Intense pulsed light (IPL) systems have evolved since they were introduced into medical practice 20 years ago. Pulsed light is noncoherent, noncollimated, polychromatic light energy emitted at different wavelengths that target specific chromophores. This selective targeting capability makes IPL a versatile therapy with many applications, from the treatment of pigmented or vascular lesions to hair removal and skin rejuvenation. Its large spot size ensures a high skin coverage rate. The nonablative nature of IPL makes it an increasingly attractive alternative for patients unwilling to accept the adverse effects associated with other procedures, which additionally require prolonged absence from work and social activities. In many cases, IPL is similar to laser therapy in effectiveness, and its versatility, convenience, and safety will lead to an expanded range of applications and possibilities in coming years.
Los sistemas de luz pulsada intensa han evolucionado desde su introducción en la medicina hace 20 años. La luz pulsada consiste en una energía lumínica policromática, no coherente y no colimada que abarca varias longitudes de onda pudiendo actuar sobre diferentes cromóforos. Este hecho permite una gran versatilidad, pudiendo tratar diferentes enfermedades, desde lesiones pigmentadas o vasculares hasta fotodepilación y fotorrejuvenecimiento, con una alta tasa de cobertura de la piel gracias al gran tamaño del haz. Al tratarse de sistemas no ablativos resulta una opción en auge actualmente, ya que los pacientes no están dispuestos a asumir los efectos secundarios de otros procedimientos que además requieren tiempos prolongados de ausencia de la vida laboral y social. Su eficacia es similar en muchos casos a los tratamientos con láser. El abanico de posibilidades y aplicaciones se incrementará en los próximos años gracias a esa gran versatilidad junto a la comodidad y seguridad.
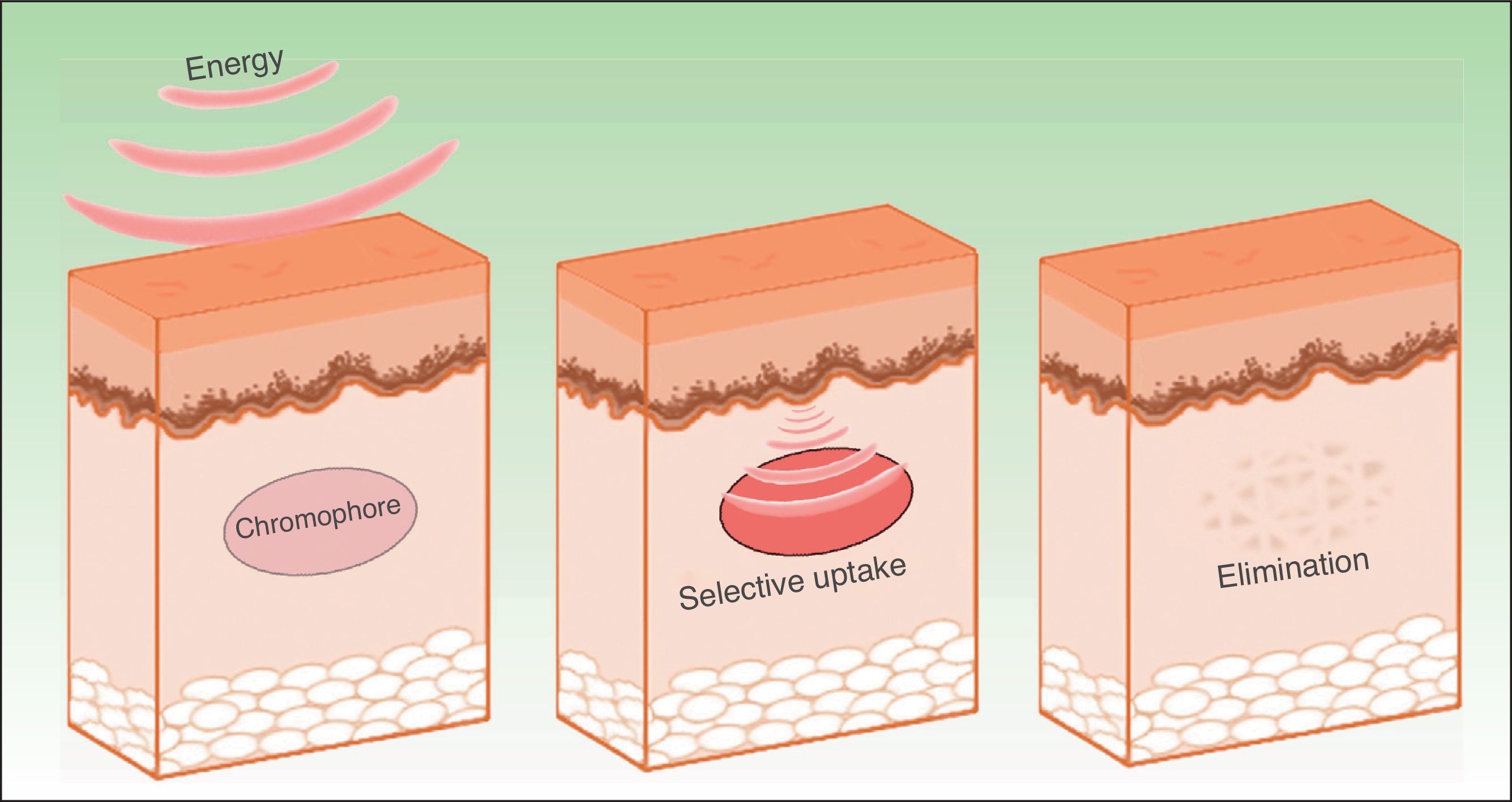
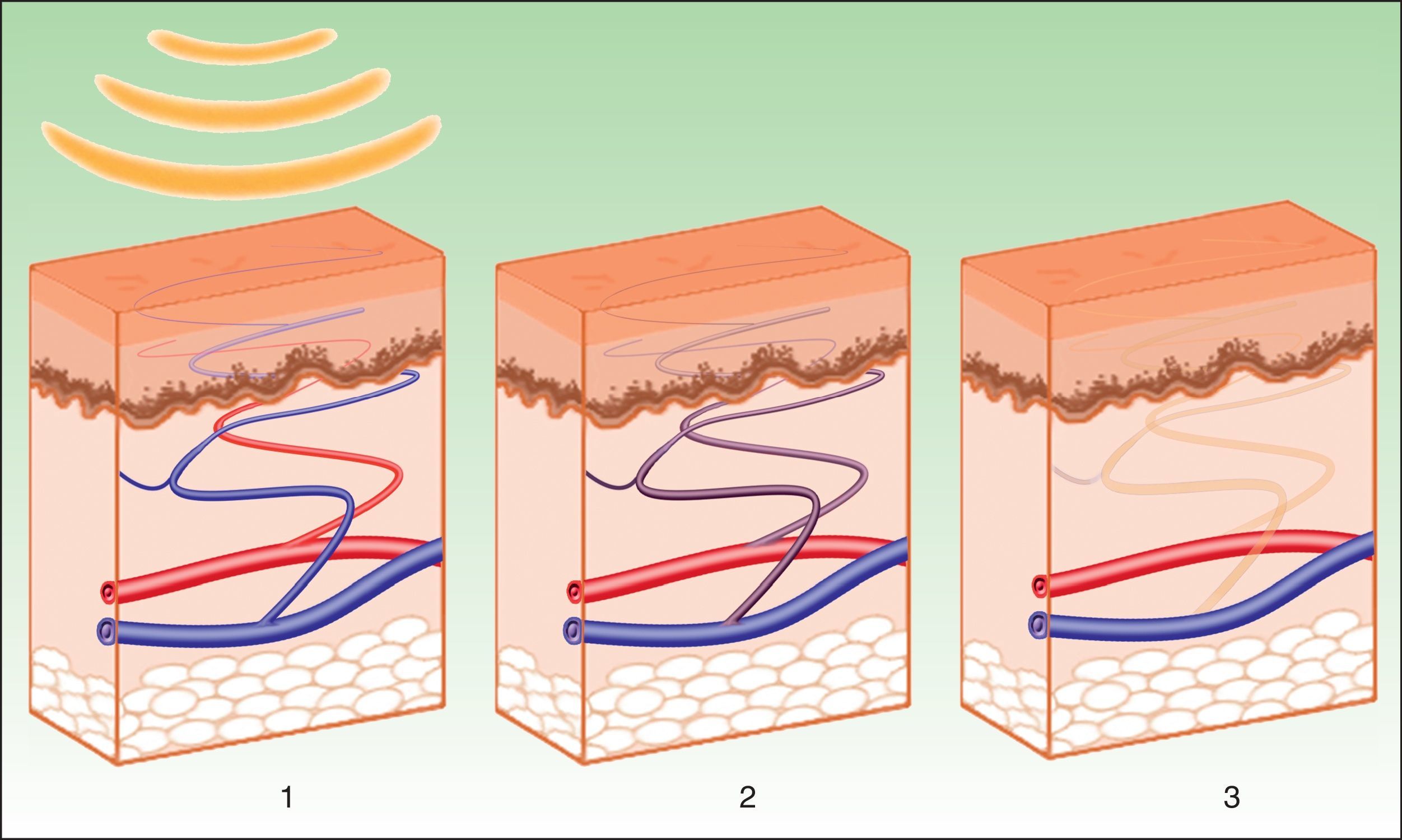
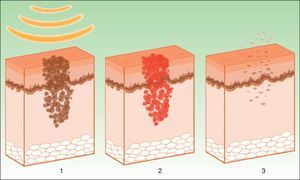
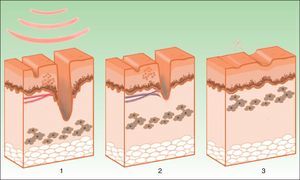
The effects produced by applying a light source to tissue can be explained by the principle of selective photothermolysis, as described by Anderson and Parrish1 in 1983. Thus, the energy supplied to a tissue has a selective action on a target molecule, denoted the chromophore, with no or minimal impact on the adjacent structures (Figs. 1 and 2). This selective action is the underlying principle by which nonablative systems such as intense pulsed light (IPL) work.
