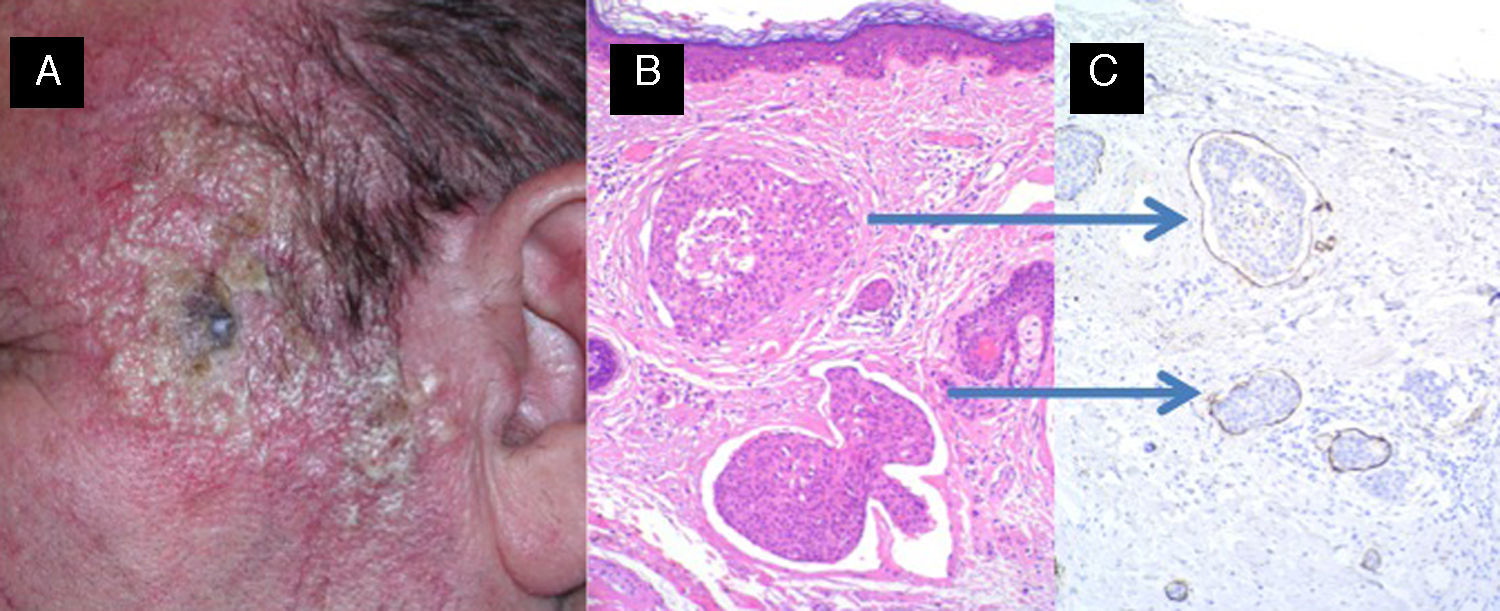
Carcinomatous lymphangitis secondary to squamous cell carcinoma previously excised from the left temple. A, Multiple papulous vesicles on the left temple. B and C, Invasion of lymph vessels by atypical squamous cells (B, Hematoxylin-eosin, original magnification x40; C, Immunostaining with D2-40, original magnification x40).
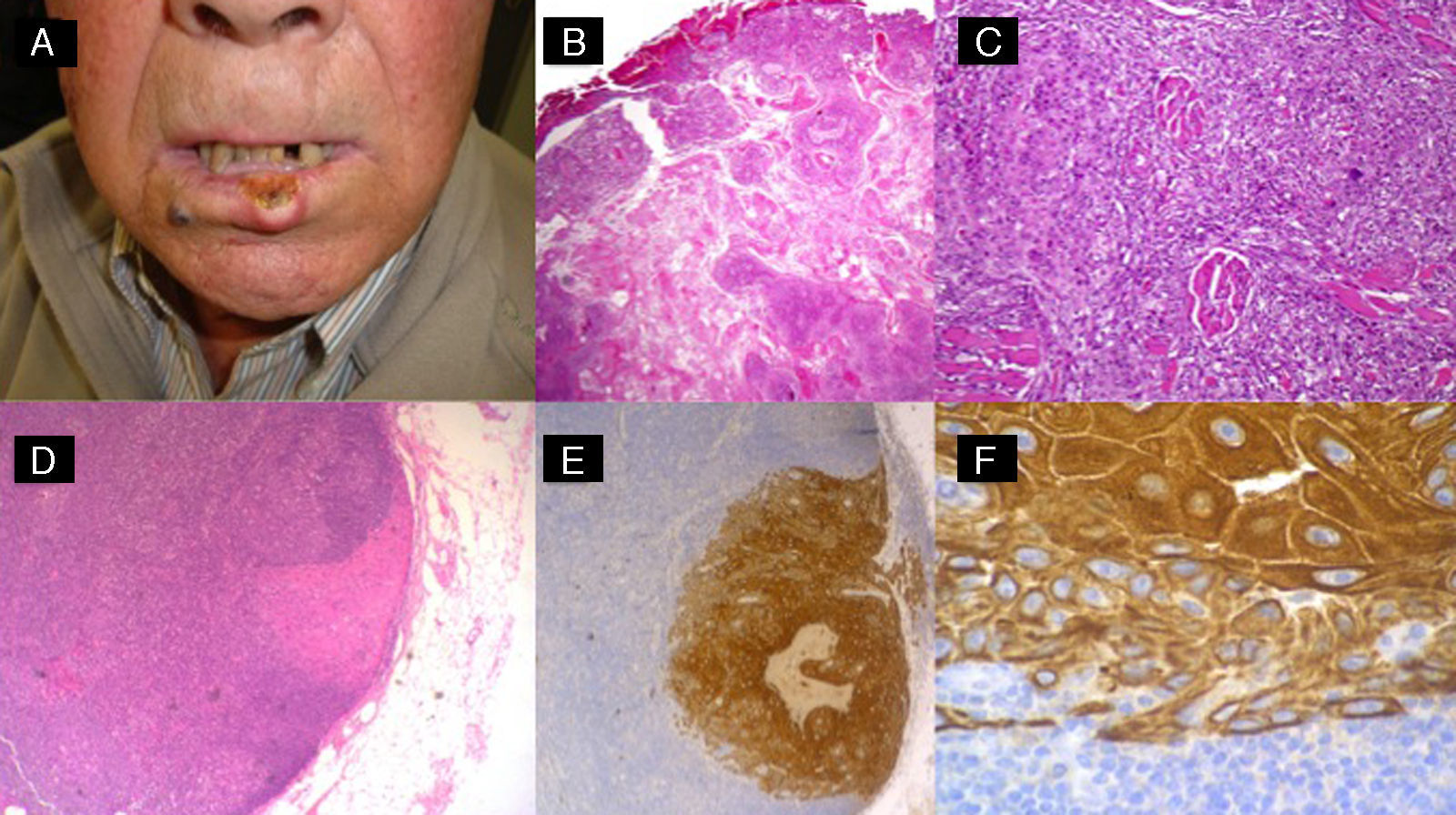
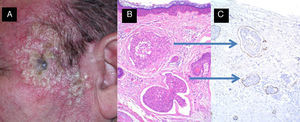
High-risk squamous cell carcinoma studied by sentinel lymph node biopsy. A, Squamous cell carcinoma of the lower lip in a 65-year-old man. B and C, Atypical squamous cell proliferation with acantholysis and perineural invasion. D-F, Invasion of sentinel lymph node by atypical squamous cells (hematoxylin-eosin, original magnification x40 [D] and x100 [E]; immunostaining with pankeratin, original magnification x100 [F]).
The main aim of this article is to accurately define this subgroup of CSCC, which is known as high-risk CSCC. A clear definition will help to refine prognosis and improve the individualized care of patients with high-risk CSCC.
Definition of High-Risk CSCCIn recent years, several authors have focused their research on analyzing differences between nonmetastatic and metastatic CSCC. The ultimate aim of such research was to predict which forms of CSCC are associated with an increased risk of locoregional and/or distant complications in order to be able to intervene promptly in patients at risk.
The findings led to the concept of high-risk CSCC, which is defined as a squamous cell carcinoma lesion, clinically staged as N0, that extends through the basement membrane and is associated with a high risk of subclinical metastasis. CSCCs that do not this meet this definition are classified as low-risk.
Defining Features of High-Risk CSCCThe factors that define high-risk CSCC can be divided into 3 subgroups: clinical factors, histologic factors, and molecular factors.
Clinical FactorsPersonal HistoryGenetic DisordersPatients with genetic disorders associated with an increased risk of CSCC typically develop tumors with a high risk of malignant transformation. Examples of these disorders are xeroderma pigmentosum, epidermodysplasia verruciformis, oculocutaneous albinism, dyskeratosis congenita, and recessive dystrophic epidermolysis bullosa. This last condition is associated with the highest mortality in patients with concomitant CSCC, with 5-year survival rates of just 80% after a diagnosis of the skin tumor.4,11–13
