Ixekizumab demonstrated greater efficacy than placebo and etanercept in UNCOVER-3. Subgroup analysis of Latin American patients was performed. We report 12-week and 60-week data.
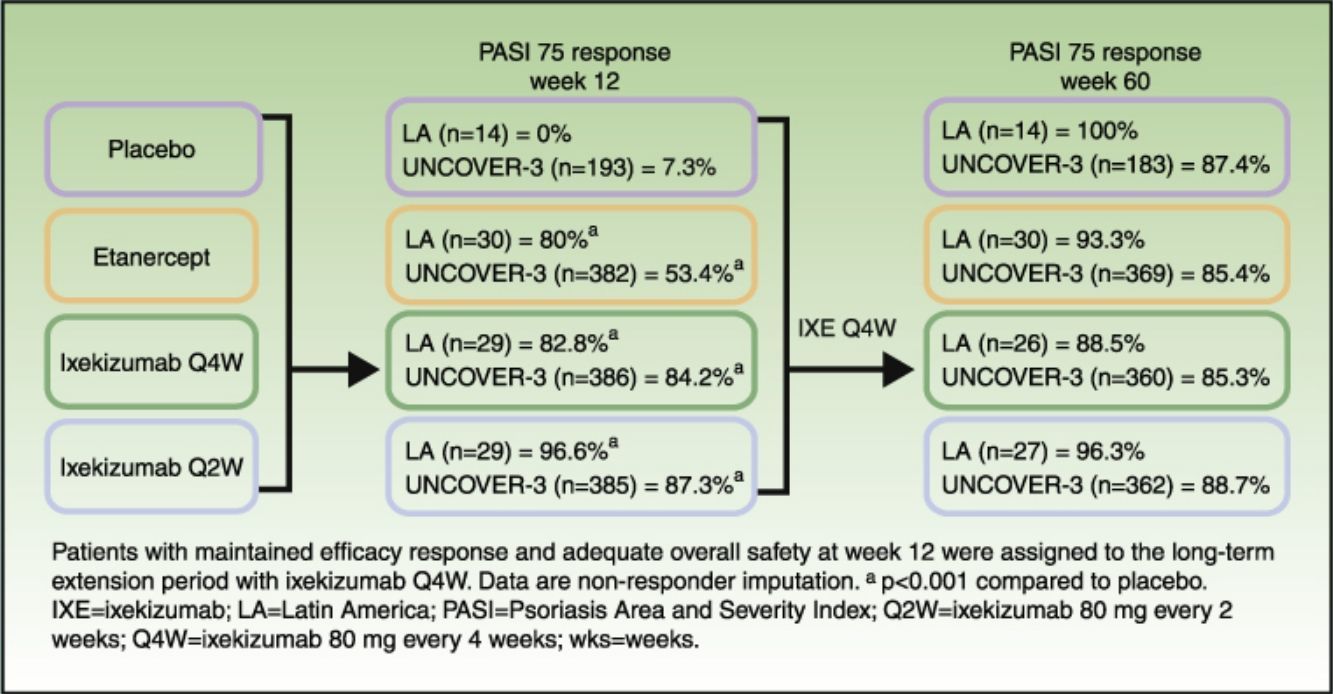
Patients and methodsAnalysis included 102 Latin American patients randomized to receive placebo (n=14), etanercept 50mg twice weekly (n=30), or ixekizumab 160-mg starting dose followed by 80mg every 2 weeks (Q2W; n=29) or every 4 weeks (Q4W; n=29). At week 12, patients maintaining efficacy response and adequate overall safety were assigned, at the discretion of the investigator, to long-term extension with ixekizumab Q4W.
