Obesity and diabetes are chronic diseases that affect people all over the world, and their incidence is increasing in both children and adults. Clinically, they affect a number of organs, including the skin. The cutaneous manifestations caused or aggravated by obesity and diabetes are varied and usually bear some relation to the time that has elapsed since the onset of the disease. They include soft fibromas, acanthosis nigricans, striae, xerosis, keratosis pilaris, plantar hyperkeratosis, fungal and bacterial skin infections, granuloma annulare, necrobiosis lipoidica, psoriasis, and atopic dermatitis.
In this review article we present the skin changes found in children with diabetes mellitus and obesity and related syndromes and highlight the importance of the skin as a tool for establishing clinical suspicion and early diagnosis of systemic disease.
La obesidad y la diabetes son 2 enfermedades crónicas de distribución mundial, de incidencia en aumento tanto en niños como en adultos. Clínicamente se caracteriza por comprometer distintos órganos, entre ellos la piel. Las manifestaciones cutáneas secundarias o agravadas por la obesidad y la diabetes son variadas, y en su mayoría están relacionadas con el tiempo de evolución. Entre ellas se incluyen: fibromas laxos, acantosis nigricans, estrías, xerosis, queratosis pilar, hiperqueratosis plantar, infecciones cutáneas por hongos y bacterias, granuloma anular, necrobiosis lipoidea, psoriasis y dermatitis atópica, entre otros.
En esta revisión presentamos los hallazgos cutáneos en niños con estas 2 enfermedades; como también en los síndromes relacionados, recordando la importancia de la piel como herramienta para la sospecha clínica y el diagnóstico temprano de enfermedades sistémicas.
Obesity and diabetes are 2 chronic diseases with worldwide distribution that affect several organs, including the skin.1 Although both diseases are more common in adults, their prevalence in the pediatric population is growing. According to the World Health Organization, 20% of children and adolescents in Europe are overweight and, of these, a third are obese.2 Currently, 3700 cases of noninsulin-dependent diabetes are diagnosed annually in children and adolescents,3 a figure clearly higher than that previously reported.4 Given the current situation, physicians can expect to encounter a growing number of patients with clinical conditions related to these diseases.
The cutaneous manifestations of diabetes and obesity are directly related to the age of onset, duration, and severity of the underlying disease. This review article is divided into 2 sections based on existing classification schemes: the first deals with the cutaneous manifestation associated with diabetes, and the second with those associated with obesity.
DiabetesDiabetes mellitus is a heterogeneous group of disorders characterized by elevated blood sugar and impaired lipid and carbohydrate metabolism.5 It is classified according to pathogenesis as type1 (DM1) or type2 (DM2), and each type has specific clinical characteristics. DM1 is the result of the destruction, probably by way of an autoimmune process, of insulin-producing B-cells in the pancreatic islets. It is characterized by abrupt onset, insulin deficiency, a tendency to progress to ketoacidosis even in the early stages, and absolute dependence on exogenous insulin to sustain life. In the case of DM2, patients may be relatively asymptomatic for many years and have a twofold defect: deficient insulin action (insulin resistance) and deterioration of B-cell function. These patients may have low, normal, or elevated insulin levels. The typical patient with DM2 is an obese person with a family history of diabetes.
The complications associated with diabetes are multifactorial in origin, occurring as a result of biochemical, structural, and functional abnormalities.6 Of particular note among the anomalies found in diabetic patients is the acceleration of the biochemical process of advanced glycation as a result of chronic hyperglycemia and increased oxidative stress. Advanced glycation involves the generation of a diverse group of chemical substances known as advanced glycation end products (AGEs), which react with specific receptors to produce adverse effects.7 The production of AGEs is secondary to a nonenzymatic reaction of glucose with proteins, lipids, and nucleic acids.
Some 30% of adult diabetic patients will present cutaneous manifestations at some time in their lives; the timing will vary depending on the type of diabetes and the patient's age at onset. Children are no exception. DM1 is the most common type of diabetes in children,4 with a mean age at onset of 8years. It can be associated with growth disorders and autoimmune diseases as well as manifestations related to microvascular changes. Owing to the increasing prevalence of obesity and insulin resistance in children, the prevalence of DM2 has also increased in the younger population, particularly among adolescents.3
Since there are very few review articles on the classification of the cutaneous manifestations of diabetes in either children or adults, we have classified them using the schema proposed in 1985 by Edidin,4 which differentiates between skin disorders secondary to diabetes, skin disorders that occur more frequently in diabetic patients, cutaneous manifestations of insulin resistance, and skin disorders associated with insulin therapy (Table 1).
Cutaneous Manifestations in Diabetic Children.
| Skin Changes Caused by Diabetes | Skin Conditions Associated with Diabetes | Skin Changes Associated with Insulin Resistance | Complications of Insulin Therapy |
| XerosisKeratosis pilarisRubeosis facieiLimitation of joint mobilityInfections:a. Fungalb. BacterialMicroangiopathy and neuropathy | Granuloma annulareNecrobiosis lipoidicaDiabetic dermopathyAssociated with autoimmune diseases:a. Hypothyroidismb. Hyperthyroidismc. Celiac diseased. Addison diseasee. Vitiligo | Acanthosis nigricansFibroepithelial polyps or acrochordaObesityHirsutismAcneSeborrhea | Localized rashLipohypertrophyScarringBlisters |
High blood sugar levels and the damage to vascular and nerve structures characteristic of diabetes produce cutaneous manifestations such as xerosis, faciei rubeosis, limited joint mobility, infections, microangiopathy, and neuropathy.