CSCC Arising at the Site of a Pre-existing LesionCSCCs that arise at the site of chronic skin damage, such as scars, slow-growing ulcers, burn sites, and chronic radiation dermatitis, have an increased risk of metastatic spread. This risk appears to be associated with a reduction in E-cadherin levels, which would favor the spread of atypical keratinocytes through the epidermis and into the dermis.14
Immunosuppression and TransplantationImmune status is a predictor of prognosis in many neoplastic conditions. Immune system alterations, for example, play an important role in the development of skin cancers, such as Merkel cell carcinoma.15
Patients who undergo solid organ transplantation (SOT) have a 65-fold higher risk of developing CSCC than the general population; furthermore, CSCC is the most common nonmelanoma skin cancer in SOT recipients and is 3 times more common than basal cell carcinoma.16
Recurrence rates, locoregional metastasis, and survival in transplant recipients with CSCC vary depending on the organ transplanted. In the field of SOT, heart transplantation is considered to carry the highest risk of CSCC and its high-risk variant,16 followed by, in decreasing order, lung, kidney, and liver transplantation. In the case of hematologic malignancies, the highest risk of both types of CSCC has been observed in patients with chronic lymphatic leukemia and small lymphocytic lymphoma.17
The cumulative incidence of CSCC increases progressively with the duration of immunosuppression, with observed rates of 7% after a year, 45% after 11 years, and 70% after 20 years. Furthermore, up to 66% of transplant recipients have been reported to develop a second CSCC after the first squamous cell carcinoma.18
CSCC recurs more frequently in immunosuppressed than in immunocompetent individuals (39% vs 15% in 5 years of follow-up),19 and mortality is also higher (5% in transplant recipients vs 1% in immunocompetent individuals).20 Organ transplant recipients with metastatic CSCC have a 3-year survival rate of 56%, which is similar to rates reported for patients with noncutaneous SCC of the head and neck.20
The fact that metastatic CSCC often has similar clinical characteristics (horizontal diameter of <2cm and vertical histologic thickness of <2mm) and favorable outcomes in immunosuppressed and immunocompetent individuals suggests that as yet unknown molecular alterations have a role in the high malignant potential of CSCC in immunosuppressed individuals.
