To the Editor:
Kathon CG (Cosmetic Grade) is a mixture of methylisothiazolinone (1.125%), methylchloroisothiazolinone (0.375%), magnesium nitrate and magnesium chloride (23%), and water (75%).1–4 Isothiazolinones are heterocyclic compounds used as biocides because of their antimicrobial properties against gram-positive and gram-negative bacteria, fungi, and algae.1–4
Kathon CG is used in a wide range of cosmetics and hygiene and personal care products in maximum permissible concentrations of 7.5ppm (stay-on products) and 15ppm (rinse-off products).1,4 It is also used in industry, where the concentrations can be higher.4
Kathlon CG was included as an allergen in the standard series in the 1980s at a concentration of 100ppm and is still one of the most common allergens in both the workplace and the home.1,4–8
We report the case of a 30-year-old woman with a personal and family history of atopic dermatitis and respiratory allergy who presented with acute eczema on both hands after contact with a neutral massage cream (+BO pro, Telic, S.A.) that she used in her work as a physiotherapist. The cream contains water, liquid paraffin, petrolatum, stearic acid, cetyl alcohol, ceteth-25, PEG-8 stearate, benzyl alcohol, ceteareth-12, fragrance, methylparaben, propylparaben, triethanolamine, and diazolidinyl urea.
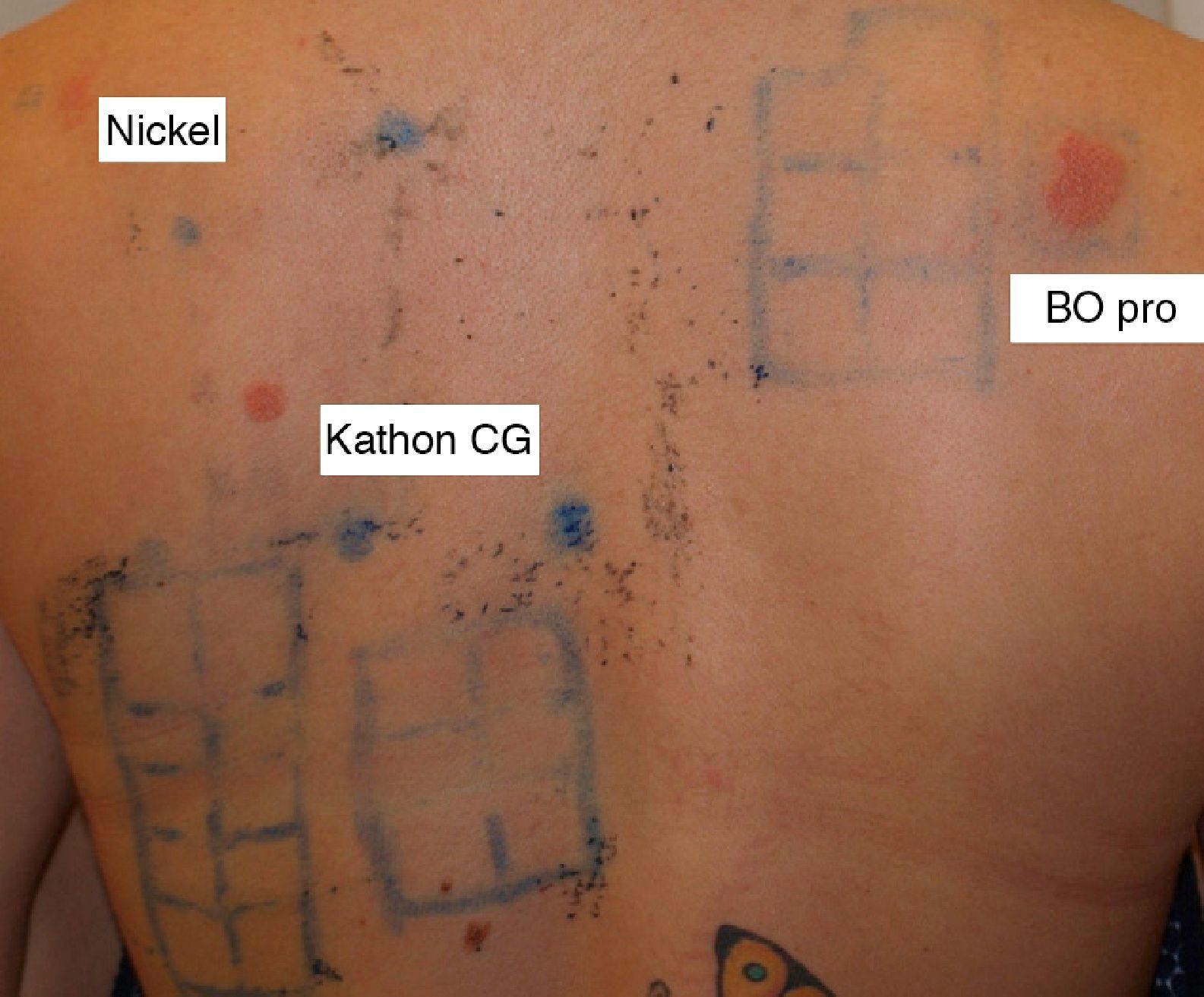
With a presumptive diagnosis of allergic contact dermatitis, we performed patch tests with a Spanish Group for Research Into Contact Dermatitis and Skin Allergies (GEIDAC) standard series, a Martí Tor cosmetics series and the product itself. Readings at 48h, 96h, and 7 days revealed sensitization to nickel sulfate (++) with past relevance, Kathon CG (+++) with unknown relevance, and the product itself (+++) with present relevance (Fig. 1).
We contacted the manufacturer, who confirmed that Kathon CG is not present in the finished product, nor is it used in the manufacture of the starting materials of which the cream is composed. We requested the components separately, which showed negative results in the patch tests at 48h, 96h, and 7 days.
The patient was asked whether the cream could have been mixed or contaminated with another substance or manipulated by another worker. She assured us that she herself had unsealed the container and applied the product with washed hands and that the eczema had appeared a few hours later.
To rule out the possibility that the product had been adulterated and was an irritant, controls were carried out on 10 volunteers, with negative results at 96h in all cases.
On suspicion that the product could contain methylchloroisothiazolinone/methylisothiazolinone, we contacted the manufacturer, who investigated other possible sources of contact and thus discovered the curious way in which small amounts of this biocide had contaminated the product.
The mixer, the metering pump, and the pipes of the packaging machine are cleaned and disinfected after each production run with hydrogen peroxide and rinsed with deionized water. As moisture may remain in the less accessible points of the system (bends and filters), Kathon CG is added to the rinse water to ensure a completely sterile process.
As a result of this finding, the company marketing the cream compensated the patient and changed the procedure for cleaning the machinery, replacing the Kathon CG in the rinse water with other biocides (methylparaben, propylparaben, and diazolidinyl urea).
We believe that the very small amount of methylchloroisothiazolinone/methylisothiazolinone that may be contained in the finished product is due to contamination. The rinse water which is used to clean the packaging machine contains Kathon CG and may briefly come into contact with the cream, especially in the first few bottles of the production run. Although it would have been advisable to determine the presence of Kathon CG in the content of the bottle used by the patient, it was not possible to do so because of the technical difficulty involved.
The patient's atopic predisposition and her work, in which she uses the cream daily, probably accentuated the problem and caused the severe acute dermatitis.
Please cite this article as: Cervigón-González I, et al. Eccema de contacto por metilcloroisotiazolinona/metilisotiazolinona (Kathon CG®) como contaminante del proceso de fabricación de una crema. Actas Dermosifiliogr. 2013;104:81-2.