Xerosis and Thickening of the SkinXerosis, or dry skin, is one of the earliest and most common signs of diabetes and is found in up to 22% of patients with DM1.8 Xerosis has been demonstrated by measuring transepidermal water loss and the high-frequency conductance of the forearm.9 Another finding of interest is that even in the absence of clinically evident xerosis, the skin of patients with diabetes exhibits abnormal desquamation and reduced elasticity10 as well as a greater than average thickness that may contribute to a reduction in elasticity.11 Skin thickening is classified clinically into 3 categories: a)benign thickening of the skin; b)scleroderma-like syndrome; and c)scleredema of Buschke. It is thought that skin thickening in this setting is caused by abnormal collagen glycation during episodes of hyperglycemia or by collagen proliferation promoted by excess insulin.12 The hands and feet are the most common sites of benign skin thickening in diabetic patients, and involvement of these sites is closely associated with joint limitation.13 Although the condition may be asymptomatic, increased skin thickness can be measured using cutaneous ultrasound.11
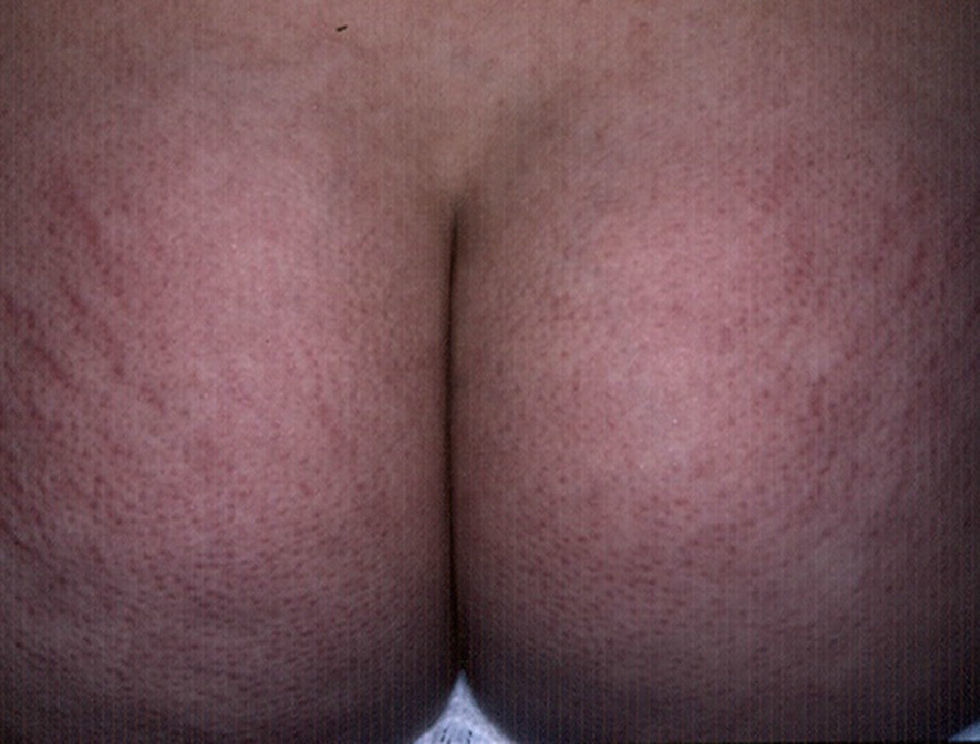
Keratosis PilarisKeratosis pilaris is a common condition in diabetic children, with a prevalence of 11.7% in patients with DM1 over 10years of age.8 The etiology is unclear, but it appears that xerosis plays an important role.14 The clinical characteristics include rough follicular papules and variable erythema located predominantly on the extensor surfaces of the arms and legs and occasionally on the face, buttocks, and trunk (Fig. 1). Keratosis pilaris tends to flare up in winter and usually improves during the summer months.15 It is mainly associated with atopic dermatitis and an high body mass index.16 Treatment includes keratolytic agents, retinoids, and low potency topical corticosteroids.17
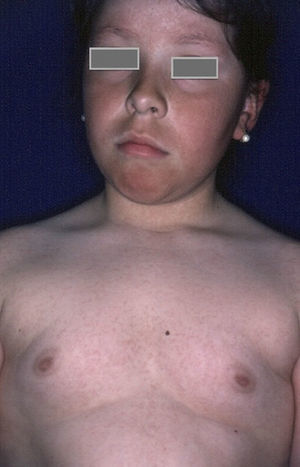
Rubeosis FacieiRubeosis faciei diabeticorum—the characteristic facial rash found in diabetic patients (Fig. 2)—is caused by the dilation of small vessels in the cheeks probably as a result of microangiopathy induced by hyperglycemia.18 The prevalence of this condition is higher in patients with DM2 (21%-59%)19 than in children with DM1 (7%),8 and it is directly related to the duration of the disease.
Limited Joint MobilityLimited joint mobility, also called diabetic cheiroarthropathy or limited joint mobility syndrome, is the earliest clinically apparent long-term complication of DM1 in childhood.20 It involves asymptomatic bilateral contracture of the joints of the digits and the large joints associated with waxy thickening of the skin and short stature. It appears to be the result of nonenzymatic glycation of collagen, which results in insoluble crosslinked collagen that produces rigidity in the dermis and joints.7 The frequency of this condition is variable; in some case series it has been found in 2.3% of patients with DM1,8 and in others it has been reported in 30% of diabetic patients aged under 21 who are short in stature.21 The incidence of this complication has decreased in recent years with improved glycemic control.
The first joints affected are the proximal interphalangeal joints of the fourth and fifth fingers, with a stiffness or flexion contracture that limits finger extension accompanied by scleroderma skin changes. This rigidity may spread to the other fingers and to the wrists, elbows, ankles, knees, toes, and cervical and thoracic spine. As the development of this complication is directly related to the duration of diabetes and glycemic control, cases in children under 10 years of age are rare.22 Patients with limited joint mobility have an increased risk of developing the other microvascular complications of diabetes, especially retinopathy, nephropathy, and neuropathy.23
InfectionsIt is well known that diabetic patients are susceptible to severe, recurrent, and atypical infections. The infections most often found in diabetic children are fungal and bacterial infections, especially with species of Candida, Staphylococcus, and Streptococcus.5No association with or predisposition to specific viral infections has been observed.
Fungal InfectionsChildren with diabetes have increased susceptibility to infections caused by dermatophytes and Candida species, especially tinea pedis, onychomycosis, and candidiasis of the mucous membranes or flexures. Tinea pedis and onychomycosis, which mainly affect the interdigital spaces and the forefinger, are present in 2.8% of these patients and, as in the general population, over 90% of cases are caused by Trichophyton rubrum and Trichophyton mentagrophytes.24
Candidal infections are relatively more frequent in these patients than in the general nondiabetic population. The most common are candidal vulvovaginitis, balanitis, and angular cheilitis, one of which is present in over 5% of diabetic patients.8 In a study of diabetic girls aged between 2 and 15years, Candida species were isolated in 56% of vulvar cultures.25 Close monitoring of blood glucose and glycosylated hemoglobin levels in patients with mycotic infections is important because the infection will be very difficult to control if the diabetes is poorly controlled.
Bacterial InfectionsStaphylococcal infections are more common in patients with DM1 than in patients with DM2 owing to the direct relationship between glycemic control and susceptibility to the infection and its severity.26 Folliculitis and impetigo are the most common infections, and these patients are often carriers of Staphylococcus in the body orifices. Boils, anthrax, and necrotizing fasciitis are all rare in children.4
Other infections that can occur in diabetic children and adolescents include erythrasma, mucormycosis, and malignant otitis externa, although these conditions are typically found in older patients who have some degree of immunosuppression.4
Microangiopathy and NeuropathyChanges related to the effects of diabetes on the vascular and nervous system occur later than those described above because they are associated with structural deterioration caused by chronic hyperglycemia and ischemia of the vasa nervorum in the case of peripheral nerves27 and a form of arteriosclerosis in the microvasculature associated with reduced elasticity and impaired endothelial vasomotor function in the peripheral arteries.28 Consequently, these effects occur more frequently during the second decade of life.