Human Immunodeficiency Virus InfectionHuman immunodeficiency virus (HIV) infection, regardless of disease stage or immune status, appears to be a marker of poor prognosis in CSCC. This possibility was suggested by Nguyen et al.21 in a retrospective study of 10 consecutively recruited HIV-positive patients aged between 31 and 54 years with high-risk CSCC. Five of the 10 patients died within 7 years of the initial diagnosis, and local recurrence, metastasis, and survival were not correlated with the number of opportunistic infections or with CD4+ T cell count.
Clinical Characteristics of CSCCLesion SizeThe size of primary lesions in CSCC has been described by many authors as being an important predictor of lymph node metastasis.12,22–25
Data from the first prospective study of CSCC, involving over 1000 patients, showed that horizontal tumor size was an independent risk factor for metastasis. Specifically, the risk of metastatic spread was 0.01% in lesions measuring 2cm or less in diameter and 10% in larger lesions. In the second group, 7% of patients with a tumor size of between 2 and 5cm developed metastasis compared with 20% of those with a tumor size of over 5cm.26–29
Based on our experience and on data from the literature, we consider a horizontal size of 2cm to be the cutoff for increased risk of lymph node metastasis in CSCC. A smaller size, by contrast, would exert a protective effect, meaning that there would not be a risk of distant metastasis in immunocompetent patients with a tumor diameter of less than 2cm.
Lesion SiteLesion sites with the highest incidence (20%-30%) of metastatic CSCC are the external ear (Fig. 1) and the nonglabrous lip (Fig. 6).19,30
Middle-risk sites include the scalp (mainly the temple), the perineal and genital areas, and acral sites (hands and feet).6,31 It is also important to consider that areas not exposed to sunlight, such as the perineum, the sacral region, and the soles of the feet, have a proportionally higher rate of metastasis than chronically sun-exposed areas.32
RecurrenceTumor recurrence tends to be associated with poor prognosis in skin cancers.
Comparative analysis of lymph node metastasis in recurrent CSCC (15%) and nonrecurrent CSCC (2%) (P<.001) has led to the conclusion that tumor recurrence is an important risk factor in CSCC.33
Clayman et al.6 observed an association between CSCC recurrence and tumor size, and reported that large tumors were associated with a significantly higher rate of recurrence (2.4vs 1.5cm, P<.0001). They also found recurrent lesions to be associated with a higher rate of perineural invasion (PNI) (24% vs 10%), lymphovascular invasion (17% vs 8%), and subcutaneous tissue invasion (30% vs 10%).6
Recurrence has also been significantly associated with positive margins in surgically excised CSCC, with recurrent tumors—and consequently increased risk of metastasis—observed in up to 50% of patients with positive margins.33
Human Papillomavirus Infectionβ-Human papillomaviruses (HPVs) are the most common type of HPVs involved in CSCC. Numerous studies have demonstrated a relationship between β-HPVs and CSCC, above all in immunosuppressed patients, although these viruses may also act as a cofactor with UV radiation in immunocompetent patients. Nevertheless, because β-HPVs appear to be involved in the etiology and pathogenesis of CSCC and not in metastatic spread, they are not considered prognostic factors.α-HPVs associated with CSCC of the genital region, the head and the neck, and acral sites might be associated with a higher risk of metastasis as they alter regulatory mechanisms, such as p53 and the retinoblastoma gene/p16. Most studies of p16 in CSCC have reported loss of p16 expression to be associated with the transformation of in situ CSCC to invasive CSCC but not with a higher risk of metastatic spread. As mentioned previously, only p16-positive cases associated with the presence of α-HPVs would carry an increased risk of metastasis.34–39
Histologic Features of CSCCTumor Thickness and Clark LevelTumor ThicknessTumor thickness is currently considered to be the most important independent predictor of metastasis in CSCC, with greater thickness associated with higher risk.26,32
Based on data from the largest prospective series of CSCC to date, conducted by Brantsch et al.,26 CSCC can be divided into 3 risk groups (low-risk, middle-risk, and high-risk) based on tumor thickness. Patients in the low-risk group have tumors with a thickness of 2mm or less, and have virtually no risk of distant metastasis. Those in the middle-risk group have a tumor thickness of between 2 and 6mm, which is associated with a 4% increased risk of metastasis in 5 years of follow-up. Finally, those in the high-risk group have tumors with a thickness of 6mm or more and a 16% increased risk of metastasis.
Based on data from later studies and on our own experience, we believe that a cutoff a 4mm provides the best sensitivity for separating low-risk CSCC from CSCC with a high risk of metastatic spread. Tumors with a thickness of less than 2mm, by contrast, would be associated with virtually no risk of distant disease.
Degree of Tumor DifferentiationDegree of tumor differentiation is another important prognostic factor in CSCC and other neoplastic diseases.
In a study of 571 patients with CSCC, a significant difference was observed for the rate of metastasis between lesions with a high degree of differentiation and other lesions (17% vs 4%, P=.004),22 and in another study involving a large number of patients, high-grade CSSS was associated with a greater risk of malignant transformation than other types of CSCC (44% vs 5%, P<.01).12
Tumor differentiation is also associated with an increased risk of early recurrence. Poorly differentiated CSCCs have a 2.9-fold increased risk of distant metastasis and death compared with well-differentiated CSCCs, although well-differentiated tumors may also be associated with the development of advanced disease.12,40
Finally certain histologic subtypes of CSCC (acantholytic, adenoid, isolated cell pattern) should be considered as a risk factor in combination with tumor differentiation (Fig. 3).