MethodologyWe undertook critical and systematic review of scientific articles published on the use of pulsed light sources in humans over the last 24 years (1990 to 2014). We searched the MEDLINE database with the Pubmed platform (United States National Library of Medicine), using the following search terms: intense pulsed light, new applications, new indications, acne, rosacea, hair removal, photodynamic therapy, and photorejuvenation. This strategy returned 627 articles. Articles were discarded if they had limited relevance, had a low impact factor, dealt with obsolete techniques, or were not primarily about the practice of dermatology or cosmetic medicine. In total, we reviewed 125 articles.
Based on the literature reviewed, we have drawn conclusions about the current indications and possible new applications.
Pulsed LightIn 1994, the Israeli engineer Shimon Ekhause managed to produce a broad-spectrum stimulated light emission, thereby creating IPL. The technique was approved by the US Food and Drug Administration for therapeutic ends in 1997. IPL systems emit noncoherent (both spatially and temporally), noncollimated polychromatic light (between 400 and 1200nm), with a pulse duration of between 2 and 200ms. These characteristics make the system polyvalent, as it can act on different chromophores with a range of therapeutic possibilities, although this versatility demands a more extensive learning process.
The light pulses generated by the majority of the current devices are produced by current arising from a high-voltage electrical discharge in a xenon gas chamber.2 The light produced is reflected towards the distal end of the device, where it it is directed onto the surface of the skin through a sapphire or quartz crystal. Cooling systems are used to protect the epidermis in contact with the crystal.
Initially, the pulses emitted were not homogenous, with interpulse and intrapulse variations in the spectral distribution caused by a variable current supplied to the xenon arc lamp. Advances in technology have managed to optimize the energy delivery pulses, making them more homogenous and giving them a square-wave form through the use of capacitors to ensure a constant current is delivered. This prevents the production of higher energy peaks that could give rise to side effects, with better outcomes and a better safety profile, without needing to resort to higher fluences. The current systems also allow each pulse to be fragmented into multiple pulses with gaps in emission between them, such that as the pulse train is emitted, the target can absorb the energy without sufficient time to release it, thereby avoiding heating and damage to neighboring structures. The first pulsed light systems worked at 0.1Hz (a pulse every 10s or more). Today, the systems work at 1-2-3Hz, with an appreciable shorting of the treatment time.
The first generation devices emitted infrared light, leading to epithelial damage and undesired effects. To make their action more selective, cut-off filters can be used to eliminate unwanted wavelengths and optimize treatment. Thus, the most recent second-generation devices can filter out the longer wavelengths corresponding to the infrared part of the spectrum, significantly reducing side effects.3
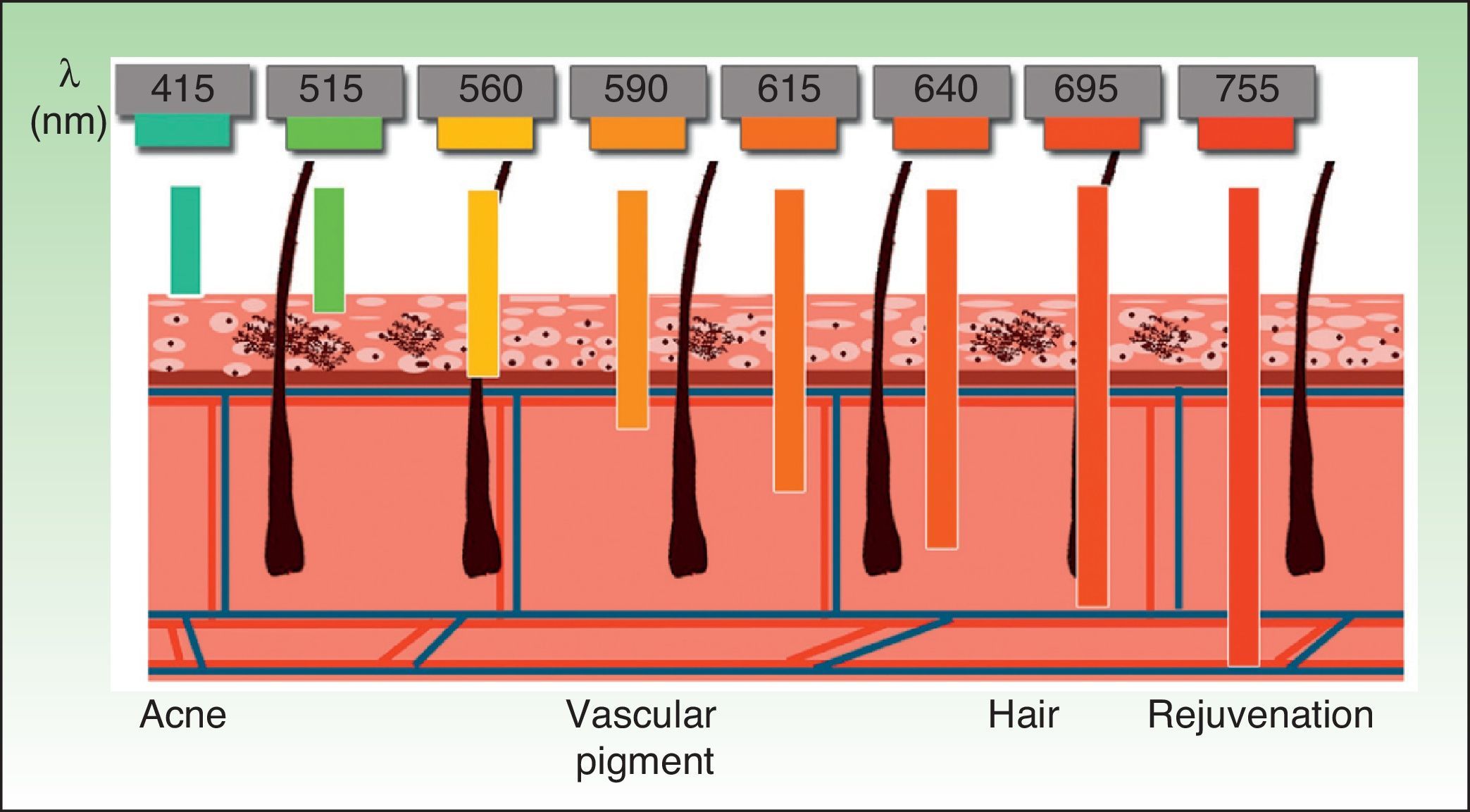
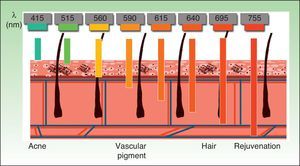
The current IPL systems offer great versatility. It is now possible to treat different conditions by targeting different chromophores with the same device, using different parameters (wavelength, pulse duration, number of pulses, interval between pulses) (Fig. 3).4,5
Another important advantage of the IPL system is its relatively large spot size, which can increase the speed of treatment given that large areas can be treated quickly with fewer pulses. However, the hand pieces are larger and have a flat surface, hindering treatment of irregular surfaces (Table 1).