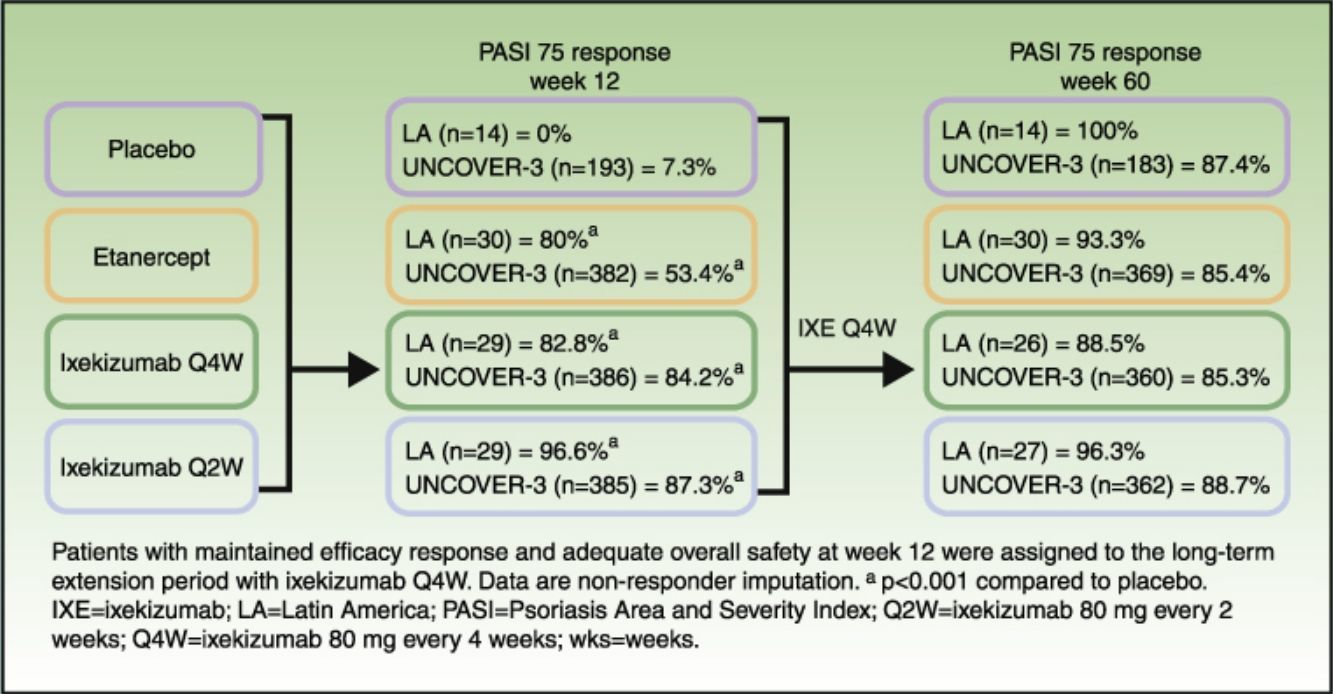
ResultsAt week 12, Psoriasis Area and Severity Index (PASI) 100 scores were 0%, 20.0% (p=0.075 vs placebo), 62.1% (p<0.001 vs placebo; p=0.001 vs etanercept), and 48.3% (p=0.002 vs placebo; p=0.023 vs etanercept) for placebo, etanercept, ixekizumab Q2W, and ixekizumab Q4W, respectively. Among patients who continued therapy up to week 60 (n=97), PASI 100 scores were 71.4%, 60.0%, 77.8%, and 57.7% for patients who received induction placebo, etanercept, ixekizumab Q2W, and ixekizumab Q4W, respectively (non-responder imputation). By week 60, ≥1 serious adverse event was experienced by 7.1% (n=1/14), 3.3% (n=1/30), 14.8% (n=4/27), and 0% (n=0/26) of patients who received induction placebo, etanercept, ixekizumab Q2W, and ixekizumab Q4W, respectively. There were no cases of active tuberculosis with ixekizumab treatment through 60 weeks.