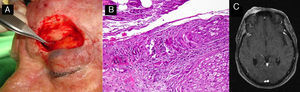
Histologically Positive Surgical MarginsIncomplete tumor excision—and consequently—disease persistence, is a predictor of poor prognosis in CSCC. Disease, and with it an increased risk of metastasis, recurs in up to 50% of patients with histologically positive margins following surgical excision.33 Recurrence following tumor excision appears to be related to a risk of subclinical tumor progression, which would, in turn, favor metastasis.41
The decision to take a watch and wait approach with patients with incompletely excised CSCCs, i.e., with a pathology report showing the involvement of 1 or more margins, should be weighed up carefully given the high rate of lymph node disease in recurrent CSCC. Several studies have shown a history of disease recurrence in between 45% and 51% of patients with CSCC and lymph node involvement.12,22,42,43
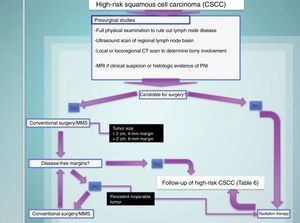
Consequently, all patients with CSCC should undergo surgery until disease-free margins are achieved,44 and if this is not possible, other treatments, mainly radiation therapy, should be considered.
Perineural InvasionPNI occurs in approximately 5% to 10% of patients with CSCC and is usually detected as an incidental finding.6,45 Nonetheless, histologic evidence of PNI appears to be associated with a significant increase in disease recurrence and distant metastasis rates.6,45 A study performed at the Anderson clinic in Texas, United States, showed that compared with CSCC patients without PNI, those with PNI had a significantly increased frequency of regional metastasis (35% vs 15%, P<.005) and distant metastasis (15% vs 3.3%, P<.005).46PNI is important not only because of the risk of locoregional spread, but also because of disease caused by perineural spread through the cranial nerves, mostly the facial and the trigeminal nerves (Fig. 4) and because PNI is associated with worse 3-year survival in CSCC (64% in patients with PNI vs 91% in those without, P=.002).47–49
The evaluation of PNI risk in CSCC should vary depending on the thickness of the nerves affected and the presence of clinical and/or radiologic signs of invasion. Infiltration of nerves with a diameter of less than 0.1mm, for instance, is associated with a low risk of local or distant complications, while invasion of nerves measuring more than 0.1mm in diameter has been associated with poor short-term and long-term prognosis (CSCC-specific death of 0% in individuals with PNI of nerves<0.1mm vs 32% in those with PNI of nerves>0.1mm, P=.003).50
PNI can manifest as an incidental finding on histology, with or without accompanying symptoms. Symptoms include pain on palpation, regional paresthesia, and acute intermittent or shooting pain. Based on data from the University of Florida College of Medicine in the United States, it has been suggested that patients with asymptomatic PNI not visible on radiography have a better prognosis than those with clinical or radiological evidence of PNI (5-year local control rate of 87% vs 55%, P=.006).51
Lymphovascular InvasionRecent studies have suggested that lymphovascular invasion may increase the risk of metastasis in CSCC. Moore et al.43 defined lymphovascular invasion as an independent predictor of lymph node metastasis in a multivariate analysis (OR, 7.54, P.<.00001), and reported that 40% of patients with metastasis had lymphovascular invasion, compared with just 8% of those without. The prognostic significance of lymphovascular invasion, however, has been questioned by some authors.12,50
The implications of CSCC in dermal lymph vessels, which has been rarely described, are unknown, but it may increase the risk of recurrence and explain in-transit metastasis (Fig. 5).
Other FactorsOther factors that have been proposed as possible prognostic factors in CSCC are peritumoral actinic keratosis,37–39 Clark level,37–39 Ki67 expression, desmoplasia,26,38 and the presence of a tumor inflammatory response with mainly eosinophils and plasma cells. The true prognostic value, however, of these factors, is still a matter of debate and needs to be investigated in further studies.
Molecular Markers in CSCCSeventy percent of patients with metastatic CSCC have 1 or more of the defining features of high-risk CSCC described above. However, between 20% and 30% do not (those with thin, small CSCCs), suggesting that other, as yet unknown variables, probably have an important role in the pathogenesis of high-risk CSCC.12,51 Of relevance in this group are certain molecular alterations that appear to be associated with a subgroup of CSCCs with more aggressive behavior. Specifically, it has been suggested that mutations in genes expressing the epidermal growth factor receptor (EFGR), and to a lesser extent, p16 and CKS1B mutations, are the main molecular alterations involved in high-risk CSCC; confirmation of this would have important therapeutic implications.52–56
Epidermal Growth Factor ReceptorTumors that overexpress EGFR tend to be associated with more advanced disease, a greater risk of lymph node metastasis, and higher rates of early recurrence and shorter survival in various malignant disorders, including squamous cell carcinoma of the mucosa of the upper aerodigestive tract.57–62
Just 1 small study has analyzed the importance of EFGR mutations in the prognosis of CSCC. In an analysis of 15 cases of metastatic CSCC in the head and neck region, EGFR overexpression was significantly associated with metastatic potential, with strong overexpression found in 79% of patients with metastatic disease and in just 36% of those without.62,63 Overexpression was independent of gene amplification. Alternative mechanisms that would explain the increase in EGFR expression include increased messenger RNA (mRNA) transcription, activating receptor mutations, increased levels of receptor ligands, and increased expression of heterologous receptors, such as Her-2.62 With respect to Her-2, abnormal Her-2 expression and alterations in the gene encoding Her-2 (chromosome 17) have been analyzed in patients with EGFR overexpression.63 While the authors did not observe Her-2 overexpression in any of the 27 cases they analyzed, they did detect Her-2 polysomy, which, like in breast cancer, was not associated with increased overexpression. The absence of overexpression leads to treatment failure with anti-Her2 drugs, such as trastuzumab.64 Although the significance of this last finding has not yet been clarified, the detection of Her-2 polysomy may have an impact on predicting therapeutic response to tyrosine kinase inhibitors.61,62 Nevertheless, EGFR overexpression has only been observed in up 65% to 75% of metastatic CSCCs, adding strength to the hypothesis that multiple factors are involved in the etiology of this form of CSCC.62,63
p16Data from several studies support a correlation between p16 overexpression and degree of malignancy, suggesting that p16 expression (like p53 expression) might be a biomarker of tumor progression.64,65 Other authors, however, have reported a correlation between loss of p16 expression and high-risk CSCC.66,67 In 1 of these studies, Chang et al.67 detected a correlation between loss of p16 expression and the development of metastasis, suggesting that loss of this protein might be a predictor of poor prognosis.