Advantages and Drawbacks of Intense Pulsed Light.
| Advantages | Drawbacks |
|---|---|
| Highly versatile | Variation in spectrum and fluence emitted |
| Lower price (except for top-of-the-range systems) | Weight of handpiece |
| Large spot size | Problems with focusing |
| High degree of skin coverage | Gel application device and cooling system (cooling sprays, contact cooling, or cold air) |
| Robust technology | Direct contact with the skin |
These devices were first developed to treat benign vascular lesions. The first article on their use in dermatology dates from 1997, when Raulin et al.6 used them successfully to treat 14 patients with telangiectasias of the face or legs or with poikiloderma of Civatte. Shortly afterwards, the same authors published 2 cases of permanent hair removal and subsequently have conducted several more standardized studies that have demonstrated the efficacy and safety of the technique.7–9 Since then, the systems have proliferated in a range of conditions, thanks to their low cost and versatility.
ILP systems mainly target hemoglobin and melanin, with greatest efficacy in the treatment of color rather than texture, according to certain authors.10,11 However, despite discussion over whether they can remodel collagen, they have also proved effective in skin rejuvenation.
Selection of patients and pre- and post-treatment care are similar to when laser systems are used. It is essential to recognize adverse effects and know how these should be managed. Initially, a large number of side effects were reported with limited efficacy (probably due to inappropriate calibration).12 However, the current systems are safer, more potent, and more reliable.13
An almost constant side effect is the sensation of pain during treatment, although this is generally not a serious problem. Cooling (during and/or after treatment) or the use of topical anesthetic can provide relief in most cases. Common side effects, which may last a few days, are edema and erythema. Blister and scab formation may arise after treatment with high fluences. In these cases, patients should avoid scratching the skin given the risk of infection and scarring. The effects that can be most permanent or irreversible are changes in pigmentation (hypopigmentation or hyperpigmentation) and hypertrophic or keloid scarring.11
Tanned patients and those with a high phototype, as well as those who cannot or do not want to avoid exposure to sunlight after treatment should usually be excluded from treatment given the high risk of hyperpigmentation.14
An increasing number of studies have examined IPL systems alone or in combination with other types of treatment.15 In the following sections, we shall discuss the current indications and the new applications, and compare them with other therapies in cases where comparative studies have been conducted.
Current IndicationsBenign Pigmented LesionsQ-switched laser systems are the treatment of choice for benign pigmented lesions, although many studies indicate that IPL systems are just as effective if not more so.16–18
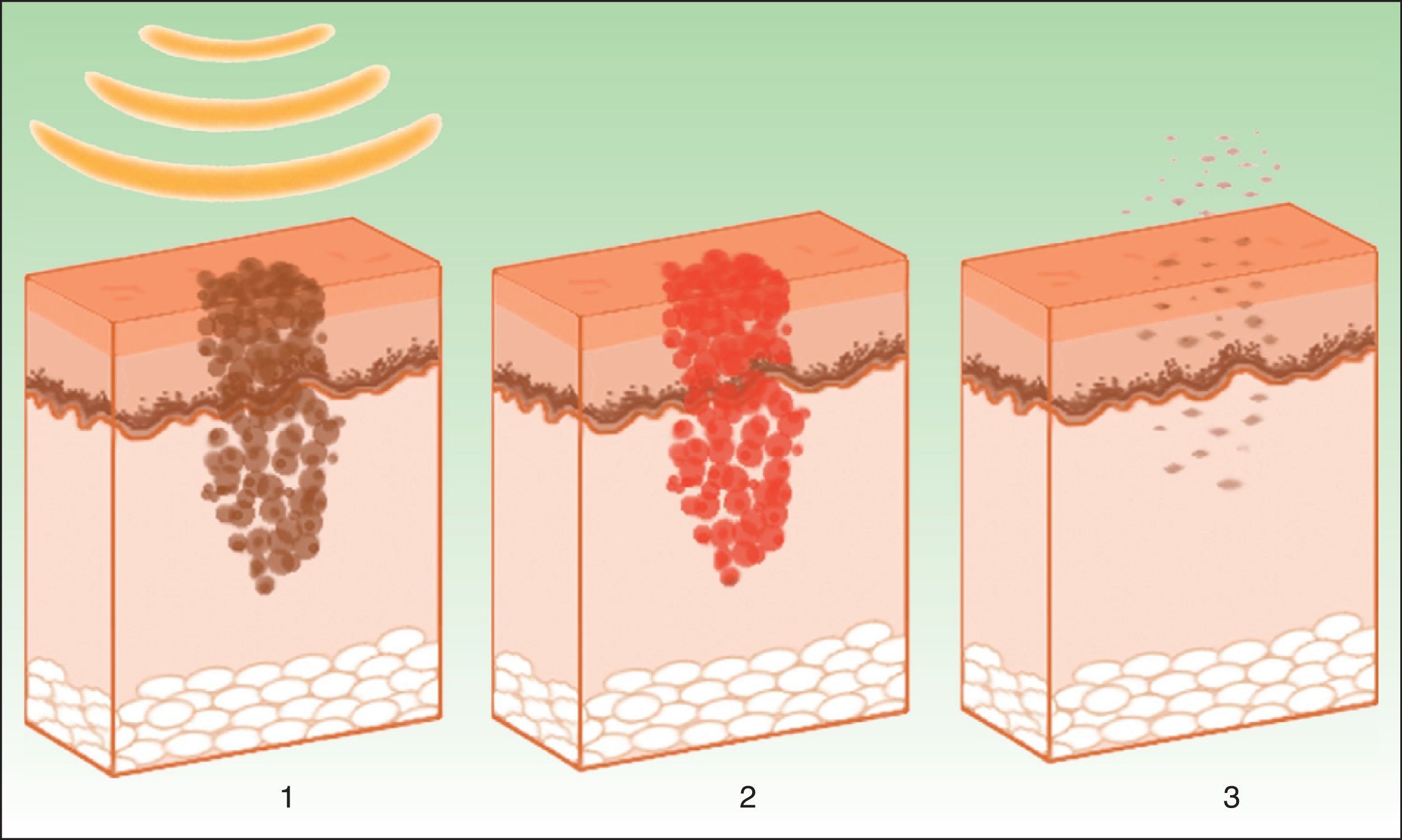
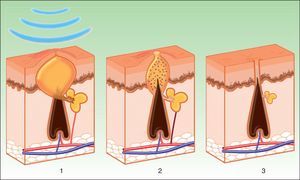
The target is melanosome, whose chromophore is melanin. This absorbs the appropriate wavelength, transforming the light into heat energy, which induces epidermolysis down to the basement membrane and rapid keratinocyte differentiation. These processes are accompanied by transfer of melanosomes towards the upper layers, where they are eliminated along with necrotic keratinocytes (Fig. 4).19
Mechanism of action of intense pulsed light in pigmented lesions.19 Melanosomes absorb the light (1), which is converted to heat (2), whereupon epidermolysis occurs and the lesion is eliminated (3).
Selective thermal damage is observed in the range of 351 to 1064nm.20 In the case of IPL systems, the wavelengths used range from 515 to 755nm. Cut-off filters can be used to select short wavelengths, which act on the epidermis and are safe for superficial lesions. Alternatively, long wavelengths can be selected. These penetrate further and are more effective with deep lesions.
In 2001, Moreno Arias and Ferrando21 published a study in which 20 patients with melanocytic lesions were treated with IPL. They concluded that greater efficacy (76%-100%) was attained with superficial lesions (ephelides, epidermal melasma, café au lait spots) compared with efficacy of less than 25% for deep lesions (Becker nevus, epidermal nevus, mixed melasma).