ConclusionsIn Latin American patients, both ixekizumab dosing regimens demonstrated greater efficacy than etanercept for treating psoriasis over 12 weeks. The safety profile of ixekizumab through 60 weeks was well tolerated and consistent with the overall profile.
Ixekizumab demostró una mayor eficacia que el placebo y etanercept en el estudio UNCOVER-3. Tras realizar un análisis del subgrupo de pacientes latinoamericanos, se presentan los resultados transcurridas 12 y 60 semanas.
Pacientes y métodosEl análisis incluyó a 102 pacientes latinoamericanos aleatorizados para la administración de placebo (n=14), etanercept 50mg 2 veces por semana (n=30), o ixekizumab 160mg como dosis inicial y 80mg cada 2 semanas (Q2W; n=29) o 4 semanas (Q4W; n=29). A la semana 12 los pacientes con buena respuesta de eficacia y ausencia de efectos adversos fueron asignados al tratamiento de ampliación a largo plazo con ixekizumab Q4W, a discreción del investigador.
ResultadosA las 12 semanas las puntuaciones del PASI fueron del 0%, 20% (p=0,075 vs placebo), 62,1% (p<0,001 vs placebo; p=0,001 vs etanercept) y 48,3% (p=0,002 vs placebo; p=0,023 vs etanercept) para placebo, etanercept, ixekizumab Q2W, e ixekizumab Q4W, respectivamente. Entre los pacientes que prosiguieron la terapia hasta la semana 60 (n=97) las puntuaciones PASI 100 fueron del 71,4%, 60%, 77,8%, y 57,7% para los pacientes a quienes se administró placebo de inducción, etanercept, ixekizumab Q2W e ixekizumab Q4W, respectivamente (imputación del no respondedor). En la semana 60 ≥1 presentaron reacción adversa grave el 7,1% (n=1/14), 3,3% (n=1/30), 14,8% (n=4/27) y 0% (n=0/26) de los pacientes a quienes se administró placebo, etanercept, ixekizumab Q2W e ixekizumab Q4W, respectivamente. No se produjeron casos de tuberculosis activa con el tratamiento de ixekizumab a lo largo de las 60 semanas.
ConclusionesEn los pacientes latinoamericanos ambos regímenes de dosificación de ixekizumab demostraron mayor eficacia que etanercept para el tratamiento de la psoriasis durante 12 semanas. En cuanto al perfil de seguridad, ixekizumab a lo largo de 60 semanas fue bien tolerado y consistente con el perfil general.
Psoriasis is a universal chronic immune-mediated skin disease, characterized by the eruption of reddish, silvery-scaled plaques, predominantly on the elbows, knees, scalp, and trunk. Clinical manifestations of skin lesions and coexisting comorbidities have substantial negative effect on quality of life.1–4
Psoriasis is a heterogenic disease with different genetic and clinical phenotypes. This is reflected in the prevalence of the disease in different geographic regions and ethnic groups. In the United States, the prevalence of psoriasis in adults 20 years or older is estimated to be 3.2%,5 while it is lower in Hispanics (1.6%) than in Caucasians (3.6%) and African Americans (1.9%).5 While the potential genetic factors may contribute to the different prevalence of psoriasis in different populations, it is still unclear whether these differences reflect a different immune pathogenesis among the populations which could lead to different responses to treatment.
Although evidence is lacking regarding the prevalence and characteristics of psoriasis in Latin America, the prevalence of psoriasis may be lower in Latin America, estimated at approximately 2%, than in Western countries.6 Studies are complicated by racial and ethnic heterogeneity of the Latin American population.6 A recent population-based cross-sectional survey evaluating psoriasis prevalence in Brazil interviewed 8947 inhabitants in all 26 Brazilian state capitals. The overall prevalence of psoriasis in the Brazilian population ranged from 1.10% to 1.51% with greater proportions in the South and Southeast regions.7 Further efforts are required to understand psoriasis and establish the effectiveness of treatments in Latin America.