The explanation behind why elevated levels of p16 might be associated with poor prognosis could be related to the coexistence of HPV infection. However, with the exception of epidermodysplasia verruciformis in immunocompromised individuals and CSCC in areas other than the head and neck, the role of HPV in the development of CSCC is still a matter of debate.
Another possible explanation for increased p16 levels might be p16 UV-induced changes. The presence of a mutated p16, with a long half-life but with a loss of anti-oncogenic function, would explain the increased risk of malignancy.
CSK1BThe CKS1B gene encodes the cyclin-dependent kinases regulatory subunit 1. The protein binds to the catalytic subunit of cyclin-dependent kinases and play an essential role in their biologic function. CKS1B mRNA appears to be expressed in different patterns throughout the cell cycle of HeLa cells, indicating a specific role for the encoded protein. The peptide plays a role in cell-cycle regulation by interacting with other proteins, mainly SKP2 and CDKN1B.68,69
CKS1B appears to have a critical role in tumor progression in CSCC. In a study of 43 CSCCs and 26 actinic keratoses, Salgado et al.70 analyzed CKS1B gene and protein status using fluorescence in situ hybridization and immunohistochemical analysis, and reported that chromosome 1 polysomy was a frequent event in both CSCC (30 of 43 cases) and actinic keratosis (13 of 23 cases). CKS1B amplification, observed in 4 cases (9.3%), was associated in all cases with aggressive tumor behavior (PNI, lymph node spread, and CSCC in transplant recipients).
In conclusion, CKS1B amplifications might be a marker of high-risk CSCC.
Towards a New Prognostic Classification of CSCCCSCC is one of the most common cancers in the world and, as such, responsible for many deaths.
The ability to clearly differentiate between high-risk and low-risk CSCC would appear to be key in improving survival and optimizing management of the disease according to risk.
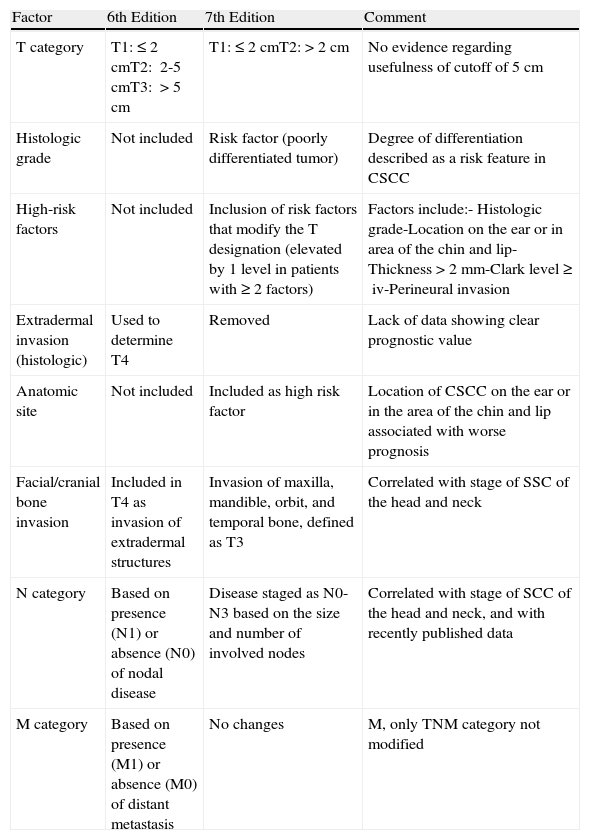
The American Joint Committee on Cancer (AJCC) recently modified its staging system for CSCC.71 The main changes are summarized in Table 1. One of the most significant changes was the introduction of a list of high-risk clinical and histologic features that modify the T designation, regardless of tumor size. These features include a tumor thickness of more than 2mm, a Clark level of IV or more, location on the external ear or nonglabrous lip, PNI, bone involvement, and poor tumor differentiation.71 Based on our experience, however, this list is not sufficient to accurately define high-risk CSCC. While anatomic location and PNI are good prognostic predictors, the situation with tumor differentiation and Clark level is less clear. Furthermore, the establishment of a cutoff of 2mm for differentiating between low-risk and high-risk CSCC deserves special mention. A high proportion of CSCCs are thicker than 2mm, meaning that this cutoff has high sensitivity but very poor specificity in terms of predicting risk. Finally, the new AJCC staging system does not include important factors such as immune system status, tumor recurrence, or lymphovascular invasion.