Findings in the lower limbs include decreased temperature, nail dystrophy, and mottled skin color on the feet, accompanied by skin atrophy and hair loss on the legs and feet. Other changes observed include anhidrosis—the result of severe vascular insufficiency or autonomic dysfunction—and poor wound healing due to vascular insufficiency and neuropathy. These changes are mainly found in older adolescents and young adults.4
Although more typical of the older diabetic patient, the structural and functional alterations that will predispose these patients to develop diabetic foot—including calluses, long nails, blisters, and dry skin—are also observed in younger patients.29 It is very important that these predisposing factors be identified so that preventive measures can be implemented. The physician should seek to reduce the incidence of diabetic foot and the prevalence of lower limb amputation in adult patients with diabetes.30
Skin Disorders That Occur More Frequently in Diabetic PatientsDisorders of Unknown OriginThere is a group of disorders of unknown origin that are associated with diabetes or are found more frequently in diabetic patients. It includes necrobiosis lipoidica, granuloma annulare, diabetic dermopathy, bullosis diabeticorum, reactive collagenosis, scleroderma diabeticorum, pruritus, and yellow skin. In this article we will only discuss necrobiosis lipoidica, granuloma annulare, and diabetic dermopathy since the other manifestations are rare and very few pediatric cases have been reported in the literature.
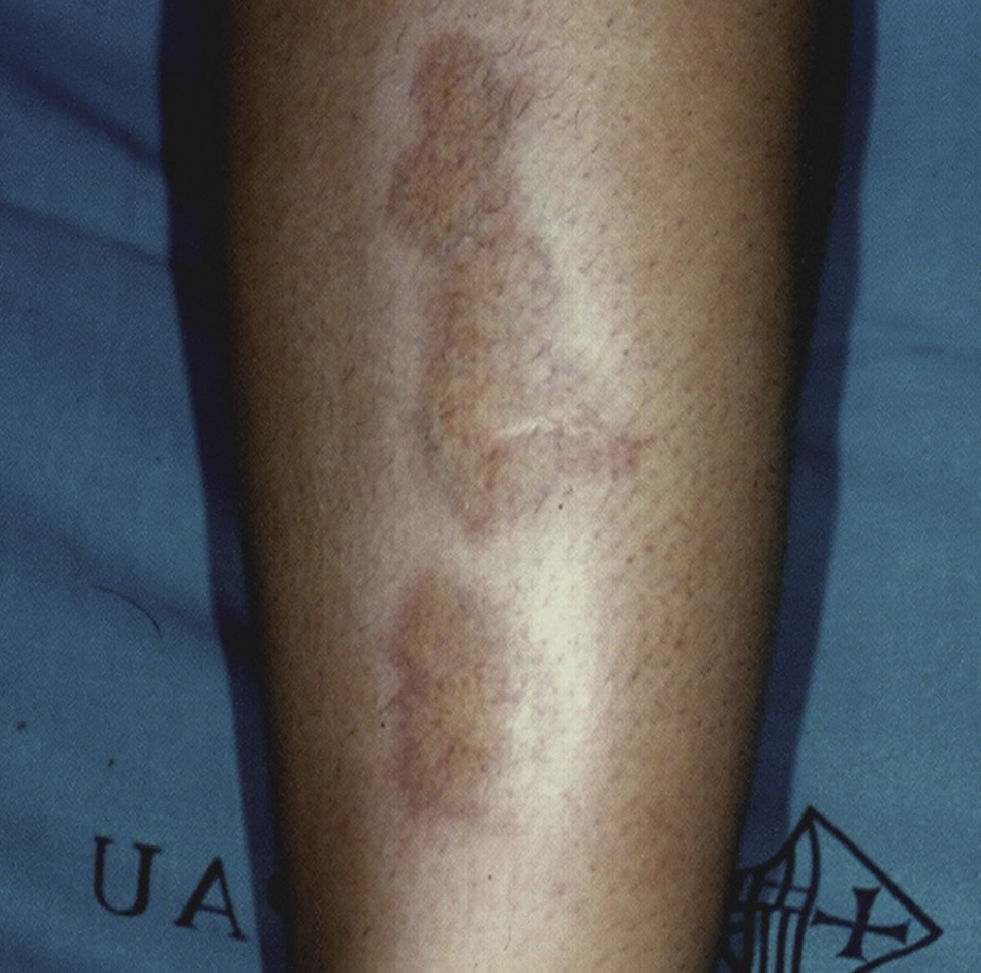
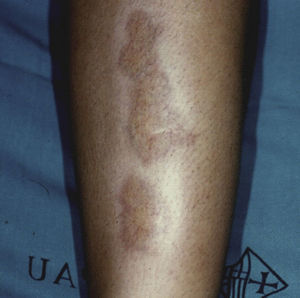
Necrobiosis LipoidicaNecrobiosis lipoidica is a rare disease, more common in women, with a prevalence of 0.3% in adult patients with diabetes; it is much less frequent in childhood, with a prevalence of only 0.06%.31,32 In diabetic patients, the presence of this disorder is associated with a higher frequency of retinopathy and nephropathy.33 Clinically, it appears as bilateral, asymptomatic, yellow-orange or red-brown plaques distributed symmetrical on the lower limbs, often on the anterior aspect of the tibia (Fig. 3); in isolated cases the lesions may be located on the upper limbs.34 The characteristic histologic features are neutrophilic necrotizing vasculitis in the early stages and amorphous degeneration and hyalinization of dermal collagen (necrobiosis) in the later stages. Treatment consists of good control of blood glucose levels. Numerous treatments have been tried with limited success, including topical or intralesional corticosteroids, pentoxifylline, topical retinoids, and calcineurin inhibitors.35
Granuloma AnnulareGranuloma annulare is a benign inflammatory disorder of unknown origin characterized by degeneration of the connective tissue and a predominantly histiocytic inflammatory infiltrate. Although the origin of this condition remains poorly understood, in adults a marked association has been observed with certain systemic diseases, particularly diabetes and rheumatic diseases.36 No such association has been established in children, but isolated cases of granuloma annulare in childhood diabetes have been reported and in the presence of these conditions physicians should assess the risk factors for other comorbidities and order the pertinent tests or studies.37
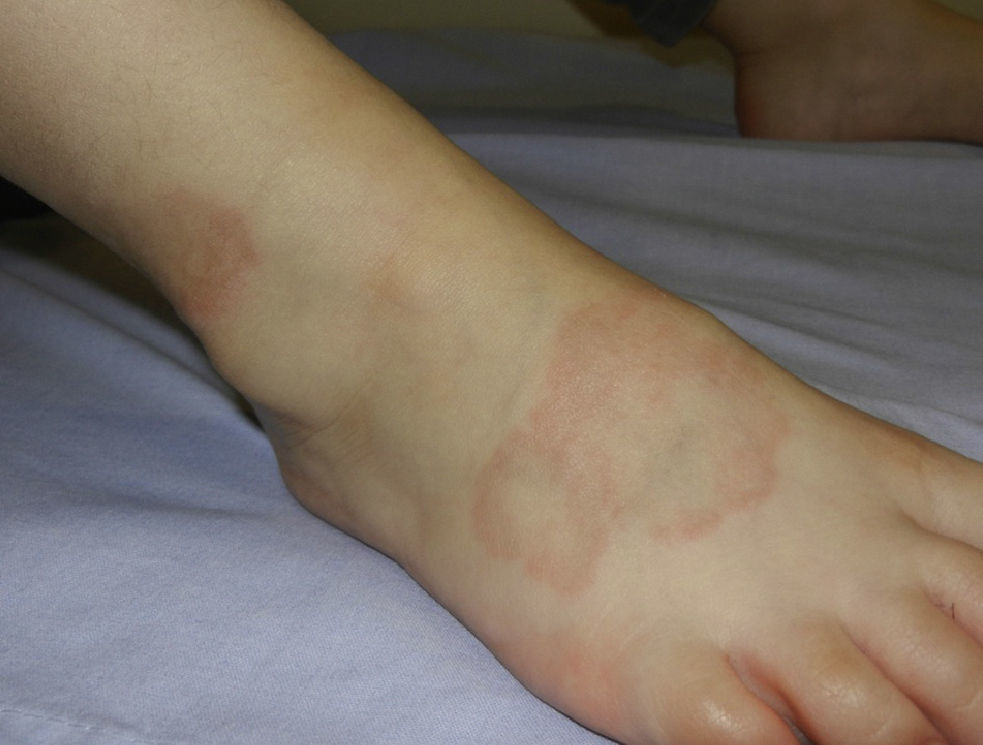
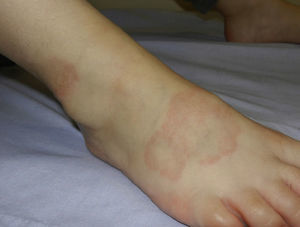