Several studies have investigated the response of melasma to IPL. Although these lesions are refractory to several therapies, many authors suggest that IPL systems are a useful and effective tool, particularly in epidermal type lesions.16–18,22–26
Other lesions that have been successfully treated, though with few publications to support their use, are Riehl melanosis,27 Mongolian spots,28 solar lentigines and ephelides,29–32 lentiginosis associated with syndromes (LEOPARD, Peutz-Jeghers),33,34 drug-induced hyperpigmentation,35 and nevus spilus.36
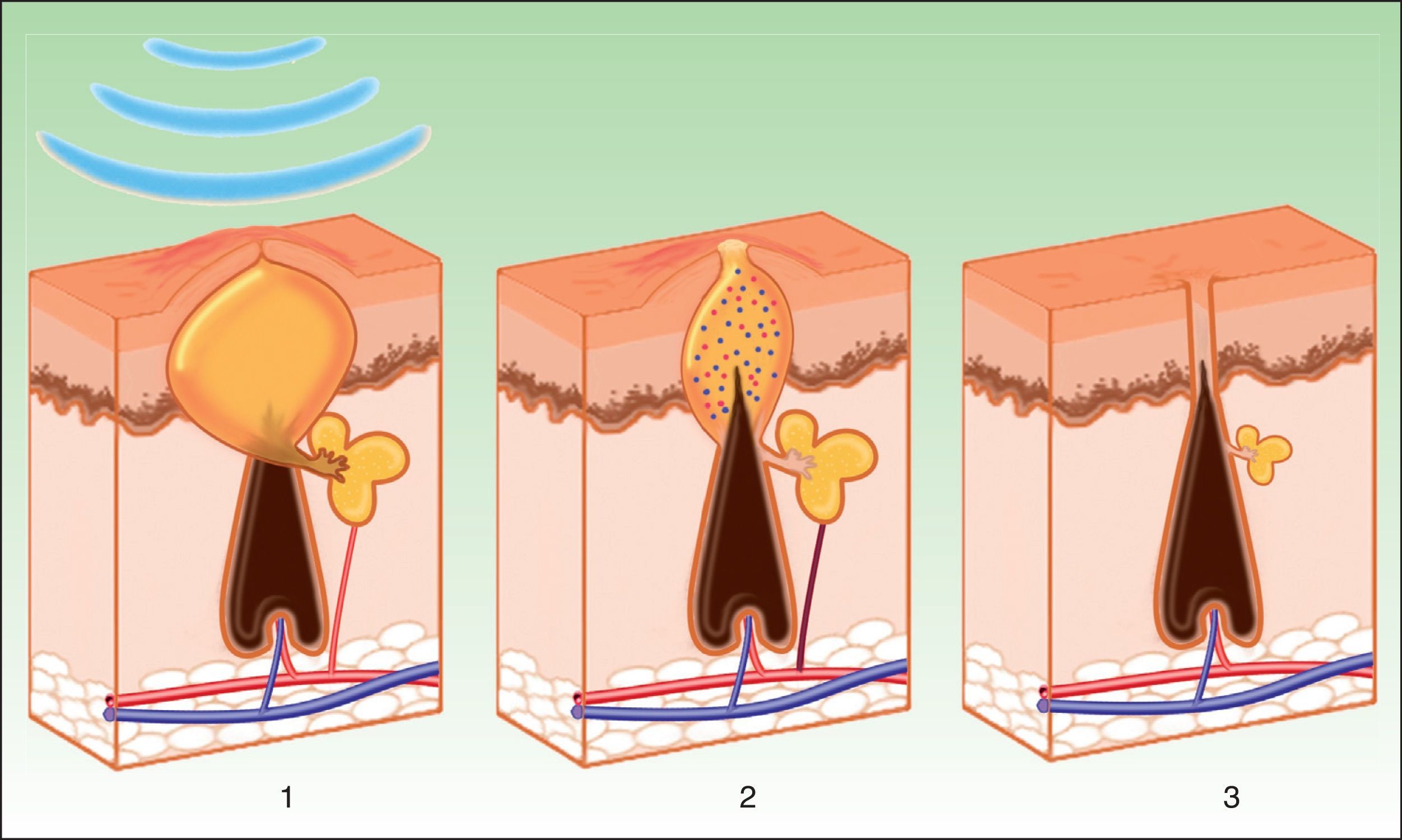
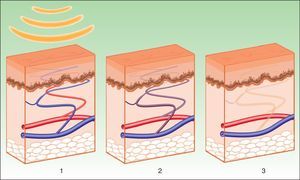
Vascular LesionsPulsed dye laser (PDL) is considered the gold standard for the treatment of vascular lesions. However, this technique is limited by the need to achieve immediate purpura that lasts 10 to 14 days. IPL systems are an alternative given the absence of purpura, reducing the down time.3 Instead of inducing purpura by destruction of erythrocytes and bursting of the vascular wall, the aim of IPL is to reach a sufficiently high temperature to cause coagulation in the vessel, with the resulting destruction and fibrosis (Fig. 5).
Several studies have reported equivalent efficacy and safety for IPL in comparison with lasers (PDL, Nd:YAG, alexandrite, KTP).37–40 McGill et al.38 conducted a comparative study in 18 patients with capillary malformations refractory to PDL, and concluded that alexandrite laser was the most effective, followed by IPL.
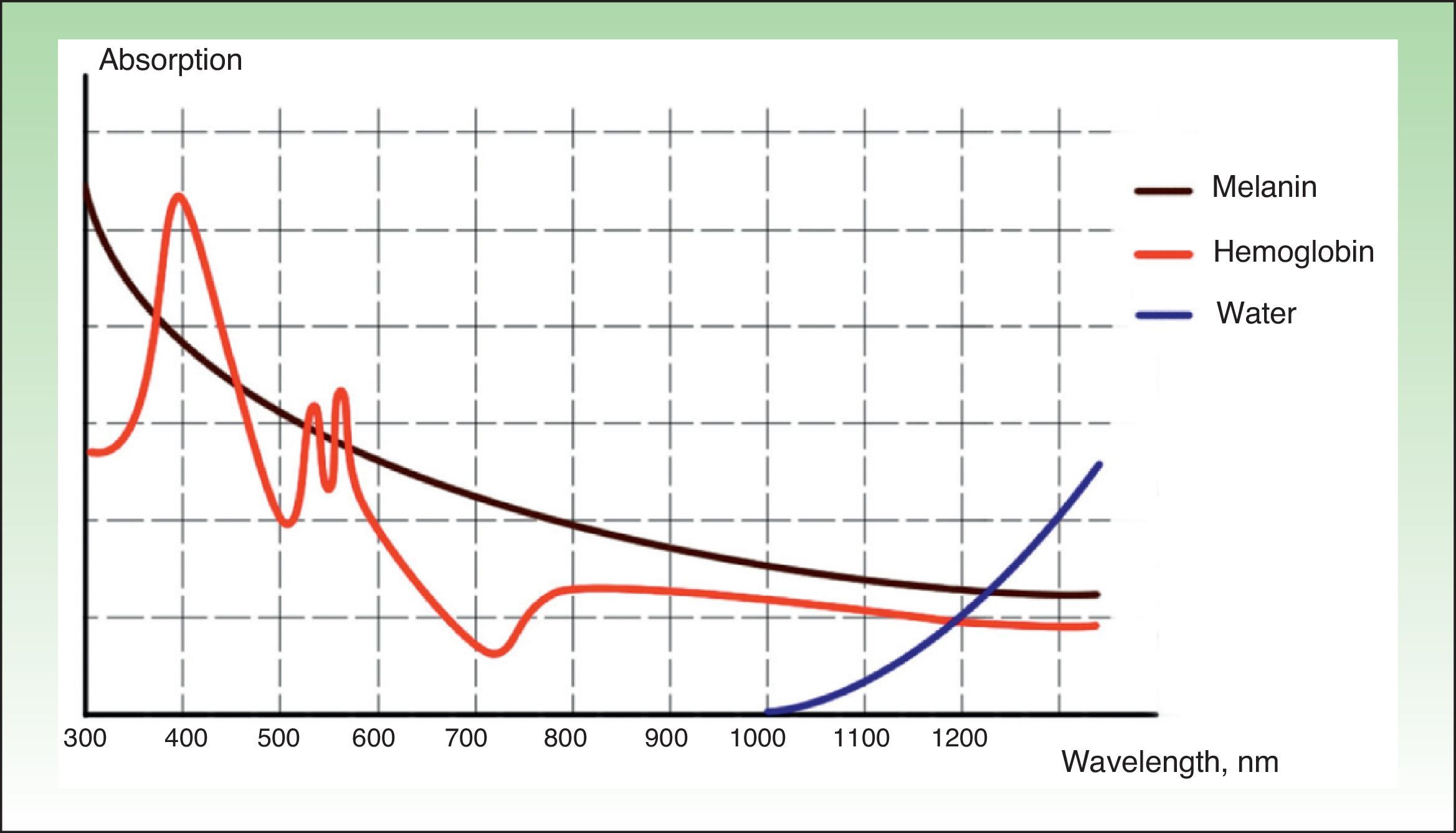
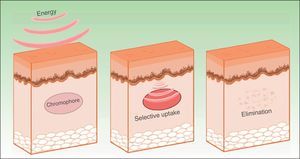
IPL acts on 3 target chromophores: oxyhemoglobin (predominant in lesions of red appearance), deoxyhemoglobin (predominant in blue lesions), and metahemoglobin, with peaks of absorption at 418, 542, and 577nm, respectively. Although the peak of maximum absorption for oxyhemoglobin is at 418nm, less penetration is achieved and, in addition, there is strong competition from melanin, whereas at 577nm, although absorption is less, the degree of penetration is greater, thereby reducing absorption by melanin and avoiding side effects, such as hypopigmentation.41 For this reason, current devices use longer wavelengths (515 to 600nm) to ensure deeper penetration while still being absorbed by oxyhemoglobin.