Human genome studies have identified polymorphisms associated with psoriasis in many immune-related genes, which support the tenet that deregulated cytokine networks play a role in the initiation and pathophysiology of psoriasis.8–11 Studies have demonstrated a high level of efficacy in large proportions of patients with psoriasis with antibodies targeting interleukin-12/23, interleukin-17A, or interleukin-17 receptor (IL-17RA) subunit.12–22
In the phase 3, randomized UNCOVER-1, -2, and -3 studies, two different dose regimens of ixekizumab, a high-affinity anti-interleukin-17A antibody, were compared with placebo and/or a tumor necrosis factor blocker, etanercept (50mg twice weekly), in the treatment of plaque psoriasis.23,24 Both ixekizumab dose regimens had greater efficacy than placebo (UNCOVER-1, -2, and -3) and etanercept (UNCOVER-2 and -3) over the first 12-week induction phase.23,24 In the three phase 3 UNCOVER studies, ixekizumab was also shown to be effective through 60 weeks of treatment.24
The UNCOVER-3 study included patients from Argentina, Chile, and Mexico. The objective of this subgroup analysis was to evaluate the efficacy and safety of ixekizumab in Latin American patients with moderate-to-severe psoriasis in the UNCOVER-3 study.
MethodsStudy design and patientsThe study design and patients for UNCOVER-3 have been published previously.23,24 Each center's institutional review board or independent ethics committee approved this study. The study followed the guiding principles of the Declaration of Helsinki and the Good Clinical Practice Guidelines of the International Conference on Harmonisation. All patients provided written informed consent before enrollment.
