In a young woman, the differential diagnosis of ulcerated skin lesions on the lower limbs that heal leaving atrophic scars should include vasculitis and occlusive vasculopathy. Cutaneous polyarteritis nodosa (CPN) is characterized by the presence of painful nodules that typically develop on the lower limbs, frequently ulcerate, and usually heal without residual hyperpigmentation.1 Its diagnosis requires demonstration of necrotizing arteritis affecting medium-sized arteries. Livedoid vasculopathy (LV) is an occlusive vasculopathy with painful lesions in the form of punched-out ulcers that heal leaving white atrophic scars with peripheral telangiectasia.2 Light microscopy shows a mild superficial perivascular infiltrate and extravasation of red blood cells without vasculitis, although some arteries may present hyalinization of the wall and intraluminal fibrin deposits.
We present a case in which the lesions showed the clinical and histological features of both disorders.
The patient was 29-year-old woman with no past medical history of interest, who had been receiving aspirin 100 mg/24 h and pentoxifylline 400 mg/12 h for the last 8 years due to a skin disorder identified in another hospital as livedo reticularis. She was seen in our department for a 4-year history of outbreaks on the lower limbs of painful isolated lesions, sometimes ulcerated with serosanguineous crusts, and that disappeared after several months, occasionally leaving residual hyperpigmentation. The outbreaks appeared at random intervals and with no seasonal relationship. She had been treated with betamethasone plus gentamicin ointment, which, according to the patient, accelerated remission. She did not present Raynaud's phenomenon or other systemic symptoms.
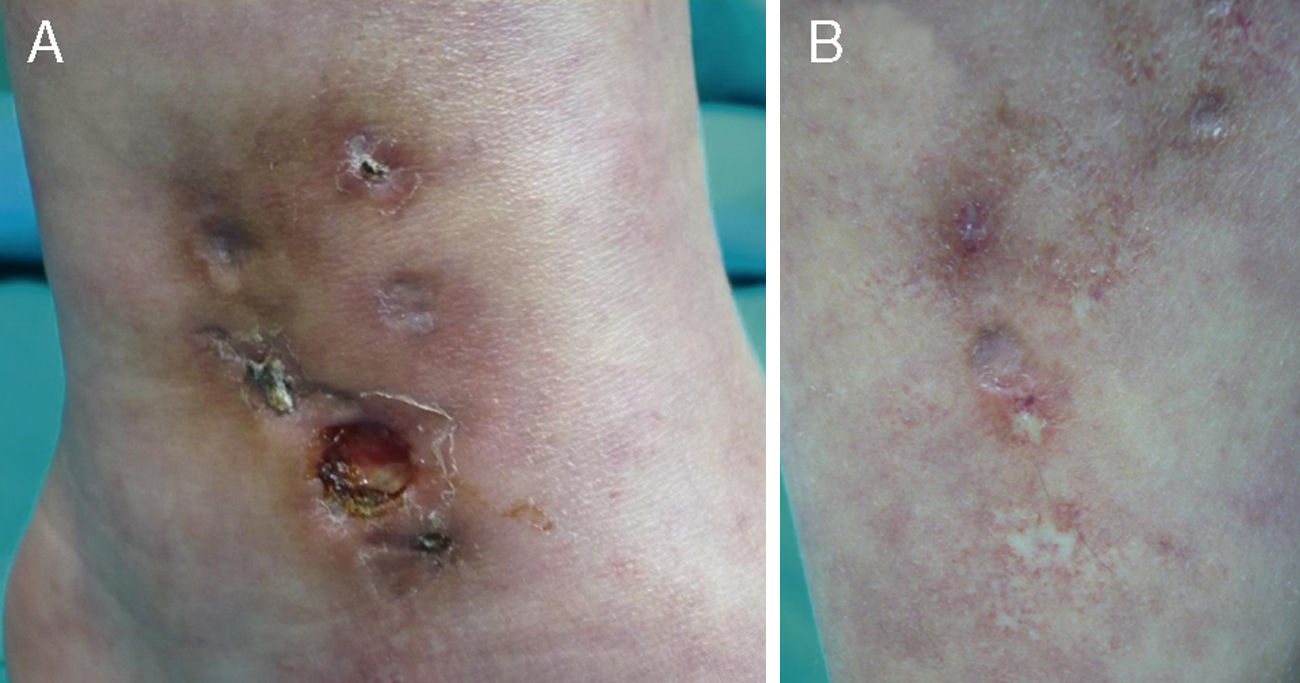
There were 3 relevant findings on physical examination: a) hypopigmented stellate macules on the ankle, dorsal surface of the foot, and left pretibial region (Fig. 1A); b) an indurated erythematous red-brown plaque on the medial aspect of the left ankle with several well-defined ulcerated lesions (the largest being 0.8 cm in diameter) within its borders (Fig. 1B); and c) very faint reticulated violaceous macules on the upper and lower limbs.