Successful treatment depends on the type and size of the vessels. Fodor et al.39 conducted a comparative study of IPL and Nd:YAG laser, and obtained better results with IPL for more superficial and smaller lesions.
In view of the above, the main indications for IPL are telangiectasias and spider angiomas, erythrosis, and erythematotelangiectatic rosacea.6,40,42–45 A study conducted by Murray et al.44 in 2012 showed an improvement in systemic sclerosis-related telangiectasias; however, these improvements were not sustained during follow-up, suggesting that other treatments should be added.
IPL can also be useful in infants with superficial hemangiomas or rapidly growing lesions >1cm,46 in capillary malformations (particularly port-wine stains),38,40,41,47–52 and poikiloderma of Civatte.43,53–56
RosaceaIPL has been shown to be an effective treatment for telangiectasias and, to a lesser extent, background erythema and papular lesions in patients with erythematotelangiectatic rosacea.43,57–59 A study by Lane et al.60 also suggests that IPL systems can be a useful additional tool for the treatment of granulomatous rosacea as the reduction in the vascular component enables a decrease in dermal inflammation.
In 2009, in a study of 29 patients, Neuhaus et al.61 found that IPL is at least as effective and safe as PDL in reducing signs and symptoms of rosacea. Papageorgiou et al.62 demonstrated an efficacy of 55% in reducing telangiectasias, sustained during 6 months of follow-up, and Schroeter et al.63 confirmed these positive findings over extensive follow-up with a mean duration of 51.6 months. Despite these promising findings, long-term efficacy studies are still required.64
Acne VulgarisSeveral articles have been published on the use of IPL in the treatment of mild-moderate inflammatory acne, although in most of these IPL was not used as monotherapy but rather in combination with another therapy or as a light source for photodynamic therapy (PDT).65–68 IPL is considered a noninvasive and rapid technique that avoids prolonged use of drugs, bacterial resistance, and associated adverse effects, although its efficacy as monotherapy remains a controversial topic.67,68
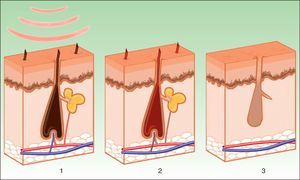
Three mechanisms of action appear to be acting during treatment with IPL (Fig. 6)69:
- -
Photodynamic effect. Propionibacterium acnes produces porphyrins (especially protoporphyrin ix). These substances act as chromophores with absorption peaks at 415 to 655nm. These wavelengths are absorbed by porphyrins, releasing free radicals with bactericidal effects. In addition, antiinflammatory cytokines, such as growth transforming factor beta (TGF-β), are stimulated.70,71
- -
Treatment induces selective photodermolysis of the blood vessels that feed the sebaceous glands (with oxyhemoglobin as the chromophore), leading to decreased gland size and reduced rate of sebum excretion.
- -
A third mechanism requires an exogenous photosensitizing agent that is topically applied. This is the basis of PDT.
Mechanism of action of intense pulsed light in the treatment of acne. Porphyrins produced by Propionibacterium acnes act as chromophores (1), and release bactericidal free radicals and stimulate antiinflammatory cytokine release (TGF- β) along with photothermolysis of the vessels that supply the sebaceous glands (2). Inflammation is eliminated and the glands are reduced (3).
Given that IPL can cover the absorption peaks of both bacterial porphyrins and hemoglobin, it could be a useful tool in the treatment of acne. The heterogeneous results from recent studies highlight that IPL is a long way from being a standard treatment, although it could be considered an alternative in patients in whom drugs are contraindicated or when other limitations apply.
Another important point to bear in mind is that the reduction of P acnes and therefore the improvement in acne is short-lasting and is only maintained by repeated sessions administered over a long time.70 This is because the response is based on an indirect, local, and superficial antiinflammatory-antibacterial effect, with no action on the systemic immunological component. As a result, the response is expected to be partial and transient, unlike in indications in which the target chromophore is the underlying cause of the process and in which destruction of the chromophore achieves a lasting result. In this respect, Choi et al.71 concluded that both PDL and IPL were effective, but with IPL the response was slightly less effective and shorter lived.
Given the lack of data and the variability in the studies conducted, a recommendation for use of IPL as monotherapy in the treatment of acne is currently not supported by the evidence.