Randomization and proceduresRandomization and procedures have been published previously.23,24 During the 12-week placebo-controlled and active-controlled induction period, patients were randomly assigned (2:2:2:1) and stratified by center to receive placebo, etanercept 50mg twice weekly, or an ixekizumab 160-mg starting dose followed by either 80mg every 2 weeks (Q2W) or every 4 weeks (Q4W). Patients with maintained efficacy response and adequate overall safety during the 12-week induction period were assigned, at the discretion of the investigator, to the long-term extension period with ixekizumab Q4W through to week 60.
Statistical analysisDetailed statistical methods have been published previously.23,24 The Latin American population was defined and analyzed as the patients enrolled at study sites in Argentina, Chile, and Mexico. A total of 76 (74.5%) patients were from Chile, 21 (20.6%) patients were from Argentina, and 5 (4.9%) patients were from Mexico. The data are presented side-by-side with data for the UNCOVER-3 intent-to-treat (ITT) population. This study is registered with ClinicalTrials.gov, number NCT01646177.
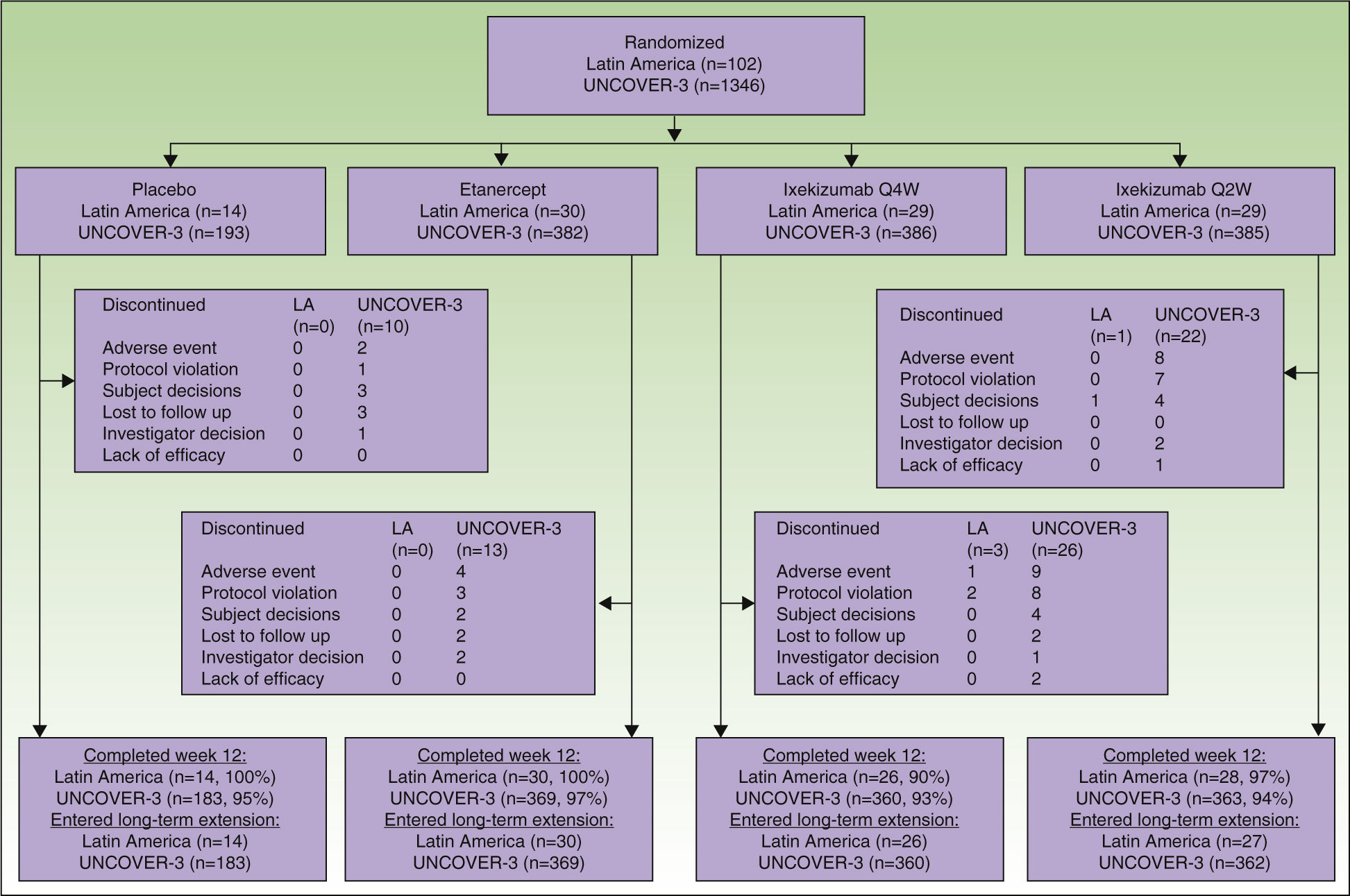
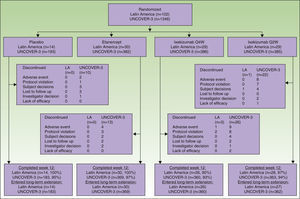
ResultsPatientsA total of 102 Latin American patients in the ITT population were randomly assigned to receive placebo (n=14), etanercept (n=30), ixekizumab Q2W (n=29), or ixekizumab Q4W (n=29) during the 12-week induction period; among the 97 patients who continued in the long-term extension period through to week 60, 14 received placebo, 30 received etanercept, 27 received ixekizumab Q2W, and 26 received ixekizumab Q4W during the 12-week induction period (Fig. 1).
A total of 1346 patients in the UNCOVER-3 ITT population were randomly assigned to receive placebo (n=193), etanercept (n=382), ixekizumab Q2W (n=385), or ixekizumab Q4W (n=386) during the 12-week induction period; among the 1274 patients who entered the long-term extension period up to 60 weeks, 183 received placebo, 369 received etanercept, 362 received ixekizumab Q2W, and 360 received ixekizumab Q4W during the 12-week induction period (Fig. 1).