Granuloma annulare can occur at any age, but is most frequently observed in children and adolescents.38 In childhood, the most common clinical variants are the localized and subcutaneous forms. Localized granuloma annulare presents as pale red or violaceous papules which are firm and smooth to the touch. The lesions fuse into single or multiple annular plaques organized around a slightly depressed and hyperpigmented center. Subcutaneous or deep granuloma annulare presents as a fixed nodule located on the legs (Fig. 4), scalp, palms, or buttocks.39 Other less common variants include disseminated granuloma annulare characterized by a diffuse papular eruption, and a perforating form that presents as umbilicated papules with a central crust or scale and transepidermal elimination of necrobiotic connective tissue from the center.
In some patients, granuloma annulare may coexist with necrobiosis lipoidica. Owing to the histologic similarity, some authors have suggested that granuloma annulare is an early phase of necrobiosis lipoidica.40
Treatment is frequently unnecessary since most of these lesions resolve spontaneously within 2years of onset. The condition may be treated for cosmetic reasons,41 and in such cases the treatment options will depend on the clinical presentation. The localized forms can be managed with high-potency topical corticosteroids, calcineurin inhibitors, cryotherapy, or pulsed dye laser.42 Generalized forms may be treated with one of a variety of systemic therapies, including dapsone, retinoids, niacinamide, antibiotics, antimalarials, phototherapy, or photodynamic therapy, all with only relative therapeutic success.43–45
Diabetic DermopathyDiabetic dermopathy is the most common cutaneous manifestation of diabetes in adults, with an incidence that varies from 9% to 55% in these patients; however, it is quite rare in children.46 It presents as well-defined, slightly indented, light brown atrophic patches of usually less than 1cm in diameter. The lesions are distributed bilaterally and asymmetrically on the anterior aspect of the lower legs and occasionally on the thighs, arms, or lateral malleolus.4 Although the etiology and pathogenesis of this condition are poorly understood, it is known that the clinical features are due to hemosiderin and melanin deposits.47
Histologic findings in the epidermis include atrophy of the rete ridges, moderate hyperkeratosis, and varying degrees of basal pigmentation. The papillary dermis reveals telangiectasias, fibroblast proliferation, and edema as well as hyaline microangiopathy, extravasated erythrocytes, hemosiderin deposits, and a moderate perivascular infiltrate made up of lymphocytes, histiocytes, and characteristically, plasmocytes.46
Treatment of these lesions is ineffective and not recommended because they are asymptomatic and their course is variable; they may persist indefinitely or spontaneously regress.48
Patients presenting with such lesions should be investigated for diabetes mellitus because, although not pathognomonic, this condition is strongly associated with and specific to diabetes. The presence of dermopathy in diabetic patients is an indication that the disease is poorly controlled.46
Cutaneous Manifestations Associated with Autoimmune DiseasesThe incidence of autoimmune diseases is higher in children with DM1 and their families, apparently due to the presence of circulating autoantibodies against specific organs.20 It is important, therefore, when taking a medical history to ask the appropriate questions and to ensure early diagnosis of these diseases. The autoimmune diseases most closely linked to diabetes mellitus are thyroid diseases, celiac disease, and primary adrenal insufficiency.