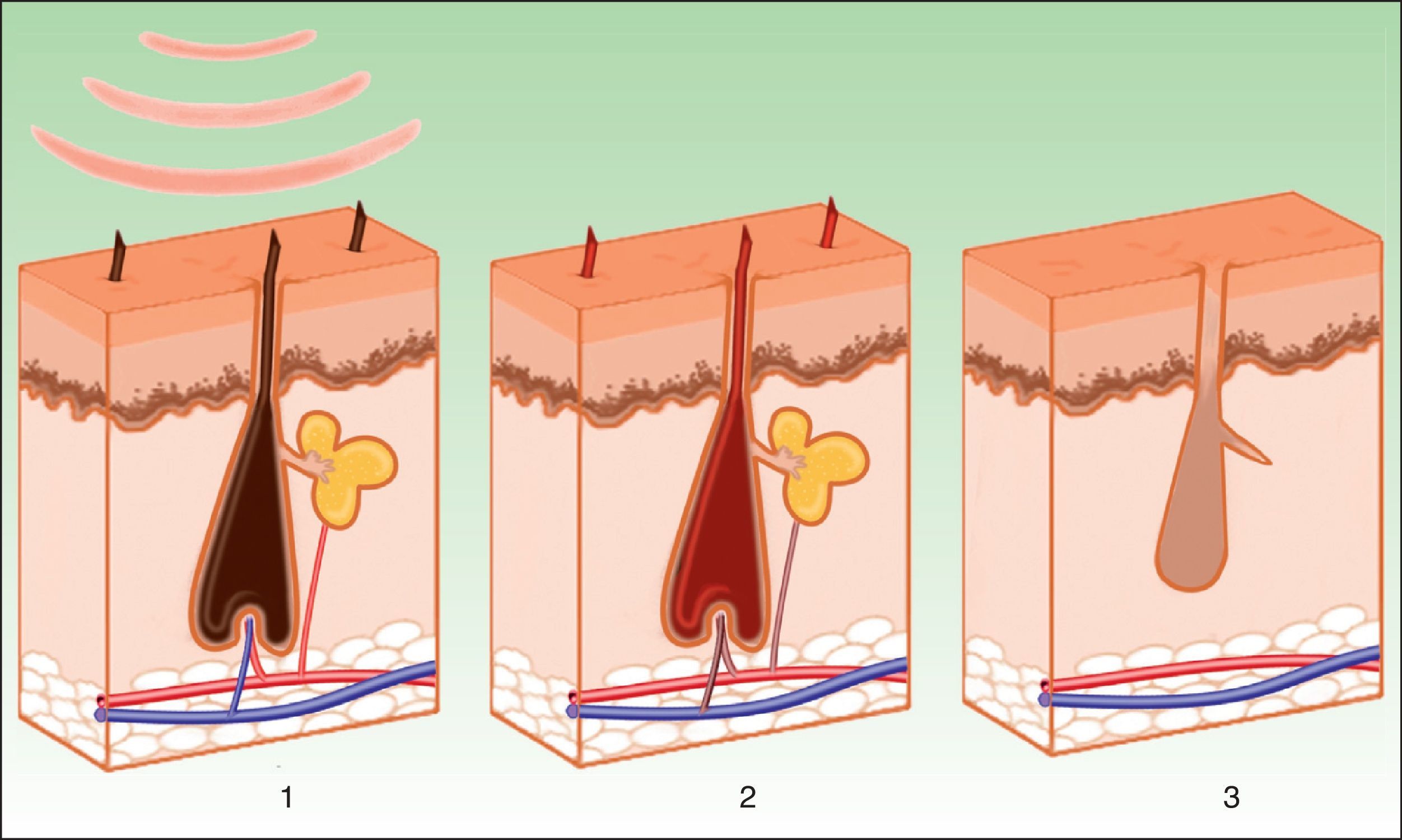
PhotoepilationIn the indication of photoepilation, melanin acts as the chromophore. It is important to selectively target the deposits in the hair shafts, leaving the epidermal melanin intact. The biggest melanin absorption peak is at short wavelengths, thereby hindering penetration towards the follicle. Longer wavelengths (590-900nm) are therefore preferred as these can act more selectively on the dense melanin deposits in hair follicles without damaging epidermal melanin. Once the melanin absorbs the light energy, the transformation into heat energy leads to necrosis of the hair bulb (Fig. 7). A hair follicle in anagen phase is more sensitive, as the shaft contains more melanin. In addition to melanin in the hair shaft, melanin in the pluripotent stem cells in the matrix of the bulb and the melanocytic stem cells in the bulge region near to the site of insertion of the arrector pili muscle and the outer root sheaf can also be targeted. The aim is to damage the follicle by destroying these cells, thereby leading to permanent hair removal. The importance of the papillary microvasculature as a target for treatment remains to be elucidated.8
Most of the side effects are minimal and transient and are usually observed when excessive energy or an inappropriate technique is applied. Such effects include erythema, burns, hyperpigmentation or hypopigmentation, leukotrichia, folliculitis, paradoxical hypertrichosis, and to a lesser extent, scarring.72–74 Paradoxical hypertrichosis could be due to activation of inactive follicles in areas close to the treated areas due to subtherapeutic doses of energy that stimulate anabolic metabolism of the follicles.72 Moreno-Arias et al.72 conducted a study in a series of 49 patients with facial hirsutism and observed this effect in 10% of cases (all associated with polycystic ovary syndrome).
Several studies have shown the efficacy and safety of these systems in photoepilation, although only a few of these were controlled, comparative studies; the most frequently used comparators were alexandrite laser, diode laser, and Nd:YAG systems.7–9,75–79 In all cases, the efficacy and safety of the different types of laser treatment are similar to IPL systems. Although the best and longest-lasting results were obtained with laser systems, significant differences were not reported and IPL caused less pain and fewer side effects in most studies.
In the case of high phototypes, energy absorption by the follicles can be compromised by the higher concentration of epidermal melanin. In addition, the risk of pigmentation disorders is greater in such patients. In these cases, it is recommended to use longer wavelengths (more than 755nm), longer pulse durations with interpulse periods of 50-100ms, and cooling systems to minimize thermal damage.3,79 Ismael79 conducted a study in 2012 with 50 women of phototypes iv-vi, and assessed the efficacy and safety of Nd:YAG laser compared to IPL in axillar hair removal, with the best results obtained for the laser system, although this treatment was more painful and led to greater inflammation.
Another important point to take into consideration is that laser systems are more effective at eliminating dark and deep hair, whereas the versatility of IPL enables it to treat fair or brown hair and fine hairs more effectively.
According to randomized, controlled, clinical trials, the evidence is strongest for alexandrite laser, diode laser, and Nd:YAG laser, whereas the evidence for IPL is limited.77
Recently, the FDA has approved the IPL systems at low fluence for home use for hair removal. Although this technology appears to be safer and effective, results from clinical trials suggest that home systems are less effective than the systems used in the clinic.80–82
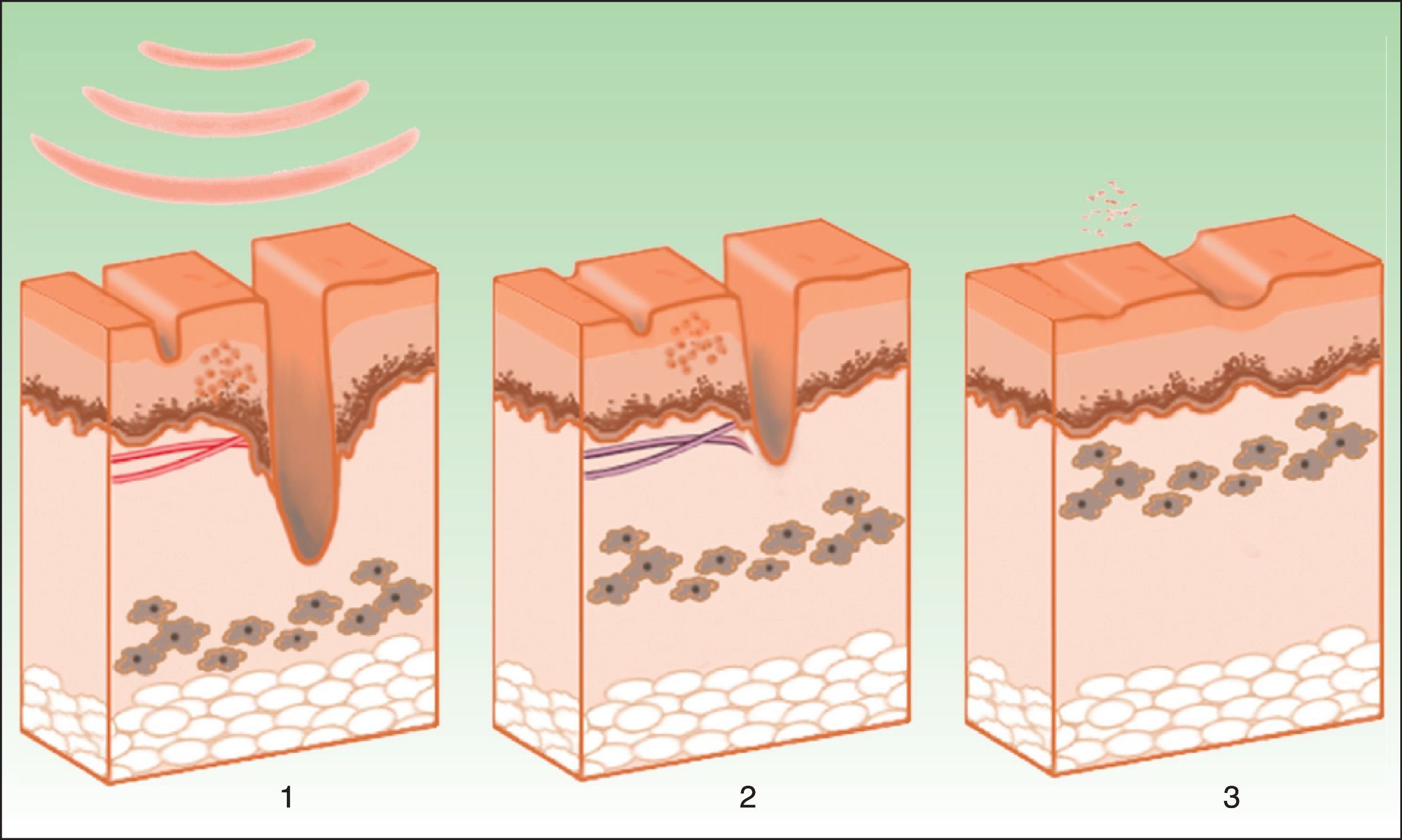
Nonablative Skin PhotorejuvenationThermal damage to the dermis induces fibroblast activation, with the resulting formation of neocollagen (Fig. 8). The synthesis and reorganization of type 1 and 3 collagen fibers are increased while elastic fibers, although present in lower quantity, are more neatly arranged.10 The wavelengths that best achieve these effects lie in the range of 515 to 1200nm The longer wavelengths are absorbed by water in the dermis, triggering a cytokine-mediated reaction that stimulates neocollagen synthesis. The shorter wavelengths are absorbed by melanin and oxyhemoglobin present in pigmentation disorders and telangiectasias associated with the ageing process. Therefore, all the visible elements of ageing (fine wrinkles, laxity, telangiectasias, irregular pigmentation) are improved with a lower rate of adverse effects and a faster recovery.