Comparison Between the 6th and 7th Editions of the American Joint Committee on Cancer Staging Manuals.
| Factor | 6th Edition | 7th Edition | Comment |
| T category | T1:≤2cmT2: 2-5cmT3: >5cm | T1:≤2cmT2:>2cm | No evidence regarding usefulness of cutoff of 5cm |
| Histologic grade | Not included | Risk factor (poorly differentiated tumor) | Degree of differentiation described as a risk feature in CSCC |
| High-risk factors | Not included | Inclusion of risk factors that modify the T designation (elevated by 1 level in patients with ≥2 factors) | Factors include:- Histologic grade-Location on the ear or in area of the chin and lip-Thickness >2mm-Clark level ≥iv-Perineural invasion |
| Extradermal invasion (histologic) | Used to determine T4 | Removed | Lack of data showing clear prognostic value |
| Anatomic site | Not included | Included as high risk factor | Location of CSCC on the ear or in the area of the chin and lip associated with worse prognosis |
| Facial/cranial bone invasion | Included in T4 as invasion of extradermal structures | Invasion of maxilla, mandible, orbit, and temporal bone, defined as T3 | Correlated with stage of SSC of the head and neck |
| N category | Based on presence (N1) or absence (N0) of nodal disease | Disease staged as N0-N3 based on the size and number of involved nodes | Correlated with stage of SCC of the head and neck, and with recently published data |
| M category | Based on presence (M1) or absence (M0) of distant metastasis | No changes | M, only TNM category not modified |
In its latest update, published in 2010, the National Comprehensive Cancer Network (NCNN) proposed that the treatment of CSCC should be guided by a series of variables. It lists a set of risk factors and considers 2 possible scenarios. First, it recommends that all CSCCs with any of the risk factors shown in Table 2 should be surgically excised with safety margins of 10mm or with Mohs micrographic surgery. And second, it recommends that patients with 3 or more of these factors should receive special attention. In our opinion, the NCCN guidelines also have shortcomings. A high percentage of patients have at least 1 of the factors shown in Table 2, meaning that suggesting treatment modifications based on this alone would appear to be quite a broad recommendation. The second scenario is even more confusing, as the NCNN considers that patients with 3 or more of the risk factors shown in Table 2 should receive special treatment. However, there is no mention of exactly how treatment or follow-up should be modified.
Factors Considered by the National Comprehensive Cancer Network for the Definition of Risk in Cutaneous Squamous Cell Carcinoma.
| The presence of any of the following risk factors is sufficient to justify excision with a safety margin of 10mm or complete assessment of all margins (Mohs micrographic surgery) |
| Tumor size ≥2cm (or 1cm on the head and 6mm on the genitals, hands, and feet) |
| Tumor thickness ≥4mm |
| Poorly defined borders |
| Recurrent tumor |
| Immunosuppression |
| Previous radiation |
| Chronic inflammation |
| Rapid growth |
| Perineural or vascular invasion |
| Moderate or poor differentiation |
Special attention should be paid to patients with 3 or more of the above risk factors.
In our opinion, the main difficulty with attempts to define high-risk CSCC to date is the fact that most studies have identified the risk factors discussed in this article in isolation. There have been no analyses of their cumulative effects.
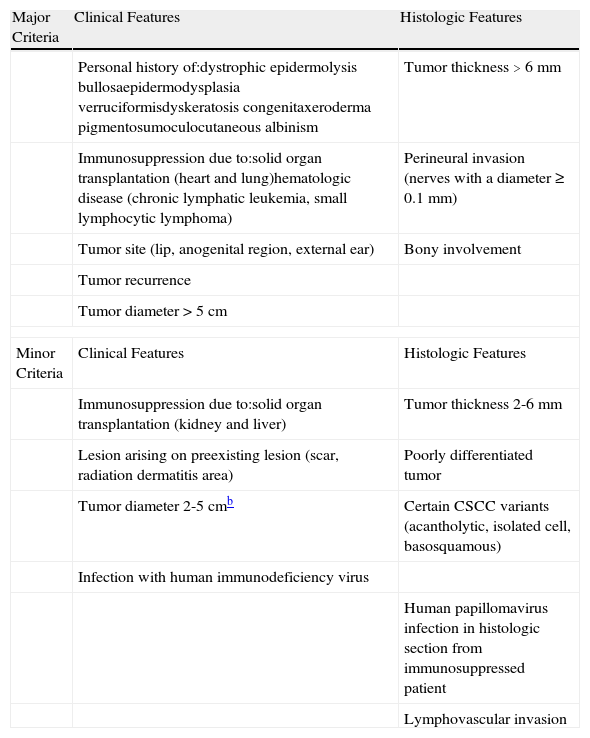
Based on data systematically stored in a database at our hospital, a specialist skin cancer center, and data from the literature, we designed a system to identify the cumulative value of each of the prognostic factors that define high-risk CSCC. We divided risk factors into major and minor criteria and created a scoring system to differentiate between low risk and high-risk CSCC (Table 3). We then considered high-risk CSCC to be any CSCC with a) 3 major criteria, b) 2 major and 2 minor criteria, and c) 1 major criterion and 4 minor criteria. Our proposed definition of high-risk CSCC, which has important prognostic and therapeutic implications, needs to be corroborated in prospective studies that analyze the different prognostic factors for CSCC from a global perspective.
Major and Minor Criteria That Define High-Risk Cutaneous Squamous Cell Carcinoma (CSCC).a
| Major Criteria | Clinical Features | Histologic Features |
| Personal history of:dystrophic epidermolysis bullosaepidermodysplasia verruciformisdyskeratosis congenitaxeroderma pigmentosumoculocutaneous albinism | Tumor thickness >6 mm | |
| Immunosuppression due to:solid organ transplantation (heart and lung)hematologic disease (chronic lymphatic leukemia, small lymphocytic lymphoma) | Perineural invasion (nerves with a diameter ≥0.1mm) | |
| Tumor site (lip, anogenital region, external ear) | Bony involvement | |
| Tumor recurrence | ||
| Tumor diameter >5cm | ||
| Minor Criteria | Clinical Features | Histologic Features |
| Immunosuppression due to:solid organ transplantation (kidney and liver) | Tumor thickness 2-6mm | |
| Lesion arising on preexisting lesion (scar, radiation dermatitis area) | Poorly differentiated tumor | |
| Tumor diameter 2-5cmb | Certain CSCC variants (acantholytic, isolated cell, basosquamous) | |
| Infection with human immunodeficiency virus | ||
| Human papillomavirus infection in histologic section from immunosuppressed patient | ||
| Lymphovascular invasion | ||
Our provisional definition of high-risk CSCC would lead to a more aggressive treatment approach and much closer follow-up.