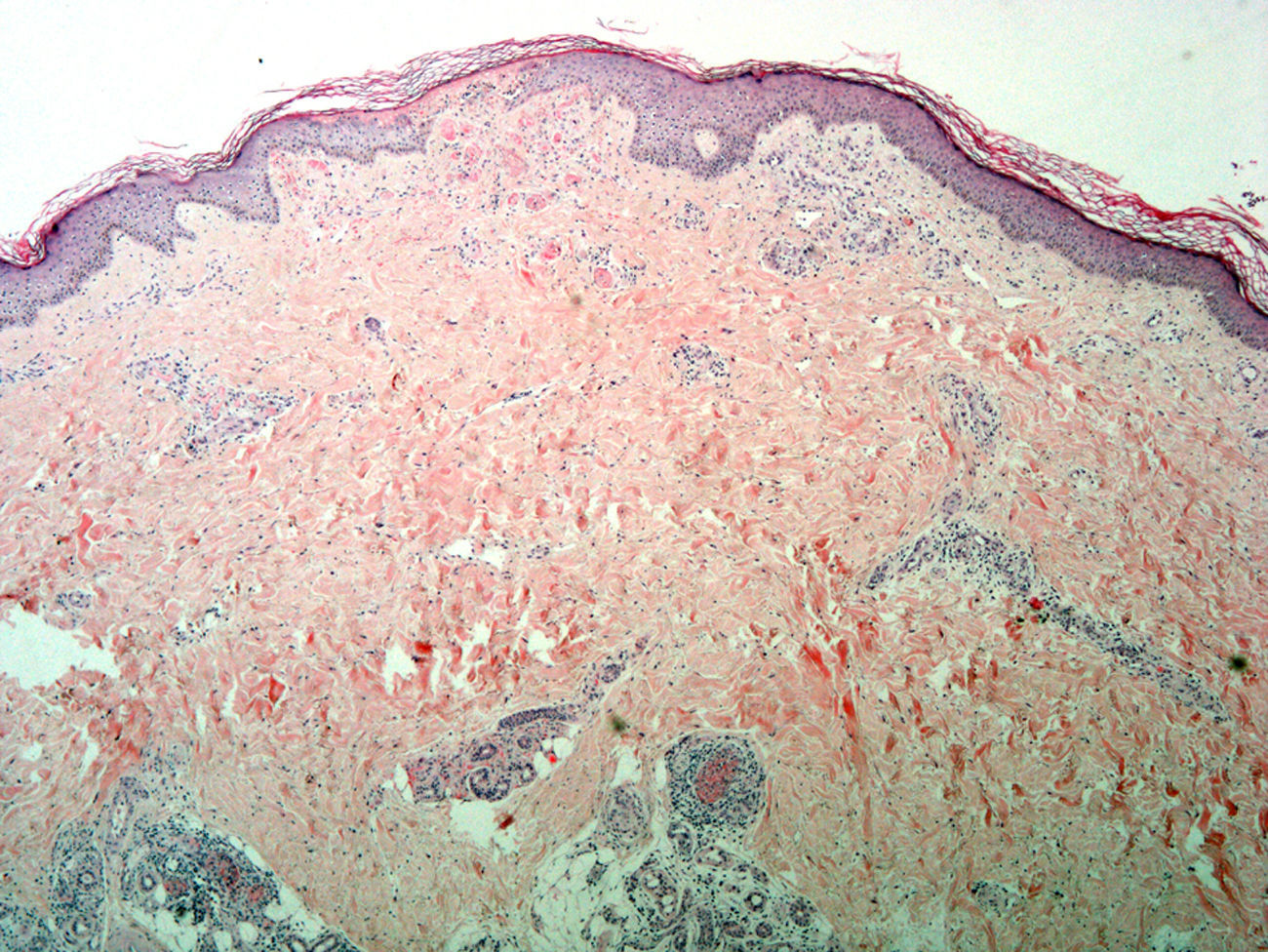
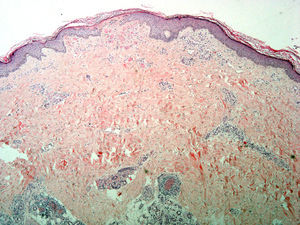
Skin biopsy from the area of the right lateral malleolus showed a necrotizing vasculitis affecting medium-sized vessels, with superficial changes consisting of vascular congestion with extravasation of red blood cells and, in some vessels, thrombosis and hyalinosis of the wall (Fig. 2).