Despite the uneven results in different studies, IPL is presented as a safe and effective alternative to traditional rejuvenation techniques with ablative lasers (CO2, erbium) while efficacy and safety results are similar to those obtained with nonablative lasers (PDL, infrared lasers, diode lasers, YAG lasers, etc), although comparative studies are lacking.84,85 There is no degree of recommendation with sufficient level of evidence; however, texture is improved and the tone more even. The technique acts primarily on vascular disorders and irregular dyschromia, while the results in terms of wrinkle reduction are more varied.10,11,83–91
Photodynamic TherapyPDT consists of topical administration of a photosensitizing agent to the target area (5-aminolevulinic acid [5-ALA] acid or its methyl ester) and subsequent stimulation using a light source. After selective uptake by the target cells (tumor cells, abnormal endothelial cells, bacteria, etc) and subsequent exposure to light, these prodrugs are transformed into their active form, protoporphyrin ix, generating cytotoxic free radicals that destroy the cells. Moreover, thanks to the use of this photosensitizing agent, which is widely distributed throughout the cell membranes, the light penetrates to deep levels where it exercises its effects. The incubation time for the photosensitizer is usually between 30minutes and 1 hour. Several studies support the use of short applications to minimize adverse effects.92–95 Prolonged studies with durations of up to 3hours have been conducted to determine whether this might improve outcomes,93 but no statistically significant differences were observed.
The traditional and approved indications are precancer and nonmelanoma skin cancer (with level A recommendation and level of evidence 1 for actinic keratosis, superficial basal cell carcinoma, and Bowen disease with an incubation time of 3h for the photosensitizer methyl aminolevulinate); the evidence is weaker and more robust studies are required in the case of acne, rosacea, and skin rejuvenation (where a large range of incubation times are also used).92–107 PDT is not currently used for photoepilation, although it was tried with greying hair, with limited effects.
The range of indications is increasing given the advantages that the technique offers. For example, it is noninvasive and the effects are specific to the target tissue. PDT is also well tolerated and can be used to treat multiple lesions in the same session while cumulative toxicity is not observed and the cosmetic outcome is good. Probably, PDT with IPL is the therapeutic indication that will undergo greatest development in the future. However, the lack of scientific evidence, along with the availability of other more appropriate therapies for other potential conditions, suggests that the list of approved indications is not going to be extended in the near future.
The efficacy of PDT has been demonstrated in several controlled studies, but given the immediate side effects, the technique is reserved for selected situations.67 The main side effects are phototoxic reactions (erythema, edema, hyperpigmentation) and pain, occasionally intolerable, during treatment. The photosensitizer used, the part of the body treated, and the type of lesion will all have a bearing whether side effects occur. Nevertheless, Babilas et al.108 reported less pain in the treatment of actinic keratosis with IPL compared to a diode light source.
Several articles have proposed PDT as an effective therapy for inflammatory acne (level B recommendation, level of evidence 2a), and it is more effective than IPL in monotherapy, with limited side effects and good tolerability, while the outcome is more long-lasting and the relapse rates are lower.92–95,99–102 Free radicals released after photoactivation of protoporphyrin ix not only destroy P acnes but also the pilosebaceous units, reducing the size of the sebaceous glands and sebum production.68,93–95 The results are not instantaneous; rather, they become manifest after 3 sessions or 4 weeks of treatment, and there may even be an initial self-limiting acneiform reaction.95,101
Taub et al.99 compared the response of acne to IPL, a combination of IPL and radiofrequency, and blue light. The best, longer-lasting, and most consistent outcomes were obtained with IPL. Hong et al.94,100 conducted a comparative study of IPL and red light after application of 5-ALA; in both cases, good outcomes were obtained, although the response was faster in the case of red light.
PDT with IPL has also been proposed as a therapeutic option with promising results and minimal side effects in photorejuvenation. Outcomes were better than with IPL alone. According to most studies, the biggest improvements are seen in overall photorejuvenation, mottled pigmentation, and, to a lesser extent, fine expression lines (with variable results in terms of roughness and paleness).103–107 Gold et al.107 compared the efficacy of PDT and IPL with IPL alone in 20 patients. They obtained an 80% improvement in the overall assessment with PDT (compared with 45% with IPL alone) and a 95% improvement in pigmentation (compared with 60% with IPL alone).
New ApplicationsHypertrophic/Atrophic ScarringFew studies have been published on the use of IPL for hypertrophic/atrophic scarring, but the encouraging results point to an effective response. Bellew et al.109 compared the use of long PDL pulses with IPL in hypertrophic surgical scars, and concluded that both treatments had a similar efficacy, although the risk of purpura was eliminated in the case of IPL. Erol et al.110 assessed the efficacy and safety of IPL in hypertrophic or keloid scars in 109 patients. Overall clinical appearance was improved in 92.5%, suggesting that this technique is effective not only for improving the appearance of scars but also for reducing height, erythema, and hardness. Color bleaching is achieved thanks to sclerosis of neovessels and reduction of interstitial edema, whereas flattening arises through breakage and remodeling of collagen, which induces fibroblast activation.
This activation, with the subsequent neocollagen synthesis and extracellular matrix, also improves atrophic acne scars, as reported by Wang et al.66 in a study of 37 patients in which IPL and fractionated CO2 treatment were combined, to increase the success rate.
Seborrheic KeratosisIPL is also effective in the treatment of seborrheic keratoses, as these lesions are superficial and slightly pigmented.16 With such lesions, filters should be used to select short wavelengths (530nm), which act on the epidermis and are safe for the treatment of superficial lesions. The mechanism of action is probably related to absorption of radiation by epidermal melanin and subsequent thermal diffusion to the surrounding cells. This results in epidermolysis that increases the rate of keratinocyte regeneration with elimination of necrotic keratinocytes through microcrusts, thereby eliminating the keratinocytic lesions.19 Although studies have not been published, the British Skin Laser Study Group presented a case series of seborrheic keratoses treated with IPL at the British Medical Laser Association Meeting in 2011. The technique was successful and the cosmetic outcomes were good.
SarcoidosisThere are no controlled studies for the treatment of cutaneous sarcoidosis with IPL, although the articles published propose IPL and laser treatment (Nd:YAG, KTP, PDL, CO2 lasers) as an alternative, with good response and tolerability in cases refractory to conventional therapy. Rosende et al.111 successfully treated a patient with lupus pernio who had shown limited response to pharmacological treatment, and Hasegawa et al.112 also successfully used PDT with IPL in a patient with facial sarcoidosis.
The mechanism of action could be related to the destruction of the blood vessels that deliver proinflammatory cytokines to the skin (as in other inflammatory dermatoses, such as lupus erythematosus, atopic dermatitis, hidradenitis suppurativa, and acne). The antiinflammatory cytokine, TGF-β, could play a key role in the resolution of inflammatory lesions (increased levels have been observed after treatment).69 This antiproliferative and antiinflammatory action, both of IPL alone and in combination with PDT, disrupts granuloma formation in sarcoidosis, but as this is a local effect, the response would be expected to be transient.111,112
Cutaneous Lupus ErythematosusIn view of the effect on vascular lesions, in 2000, Levy113 published the case of a patient with lupus erythematosus who presented with chronic facial erythema despite prior therapy. Clearance of 75% was attained. In the case of chronic diseases with erythema such as lupus, IPL and laser treatments have the advantage of being well tolerated and they avoid prolonged use of drugs. They can achieve sustained outcomes, provided regular sessions are scheduled. As commented earlier, IPL has a transient antiinflammatory effect.113 In 2013, Troilus et al.114 performed a retrospective study of cases of treatment-refractory chronic discoid lupus erythematosus. The patients were treated with IPL or PDL. Fourteen of the 16 patients experienced an improvement in pruritus, erythema, scaling, scar formation, and pain. Although more studies are necessary, the authors concluded that both IPL and PDL could be an early and safe adjuvant therapy for preventing disfiguration in discoid lupus.