HypothyroidismPrimary hypothyroidism in the presence of antithyroid antibodies occurs in approximately 3% to 8% of patients with DM1.49 Antithyroid antibodies are positive in almost 25% of children with diabetes, and the incidence of positivity is higher in girls.50 Hypothyroidism should be suspected in patients who present asymptomatic goiter, excessive weight gain, dry skin, cold intolerance, lethargy, abnormal fatigue, and bradycardia. Diagnosis is made by demonstrating the presence of autoantibodies, low levels of free thyroxine, and elevated levels of thyroid stimulating hormone. This condition requires medical treatment with levothyroxine replacement. It does not usually alter insulin requirements or glycemic control.20
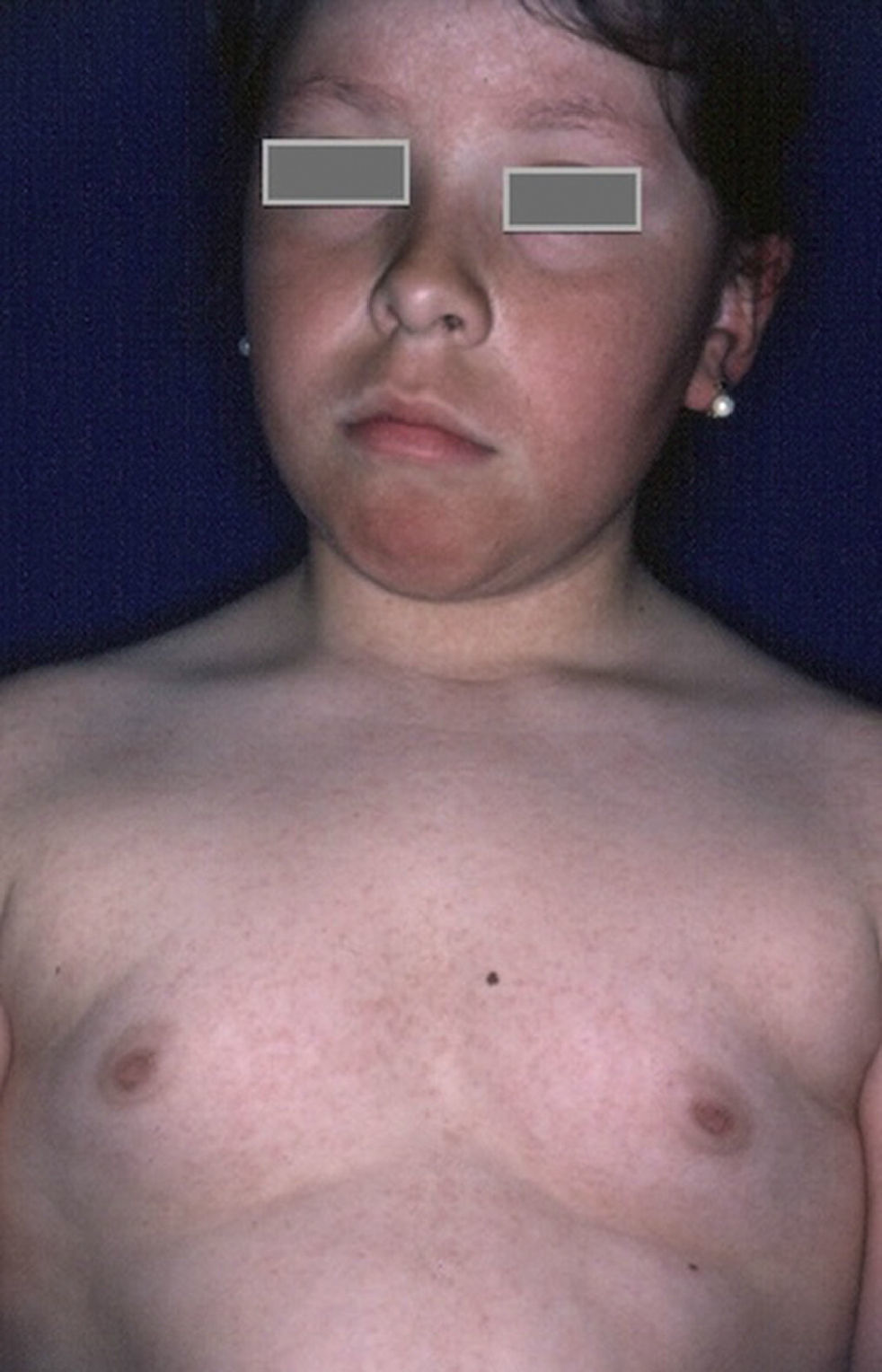
HyperthyroidismThe association between diabetes and hyperthyroidism is much less common than between diabetes and hypothyroidism, but hyperthyroidism is, nevertheless, more common in patients with diabetes than in the general population, and its presence may indicate the initial stages of either Graves disease or Hashimoto thyroiditis.51 Hyperthyroidism should be suspected in patients whose blood glucose levels are poorly controlled despite adequate treatment and who also have significant weight loss unrelated to a change in diet, increased sweating and irritability, tremors, tachycardia, and heat intolerance, as well as the characteristic enlarged thyroid and exophthalmos.20 Appropriate antithyroid treatment should be prescribed by an endocrinologist.
Celiac DiseaseThe prevalence of celiac disease in diabetic children and adolescents ranges from 1% to 10%, and the earlier the onset of diabetes the higher the risk of celiac disease.52 In most cases, celiac disease is asymptomatic and not necessarily associated with growth retardation or poor control of diabetes; however, in either of these situations the physician should investigate the possibility of celiac disease.20 Celiac disease should also be suspected in any child presenting gastrointestinal signs and symptoms—such as diarrhea, abdominal pain, flatulence, dyspepsia—anemia or recurrent thrush. Celiac disease is associated with hypoglycemic episodes and reduced insulin requirements.53 It should be diagnosed and managed with the support of a pediatric gastroenterologist.
Addison DiseasePrimary adrenal insufficiency or Addison disease is caused by dysfunction or reduced function of the adrenal cortex and results in insufficient production of glucocorticoids, mineralocorticoids, and androgens accompanied by high levels of both adrenocorticotropic hormone (ACTH) and plasma renin activity. In developed countries, the prevalence of Addison disease is 110 to 144 cases per million inhabitants.54 Diabetic patients have an increased risk of developing Addison disease, and adrenal antibodies have been detected in just over 2% of patients with DM1.55 The disorder should be suspected in diabetic patients who present increased skin pigmentation or report unexplained decreases in insulin requirements, weight loss, hyponatremia, or hyperkalemia.20 Addison disease in childhood should be diagnosed and managed by a pediatric endocrinologist.
VitiligoThe global prevalence of vitiligo ranges from 0.1% to 4%, with onset before the age of 20 in about half of these cases.56 The association between vitiligo and DM1 is common, with concomitant vitiligo occurring in about 6% of diabetic children20 and diabetes occurring in 0.6% of patients with vitiligo.57 The types of vitiligo most frequently associated with diabetes are the treatment-resistant generalized forms.56
Cutaneous Manifestations Due to Insulin Resistance SyndromeIn recent years, the growing prevalence of insulin resistance syndrome and the worldwide increase in the prevalence of DM2 has raised concerns about this epidemic. In 2007, the Search diabetes research group reported that in the United States some 3700 children and adolescents are diagnosed every year with DM2, mostly aged between 10 and 19years.58 Although the etiology and pathophysiology of this syndrome are not the subject of the present review, it is important to understand that insulin resistance is a state in which a given quantity of insulin produces a less-than-expected biological response, which is followed by compensatory hyperinsulinemia to maintain normal glucose levels and lipid homeostasis.59 Thus, the syndrome results in a series of risk factors for diabetes as well as for cardiac and central nervous system disease.