Incipient necrosis of the epidermis and, in the dermis, signs of vascular congestion with extravasation of red blood cells, thrombosis, and hyalinosis of the vessel walls. Patchy foci of segmental fibrinoid necrosis with leukocytoclasia and mixed infiltrates in the dermal and hypodermal vessels (hematoxylin-eosin, original magnification x4).
Blood analysis showed that antinuclear antibodies were negative (titer of 1:40) and cytoplasmic antineutrophil cytoplasmic antibodies (cANCA) were positive at a titer of 1:20 (antiproteinase 3 and antimyeloperoxidase were negative on enzyme-linked immunosorbent assay). A complete coagulation study was conducted which included prothrombin activity, cephalin and thrombin times, functional fibrinogen, protein C and protein S, antithrombin III, homocysteine, anticardiolipin and anti-β2-glycoprotein I antibodies, lupus anticoagulant, and cryoglobulins and cold agglutinins; the results led to the conclusion that the patient was heterozygous for the prothrombin gene 20210. The possibility of systemic involvement was excluded based on the results of blood and urine analysis, electroneurogram, chest x-ray, and assessment by the nephrology and hematology departments. No palpable skin nodules were detected during follow-up. Treatment for CPN was started with oral prednisone (tapering regimen, commencing with 0.7 mg/kg/d) with the subsequent addition of azathioprine 200 mg/d, leading to partial control of the symptoms; subsequent tests for cANCA were negative.
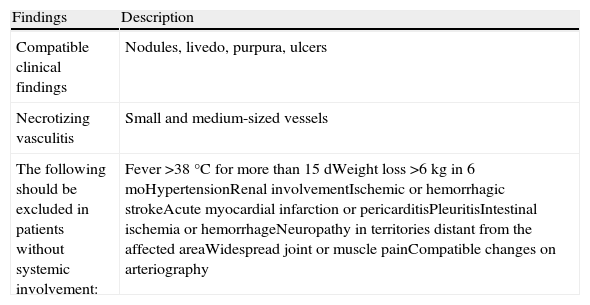
The recently established diagnostic criteria for CPN (Table 1) allowed us to consider that this was the most likely diagnosis in our case. However, the patient also presented clinical and histological features characteristic of LV. The coexistence of clinical and histological features of CPN and LV has rarely been described, although this may be due to an underestimation of their incidence. In a study of 29 patients with atrophie blanche lesions (mean follow-up, 18 years), skin biopsy demonstrated the presence of arteritis affecting medium-sized arteries, consistent with a diagnosis of CPN, in 21% of cases.3 Those authors suggested that occlusion of the dermal arteries by fibrin thrombi could be secondary to the partial obliteration of larger arteries affected by a primary necrotizing vasculitic process. The phenomenon is considered to be a reactive angioendotheliomatosis.4 In the case described, heterozygosity for prothrombin gene 20210, present in 1% to 5% of the white population, could be implicated in the occlusion of the dermal arteries that was observed concomitantly with the vasculitic changes. In this regard, there is a case report that describes a patient with LV who had the same genetic anomaly and a similar clinical presentation to that of our patient5; however, vasculitis was absent. It is well known that the pathogenesis of LV involves coagulation abnormalities such as decreased levels of activated protein C or increased levels of fibrinopeptide A.6 Its diagnosis must fulfill both clinical and histological criteria in the absence of signs or symptoms of systemic involvement.
Diagnostic Criteria for CPN.
| Findings | Description |
| Compatible clinical findings | Nodules, livedo, purpura, ulcers |
| Necrotizing vasculitis | Small and medium-sized vessels |
| The following should be excluded in patients without systemic involvement: | Fever >38°C for more than 15 dWeight loss >6kg in 6 moHypertensionRenal involvementIschemic or hemorrhagic strokeAcute myocardial infarction or pericarditisPleuritisIntestinal ischemia or hemorrhageNeuropathy in territories distant from the affected areaWidespread joint or muscle painCompatible changes on arteriography |
Source: Adapted from Nakamura et al.10
In the case described, it appears improbable that the LV progressed to CPN, given the pathogenic differences between the 2 disorders,7 although some authors have suggested that the initial thrombotic phenomenon of LV could trigger a necrotizing vasculitis in susceptible patients.8
Finally, a case of pANCA-positive CPN associated with minocycline therapy has been described.9 In our case, we identified a low titer of cANCA which, together with the negative results of further tests following treatment, suggests that these antibodies were not relevant from a clinical point of view.
In summary, atrophie blanche may be a clinical manifestation of CPN and may coexist with other more typical manifestations. Skin biopsies that include the deep subcutaneous tissue are required for diagnosis.
Please cite this article as: Llamas-Velasco M, et al. Panarteritis nodosa cutánea con clínica de vasculopatía livedoide Actas Dermosifiliogr. 2011;102:477-479.