Hidradenitis SuppurativaPDT with IPL has been successfully used in the treatment of acne and could be an alternative therapy or adjuvant therapy for hidradenitis suppurativa, as the 2 diseases have a similar etiopathogenesis. In 2011, Schweiger et al.115 investigated the efficacy and safety of PDT using blue light or IPL in 14 patients. They observed a decrease in the number of lesions and improvement in quality of life, with improved tolerability in the case of blue light. In the same year, Highton et al.116 used IPL alone in 18 patients, with significant improvement and a high degree of satisfaction among the patients. The authors suggested that IPL could be an alternative to surgery. However, more studies are required to support the efficacy of the technique and clarify the mechanism of action (possibly related to the antiinflammatory-antibacterial effect and selective vascular photothermolysis).
Atopic DermatitisCharacteristic eczematous lesions are related to endothelial activation and release of proinflammatory mediators. IPL has been used successfully in rosacea, where it acts through ablation of abnormal vascularization, leading to a reduction in the dermal inflammatory component.62 Telangiectasias induced by overuse of topical corticosteroids are similar to rosacea lesions, thereby supporting the hypothesis that IPL could be a promising option for the treatment of facial reddening in atopic dermatitis. In 2010, Oh et al.117 used this technique to treat 11 patients with chronic facial atopic dermatitis refractory to treatment. Good results were obtained both in terms of clinical improvement and quality-of-life indices. Furthermore, IPL has been used to good effect for cutaneous rejuvenation and pigmented lesions; therefore the authors also postulated that, through collagen remodeling, this technique improves lichenification and postinflammatory hyperpigmentation caused by chronic scratching. Severe adverse effects were not observed although one of the causes of atopic dermatitis is disruption of the cutaneous barrier and IPL could in principle worsen this process through epidermolysis.
Recalcitrant Viral WartsIPL and lasers (especially PDL) are being used as a therapeutic option; however, there is no evidence of their effectiveness and clinical trials to assess these techniques are lacking. The mechanism of action appears to be selective destruction of the superficial capillaries of the viral papillomas, as well as thermal damage to the human papillary virus. A randomized clinical trial conducted by Togsverd-Bo et al.118 did not find significant differences in the treatment of recalcitrant warts by scraping alone or scraping with IPL. In addition, patients treated with IPL experienced more pain. However, Kalil et al.119 published a case of a recalcitrant hand warts successfully treated with a combination of PDT and IPL (the mechanism of action may involve the selective accumulation of protoporphyrin ix in the keratinocytes of virus-infected skin).
Keratosis Pilaris Atrophicans FacieiKeratosis pilaris atrophicans faciei is a variant of keratosis pilaris characterized by erythema and follicular hyperkeratosis that can progress to atrophy. It usually has a large psychological impact and treatment is unsatisfactory. Rodríguez-Lojo et al.120 treated 4 patients, all of whom achieved an improvement in both erythema and roughness, without any side effects. The etiopathogenesis of the condition is thought to involve keratinocyte abnormalities caused by proinflammatory cytokines that lead to perifollicular inflammation followed by fibrosis and atrophy. Therefore, the antiinflammatory effect of IPL could be beneficial.
This condition has been successfully treated with PDL; however, IPL does offer some advantages in that purpura does not occur. Therefore, IPL can be considered a safe and well-tolerated therapeutic option for the treatment of keratosis pilaris atrophicans.120
Pigmented Actinic Lichen PlanusPigmented actinic lichen planus appears to correspond to the final phase of actinic lichen planus. Histology reveals abundant melanophages and incontinentia pigmenti of the papillary dermis (similar to postinflammatory hyperpigmentation). IPL has been used successfully in other hyperpigmentation disorders, such as poikiloderma of Civatte; therefore, it can also be considered a therapeutic option for this condition. Santos-Juanes et al.121 used IPL with good outcomes and without side effects in a 55-year-old woman diagnosed with pigmented actinic lichen planus refractory to conventional treatments (acitretin, topical corticosteroids, hydroxychloroquine, ciclosporin).
Striae DistensaeThanks to the phenomenon of neocollagenesis after fibroblast activation, IPL was successfully used in the treatment of striae distensae. In a study conducted by Hernandez-Pérez et al., 122 the abdominal striae distensae of 15 patients showed a statistically significant improvement, both clinically and histologically, on using IPL. The authors suggested that IPL could be a promising treatment for this common problem, with minimal side effects and a wide safety margin. In a more recent study, Basile et al.123 reported the incidence of striae distensae after breast augmentation to be 5%. Treatment was initially with a combination of IPL or erbium laser along with mesotherapy.
Plaque and Nail PsoriasisPDT has been used in inflammatory dermatoses such as acne vulgaris, psoriasis granuloma annulare, localized scleroderma, and lichen sclerosus. With the technique, proinflammatory cytokines have decreased while antiinflammatory cytokines (TGF-β, IL-10) have increased. Therefore, an antisclerotic and antiinflammatory effect is observed, explaining the therapeutic benefit of PDT in plaque psoriasis.124 A recent study has proposed IPL as an effective and promising alternative in the treatment of nail psoriasis, with significant improvement in the nail bed (71.2%) and matrix (32.2%), with long-standing remission.125
ConclusionsSeveral trials have pointed to the efficacy and compatibility of IPL devices in a wide range of skin conditions. Comparative trials have confirmed similar efficacy (and in some cases superior efficacy) compared to laser systems, although more randomized, controlled, trials are needed with prolonged follow-up to allow an assessment of the benefit-risk ratio of IPL compared with each type of laser system in the different indications.
The main advantage of IPL systems, particularly with top-of-the-range models, is their versatility, safety, and favorable cost compared to laser systems. However, these systems are more complex and require more extensive training to obtain better outcomes.
Thanks to the versatility of the IPL systems, they can act on different chromophores and so are able to treat pigmented lesions, vascular lesions, acne, and rosacea. Moreover, they can also be used in photoepilation, photorejuvenation (alone or in combination with other procedures to attain better outcomes), or as a light source for PDT in the treatment of actinic keratoses, basal cell carcinomas, and acne, among other conditions. In addition, a range of new applications are emerging (although more clinical studies are required to support their efficacy).
As IPL systems are nonablative, they are the procedure of choice in patients who find the risks of other more aggressive techniques unacceptable. These techniques also require longer recovery times, even if they may be more effective.
Technological advances have enabled current top-of-the-range systems to emit a square-wave pulse, with a series of benefits in terms of clinical effectiveness and safety, while minimizing side effects.
With regards the medical-legal implications, IPL treatments must be carried out in approved installations by trained staff with suitable safety equipment available. To this end, regulations for the use of these devices should be created, with a focus on home use, as these systems are currently considered freely available products, without the need for a medical prescription.
In our opinion, in view of the above review, IPL systems are medical-esthetical procedures of growing importance thanks to their versatility, efficacy, safety, and ease-of-use. More randomized, controlled trials are, however, needed with larger sample sizes and longer-term follow-up. These studies need to follow unified strategies and protocols specific to each type of disease or indication. The designs of the published studies vary greatly and it is impossible to derive standard parameters.
Given the limited and variable findings from the published studies, it is impossible to make any recommendations on the use of IPL with a sufficient level of evidence, except in the case of PDT for traditional indications (actinic keratosis, Bowen disease, and superficial basal cell carcinoma [level A recommendation and level of evidence 1]). For the remaining indications, the results show significant efficacy but there is insufficient or limited scientific evidence, and better quality studies are needed to recommend its use.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: González-Rodríguez A.J., Lorente-Gual R. Indicaciones actuales y nuevas aplicaciones de los sistemas de luz pulsada intensa. Actas Dermosifiliogr. 2015;106:350–364.