The most common cutaneous manifestations of insulin resistance are fibroepitheliomas and acanthosis nigricans (AN), which are described below. These disorders are observed in up to one-third of patients. Other manifestations include keratosis pilaris, hirsutism, and signs of hyperandrogenism, including acne and seborrhea, 2 conditions that are accentuated by the presence of obesity. In adults, the condition also exacerbates the severity of infections and other disorders affecting the limbs, such as plantar hyperkeratosis and ulcers, among others.60
The presence of clinical manifestations and risk factors should prompt the physician to evaluate environmental risk factors, such as home schooling, obesity in the patient's parents, the lifestyle habits of both parents and children, outdoor activity, and diet. This should be done to ensure early diagnosis and prompt treatment, which will involve changes in lifestyle and diet as well as pharmacological therapy such as metformin or insulin, when required.3
Skin Disorders Associated With Insulin TherapyThe growing incidence of diabetes in the world in general and among children in particular has led to the initiation at a younger age of insulin therapy, whether by continuous subcutaneous insulin infusion or multiple daily injections.61 The prevalence of skin complications secondary to insulin injection is variable: in one study lipohypertrophy was found in 1.8%, but the authors of 2 other studies reported lipoatrophy in 29% and lipohypertrophy in 48% of patients.8 In most cases, these skin reactions are of sufficient clinical importance to warrant withdrawal of treatment.62 The clinical findings most frequently observed in Canadian children using insulin infusion pumps were scars measuring less than 3mm, in length, erythema with no associated nodules, subcutaneous nodules, and lipohypertrophy.62 Less common findings include redness, blistering, and local infection at the injection site.61
ObesityObesity is a chronic disease characterized by increased body weight due to abnormal or excessive fat accumulation. In children, there are two different ways of defining the overweight or obese state. In the first method, the body mass index (BMI) is corrected for sex and age and the child is considered overweight if the BMI is greater than 25 and obese when BMI is over 30.63 The second method, used more frequently, defines a child as overweight if he or she falls into the range between the 85th and 95th percentile, and as obese if they are in the group above the 95th percentile as specified by the charts published by the International Obesity Task Force (IOTF).64
It is currently known that in the USA in the age group comprising children and adolescents, 1 out of every 6 individuals is overweight.65 Although the exact prevalence of the skin manifestations of obesity is not known, it has been shown that they are all directly related to the severity and duration of obesity.66 Moreover, the incidence is greater when obesity is associated with diabetes and/or insulin resistance syndrome.67
The cutaneous findings can be classified into 3 groups (Table 2): changes in skin physiology, skin changes associated with obesity, and obesity-related skin disorders.
Cutaneous Manifestations in Obese Children.
| Physiological Skin Changes | Skin Disorders Associated with Obesity | Skin Disorders Exacerbated by Obesity | Complications of Treatment |
| Dry skinHeat intoleranceExcessive sweatingLocalized rash in areas of friction | Acanthosis nigricansacrochorda or fibroepitheliomasStriaeKeratosis pilarisPlantar hyperkeratosis | Skin infectionsa. Fungalb. BacterialPsoriasisAtopic dermatitis | Lichenoid reactionErythema ab igne |
Numerous changes in skin physiology are found in obese patients, including the following: excess sweating and alteration of barrier function leading to higher transepidermal water loss and dry skin68; alteration of collagen function and structure; increased lipokine activity and leptin resistance, probably due to an imbalance in the production of cytokines by adipocytes; and finally, the production of tumor necrosis factor-α, transforming growth factor-β, interleukin (IL) 1, IL-6, and leptin by adipocytes, giving rise to a proinflammatory state.69
Leptin is of particular interest because it is the protein product of the gene for obesity, which is synthesized particularly in adipocytes and is closely linked to obesity and insulin resistance.70 Leptin is considered to be a growth factor because in vitro it stimulates the proliferation of various cell types71; consequently, leptin deficiency could lead to impaired wound healing in the skin.69 A direct relationship has been found between leptin levels and obesity, insulin resistance, and the development of fibroepitheliomas.71
Other physiological effects of obesity on skin include increased physiological folds, decreased sensitivity, a decrease in the reactivity of the microvasculature, and inhibition of lymphatic circulation.66 All of these effects correlate with the severity and duration of obesity, and are probably the causative agents of secondary dermatosis.72
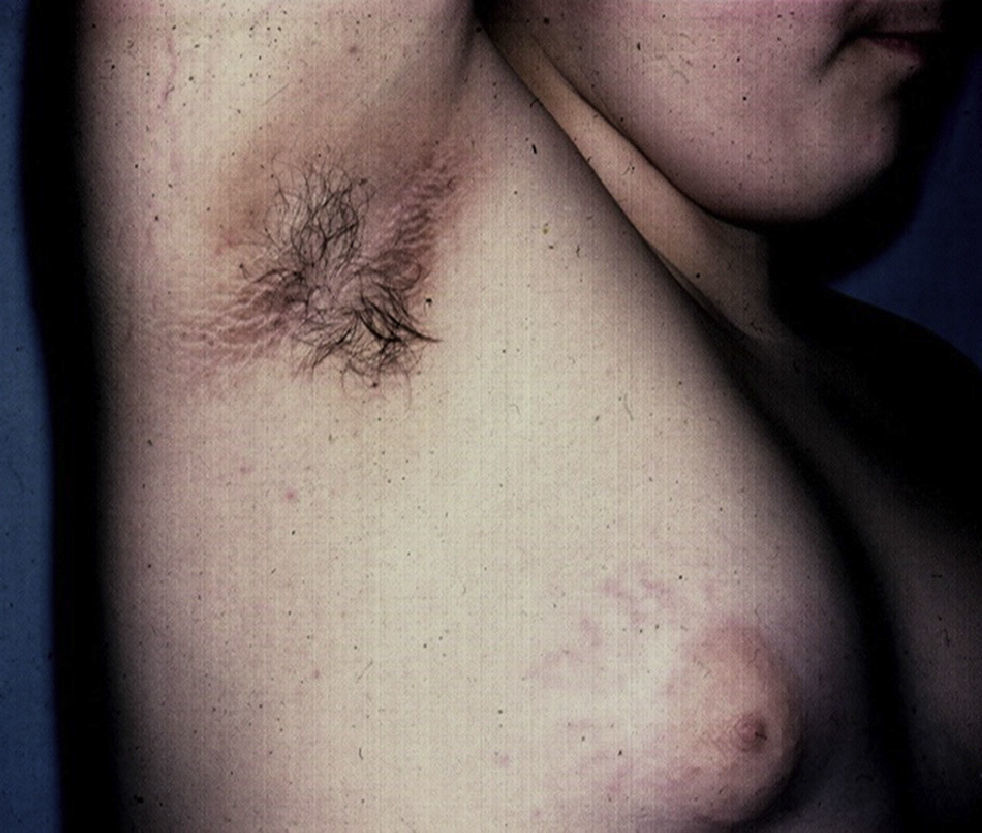
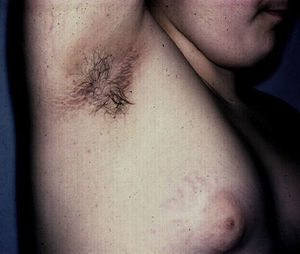
Skin Changes Associated With Obesity in ChildrenAcanthosis NigricansAcanthosis nigricans is the most common dermatologic manifestation of pediatric obesity, occurring in 66% of overweight adolescents73 and in 56% to 92% of children and adolescents with DM2. It is considered to be an important clinical marker.74 The condition presents as pigmented velvety patches or plaques, sometimes with a hypertrophic and verrucous surface. Distribution is usually bilateral, symmetrical, and localized to the armpits (Fig. 5), the posterior neck fold, the flexures of the upper and lower limbs, the umbilicus and the inguinal and inframammary folds.75 Acanthosis nigricans is the most common early symptom observed in children who present obesity and/or insulin resistance syndrome. In most cases, treatment involves weight reduction and adequate control of blood sugar levels.