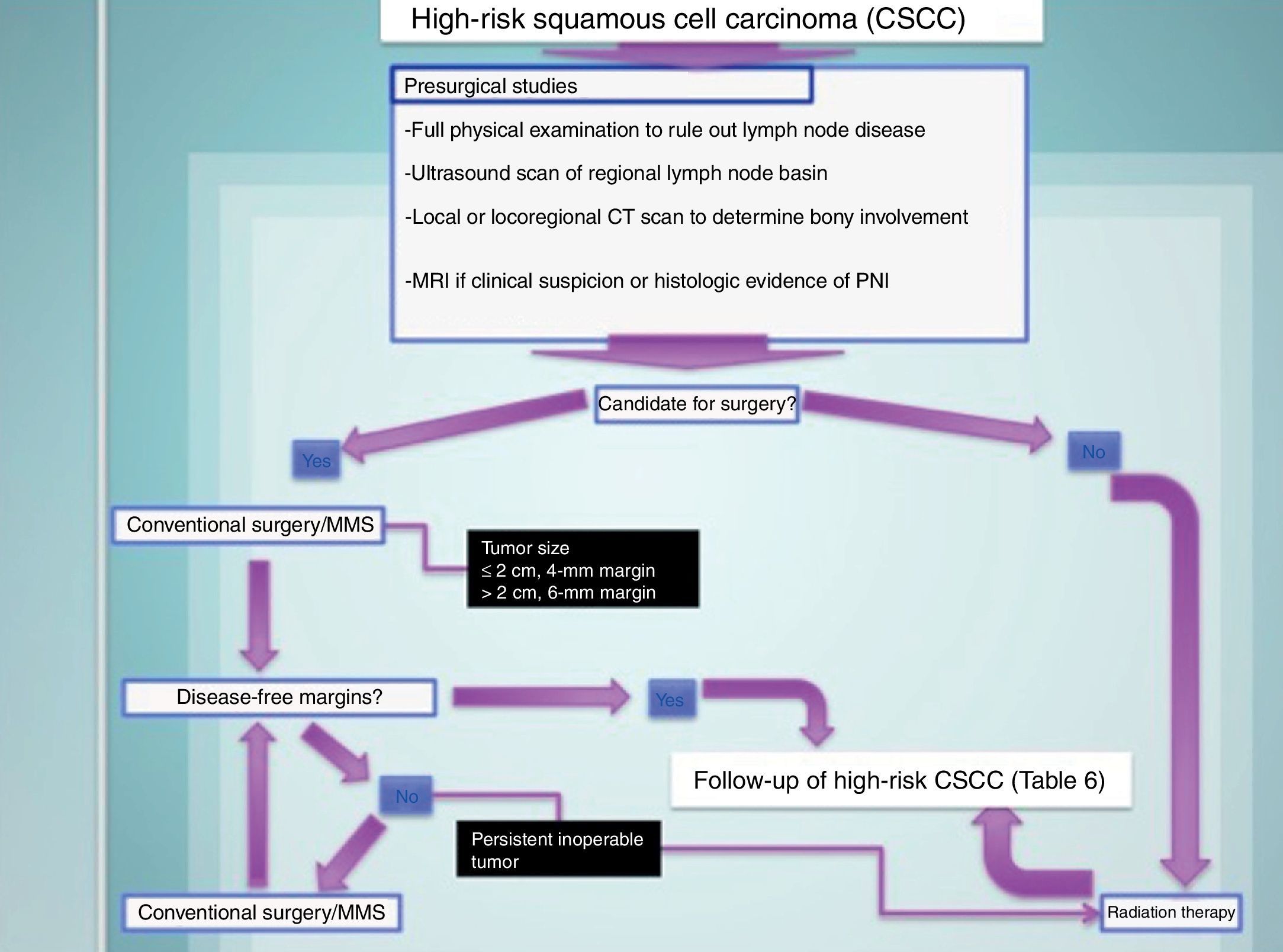
Treating High-Risk CSCCThe treatment of choice for high-risk CSCC is surgical excision. In conventional excision, safety margins should range from 4mm for tumors measuring 2cm or less to 6mm for larger tumors. Mohs micrographic surgery is the treatment of choice for tumors in locations with a risk of cosmetic or functional sequelae and for recurrent tumors42,71–75 (Fig. 7).
Radiation therapy, which is considered by several authors to be a first-line treatment for high-risk CSCC, produces poorer outcomes than surgical excision and is associated with a high percentage of earlier and more aggressive recurrence and considerable direct and indirect costs. Furthermore, it can cause iatrogenic carcinogenesis in the irradiated area. Radiation therapy, thus, should be reserved for patients who are not candidates for surgery, either because of poor general health status or the inability to achieve disease-free margins by surgical techniques.42,71–73
Additional Strategies for the Management of High-Risk CSCCAdditional management strategies are necessary in patients with high-risk CSCC given the high associated risk of lymph node invasion and mortality.
Sentinel Lymph Node BiopsyIn 2006, Ross et al.74 concluded that sentinel lymph node biopsy (SLNB) was associated with a reliable diagnosis of locoregional invasion and with low morbidity in CSCC provided that the surgeon had sufficient experience. This is also the case with cutaneous melanoma.
Indeed, SLNB provides better results in CSCC than in melanoma as the early detection of lymph node involvement in high-risk CSCC leads to a significant reduction in mortality.