Acanthosis nigricans also occurs in children with multisystem disorders and in such cases is a useful diagnostic indicator. In type A acanthosis nigricans or HAIR-AN syndrome (hyperandrogenism, insulin resistance and acanthosis nigricans), hyperandrogenism is associated with polycystic ovaries, signs of virilization, hirsutism, seborrhea, and episodes of acne. In type B acanthosis nigricans, more common in women and older patients (age range from 12 to 60 years),4 marked hyperandrogenism is found in association with other autoimmune diseases, primarily lupus erythematosus.76 In this setting, the acanthosis nigricans may be periorbital. Other more rare syndromic forms are the congenital Berardinelli-Seip syndrome and acquired generalized lipodystrophy or Lawrence syndrome, characterized by a complete absence of subcutaneous fat due to severe insulin resistance and hyperandrogenism.77
The detection of acanthosis nigricans is useful in that it can lead to early referral to a specialist, which in turn leads to advice on lifestyle modifications that can improve the patient's risk factors.78
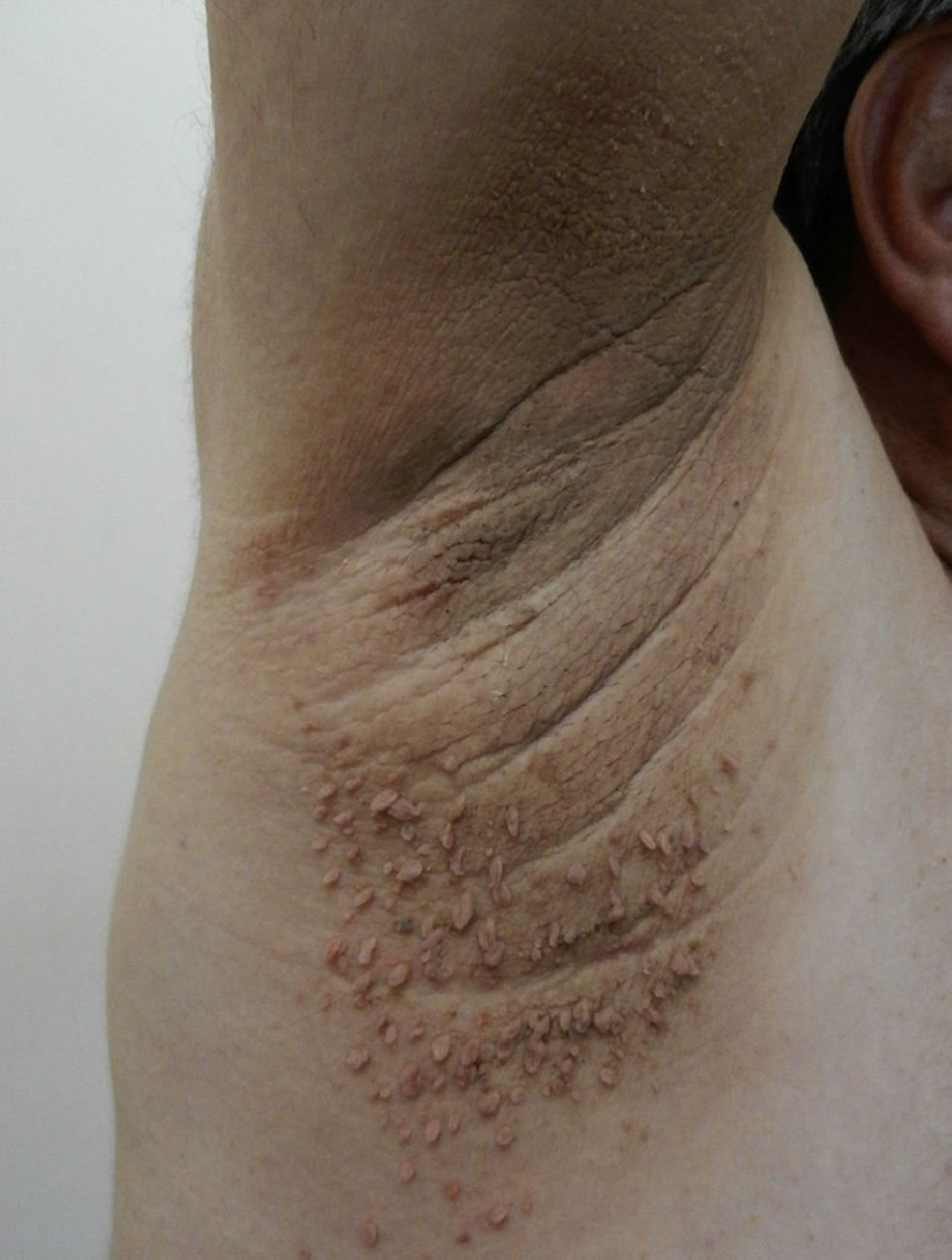
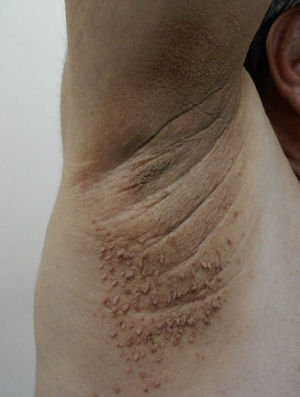
AcrochordonAcrochorda, fibroepithelial polyps, or pedunculated fibroids are soft, coffee colored, pedunculated papules often located on the neck, in the axillae, (Fig. 6) or in the groin. They are associated with acanthosis nigricans. Few studies have been published on the incidence of this condition in children. The origin of these skin tags is unknown, but their appearance in adults is closely linked to excessive rubbing of the skin, hormonal imbalance, hypertension, metabolic syndrome, and obesity in adults.79 It has been observed that patients who have more than 10 acrochorda are usually obese16 and have elevated leptin levels.71 Treatment options are simple excision with cold scissors, electrodessication, or cryotherapy.