We therefore believe that SLNB is justified in CSCCs defined as high-risk according to our provisional definition (Table 3 and Fig. 6).
Follow-up in Patients With a History of High-Risk CSCCHigh-risk CSCC is associated with a higher risk of recurrence and lymph node metastasis than low-risk CSCC in the first 5 years after treatment. The early detection of recurrence and lymph node metastasis is of supreme importance, as has been shown in multiple studies. Of particular relevance in this respect is a study by Ebrahimi et al.75 that showed that in patients with CSCC and lymph node involvement, a single diseased lymph node measuring 3cm or less in diameter without extracapsular nodal spread had a low risk of distant metastasis and mortality.
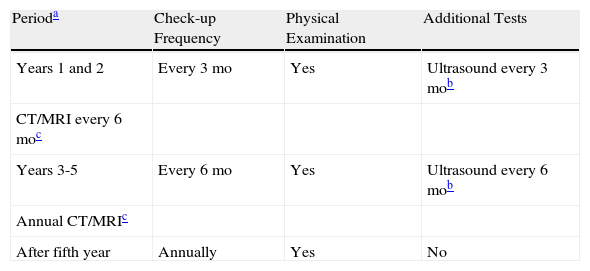
We therefore propose a follow-up approach based on risk (Table 4), whereby patients with high-risk CSCC should be followed very closely during the first 5 years after surgery. Close monitoring is particularly important during the first 24 months, which is when the risk of lymph node invasion is highest.
Follow-up Protocol for Squamous Cell Carcinoma Based on Risk.
| Perioda | Check-up Frequency | Physical Examination | Additional Tests |
| Years 1 and 2 | Every 3 mo | Yes | Ultrasound every 3 mob |
| CT/MRI every 6 moc | |||
| Years 3-5 | Every 6 mo | Yes | Ultrasound every 6 mob |
| Annual CT/MRIc | |||
| After fifth year | Annually | Yes | No |
Abbreviations: CT; computed tomography; MRI, magnetic resonance imaging.
The incidence of CSCC has increased considerably in recent years. CSCC is the second most common nonmelanocytic skin tumor in the general population and causes a similar number of deaths as melanoma.
A clear definition of epidemiological, clinical, and histologic factors of CSCCs with high rates of systemic spread (high-risk CSCC) will lead to a different management approach when dealing with this subgroup of patients. Such an approach should include more exhaustive staging at the time of diagnosis, more aggressive treatment, including SLNB, and closer follow-up. Individualized care will help to reduce the mortality associated with this malignant tumor.
In the not-so-distant future, the characterization of the molecular biology of high-risk CSCC and the analysis of genetic differences with respect to low-risk CSCC will probably help to define the malignant potential of this high-risk variant and allow the optimization of treatment via drugs that act on key molecular targets in the pathogenesis of squamous cell carcinoma.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Please cite this article as: Martorell-Calatayud A, et al. Carcinoma epidermoide cutáneo: definiendo la variante de alto riesgo. Actas Dermosifiliogr. 2013;104:367-79.


![Different histologic subtypes associated with high-risk cutaneous squamous cell carcinoma. A, Isolated-cell pattern (hematoxylin-eosin, original magnification x40). B and C, Squamous cell carcinoma with perineural invasion (nerves >0.1mm) (hematoxylin-eosin, original magnification x40 [B] and x100 [C]). D, Squamous cell carcinoma with marked lymphovascular invasion (hematoxylin-eosin, original magnification x100). E, Adenoid squamous cell carcinoma (hematoxylin-eosin, original magnification x40). F, Acantholytic squamous cell carcinoma (hematoxylin-eosin, original magnification x40). Different histologic subtypes associated with high-risk cutaneous squamous cell carcinoma. A, Isolated-cell pattern (hematoxylin-eosin, original magnification x40). B and C, Squamous cell carcinoma with perineural invasion (nerves >0.1mm) (hematoxylin-eosin, original magnification x40 [B] and x100 [C]). D, Squamous cell carcinoma with marked lymphovascular invasion (hematoxylin-eosin, original magnification x100). E, Adenoid squamous cell carcinoma (hematoxylin-eosin, original magnification x40). F, Acantholytic squamous cell carcinoma (hematoxylin-eosin, original magnification x40).](https://static.elsevier.es/multimedia/15782190/0000010400000005/v1_201307051101/S1578219013000917/v1_201307051101/en/main.assets/thumbnail/gr3.jpeg?xkr=ue/ImdikoIMrsJoerZ+w9/t1/zx4Q/XH5Tma1a/6fSs=)
![High-risk squamous cell carcinoma studied by sentinel lymph node biopsy. A, Squamous cell carcinoma of the lower lip in a 65-year-old man. B and C, Atypical squamous cell proliferation with acantholysis and perineural invasion. D-F, Invasion of sentinel lymph node by atypical squamous cells (hematoxylin-eosin, original magnification x40 [D] and x100 [E]; immunostaining with pankeratin, original magnification x100 [F]). High-risk squamous cell carcinoma studied by sentinel lymph node biopsy. A, Squamous cell carcinoma of the lower lip in a 65-year-old man. B and C, Atypical squamous cell proliferation with acantholysis and perineural invasion. D-F, Invasion of sentinel lymph node by atypical squamous cells (hematoxylin-eosin, original magnification x40 [D] and x100 [E]; immunostaining with pankeratin, original magnification x100 [F]).](https://static.elsevier.es/multimedia/15782190/0000010400000005/v1_201307051101/S1578219013000917/v1_201307051101/en/main.assets/thumbnail/gr6.jpeg?xkr=ue/ImdikoIMrsJoerZ+w9/t1/zx4Q/XH5Tma1a/6fSs=)