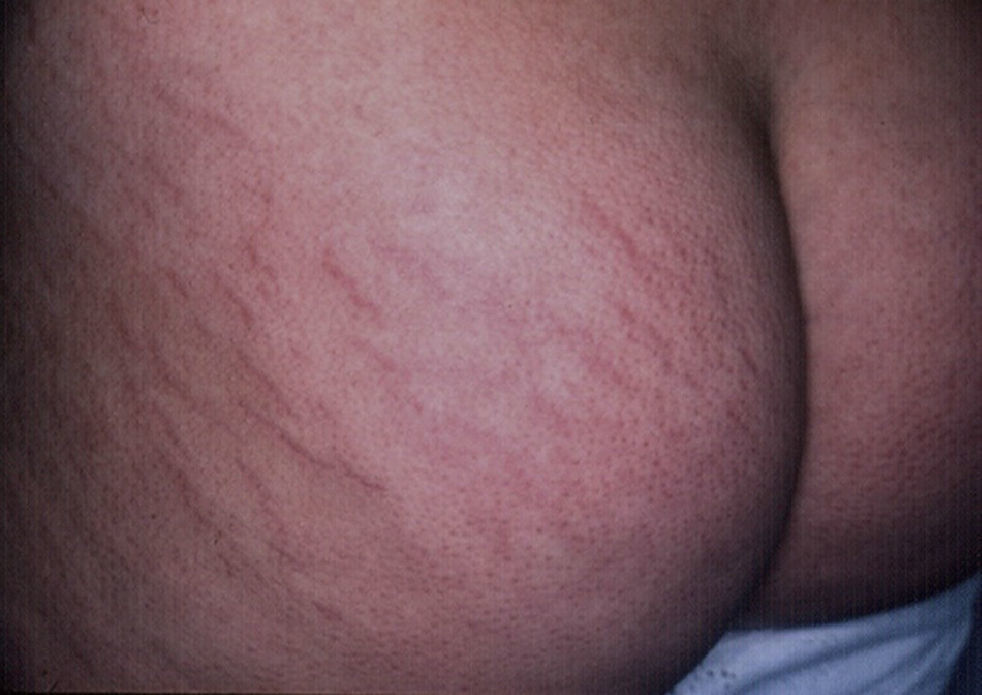
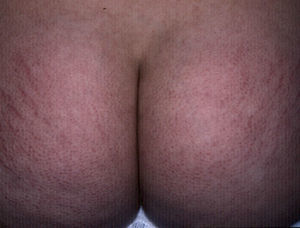
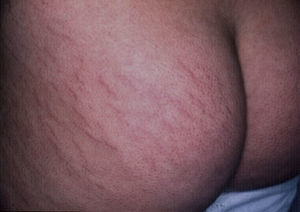
StriaeIn childhood, the presence of striae, like that of acrochorda, is directly related to excess weight,16 with an incidence of up to 40% in children with moderate to severe obesity.80 Striae are linear atrophic plaques distributed perpendicular to the force of greatest tension. They are found predominantly on the breasts, buttocks (Fig. 7), abdomen, and thighs.71 In the initial stages the marks are erythematous, but they later turn violet and finally become white depressed patches. Treatment options are numerous, and include tretinoin, alone or combined with glycolic acid, microdermabrasion, pulsed dye laser, and fractionated laser (CO2 and erbium). None of these treatment has been shown to be more successful than the others.81
Plantar HyperkeratosisIn obese patients, the shape of the foot may be altered with the loss of the arch, a wider and more obvious footprint, and greater pressure evident when the patient is standing or walking.66 Plantar hyperkeratosis, a condition caused by physical pressure mechanisms, is associated with the duration and severity of obesity16 and is a precursor lesion to diabetic foot in patients with diabetes mellitus.30
Skin Diseases Exacerbated by ObesityUnlike the many dermatological diseases associated with obesity in adult patients, these associations are infrequent in children, and further studies are needed to demonstrate them. The most common associations are intertrigo, psoriasis, and atopic dermatitis. Other diseases in which obesity may be an etiological or risk factor are suppurative hidradenitits,82 livedo reticularis,83 cutis verticis gyrata,84 and pilonidal cyst.85
Obesity and InfectionsInfections are the most frequent complications in obese patients. The risk factors are diverse, as mentioned above, and include impaired barrier function, humid and macerated microenvironments, as well as limitations in mobility and basic hygiene activities.
Intertrigo, the most common manifestation, presents as marked redness in the body folds accompanied by secretion and occasionally odor. The condition requires medical treatment with topical antibiotics. In some cases, low potency corticosteroids are used to reduce symptoms. Treatment also requires a high degree of cooperation from the patient, who must change hygiene routines and use a drying medication to reduce friction. A common complication of intertrigo is superinfection with yeasts, mainly Candida albicans; such infections require additional treatment with topical antifungals.
Other infections found in this setting include pachyonychia, furunculosis, and anthrax caused by staphylococci, and erythrasma caused by Corynebacterium minutisimi. Obese patients also exhibit a greater tendency to cellulitis in the extremities and vascular ulcers due to lymphedema and venous insufficiency.
Obesity and PsoriasisThe association of obesity and psoriasis was first described in 1986, when a higher prevalence of obesity was observed in a group of women with psoriasis than in a group of healthy women.86 It has now been shown that the two conditions share a common pathogenic substrate in the form of inflammation pathways and excess cytokines.87 It should be remembered that obesity is a chronic low-grade inflammatory disease. The elevation of proinflammatory cytokines—mainly tumor necrosis factor-α, IL-6, and acute phase proteins, such as C-reactive protein—could explain the association of obesity with psoriasis. Since obesity is associated with other diseases, it is therefore a component of metabolic syndrome.88
Worldwide, children with psoriasis tend to be overweight and carry the excess fat around the waistline, regardless of the severity of psoriasis.89 Early identification of this metabolic risk factor and education about modifiable factors (healthy eating, maintaining a healthy weight, and physical activity) are the cornerstone of the treatment of these patients and a key intervention in the prognosis of patients with psoriasis.90
Obesity and Atopic DermatitisThere is a strong association between obesity, atopic dermatitis, and bronchial asthma, especially when the patient is obese and under 5 years of age and the condition is prolonged.91 While the physiological and pathogenic mechanisms involved are not yet entirely clear, they may be explained in part by the presence of an abnormal inflammatory response in obese patients and the synthesis by white adipose tissue of mast cells; the increase in mast cells correlates with increased leptin levels and greater sensitization to allergens.72 The most important aspect of this association is that atopic obese patients experience more severe flares of dermatitis and require more treatment.92
Obesity and Hidradenitis SuppurativaHidradenitis suppurativa is a chronic and recurrent inflammatory skin disease. It begins at puberty and is characterized by deep and painful lesions located in the apocrine glands of hair-bearing parts of the body, especially the axillas and the inguinal and anogenital regions.93 The condition is rare, with a global prevalence of 1% and a male-to-female ratio of 1:3.3. It is now accepted that hidradenitis suppurativa is caused by a defect in the pilosebaceous unit and not in the sweat glands.94 The first event in the pathogenesis is hyperkeratosis of the infundibulum, which leads to follicular occlusion and bacterial superinfection with staphylococci, Escherichia coli, or streptococci. Tobacco consumption is an added risk factor, with a direct dose-effect relationship observed mainly in severe cases.82
ConclusionsIn summary, diabetes and obesity are 2 major systemic diseases with growing prevalence that have a marked effect on the skin. We, as dermatologists, must be aware of the cutaneous manifestations of these condition to ensure early diagnosis, prevent sequelae, and further expand our knowledge of existing and new associations in this setting.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
We would like to acknowledge the support of the Hospital de la Santa Creu i Sant Pau
Please cite this article as: Baselga Torres E, Torres-Pradilla M. Manifestaciones cutáneas en niños con diabetes mellitus y obesidad. Actas Dermosifiliogr. 2014;105:546–557.