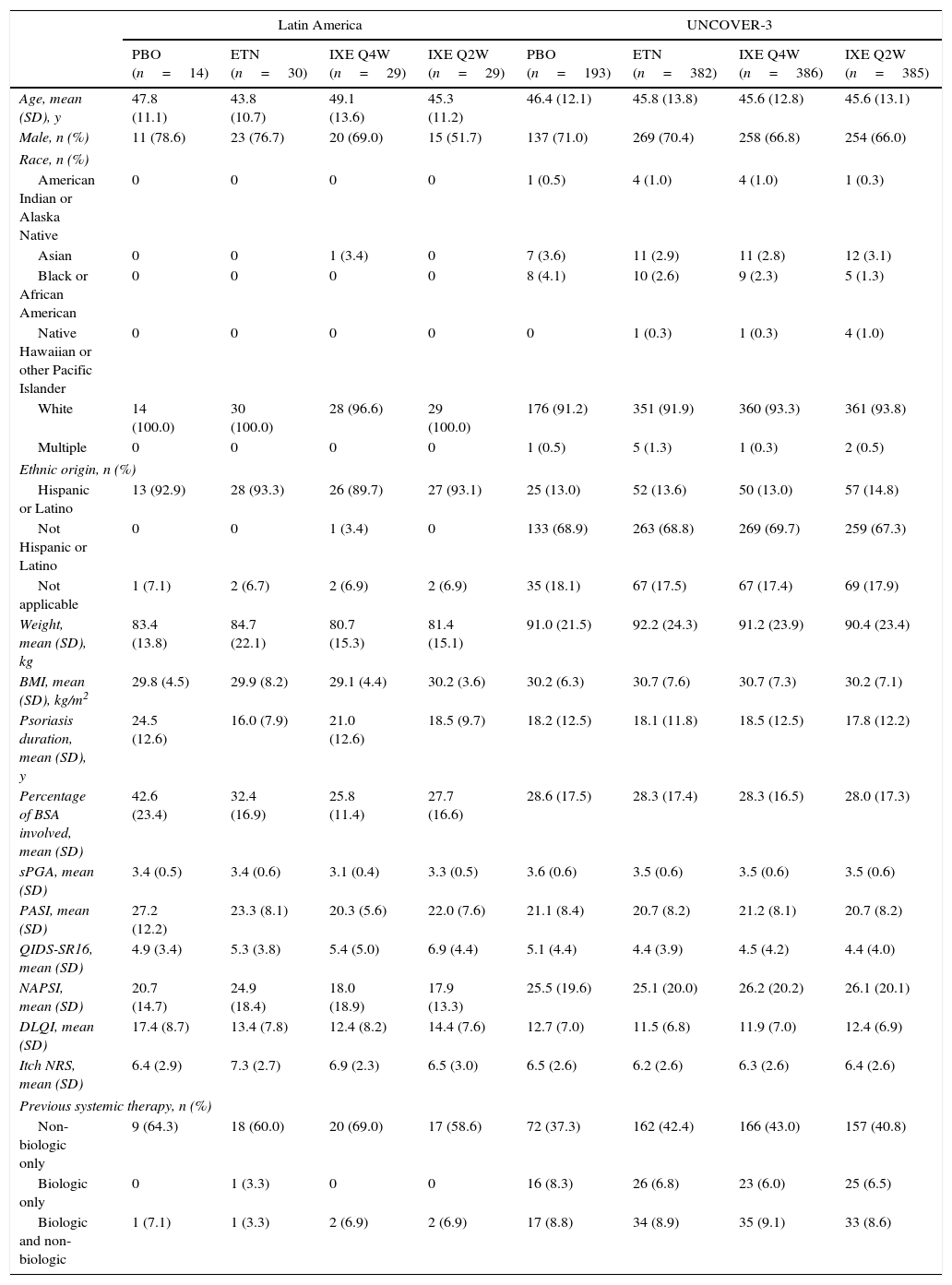
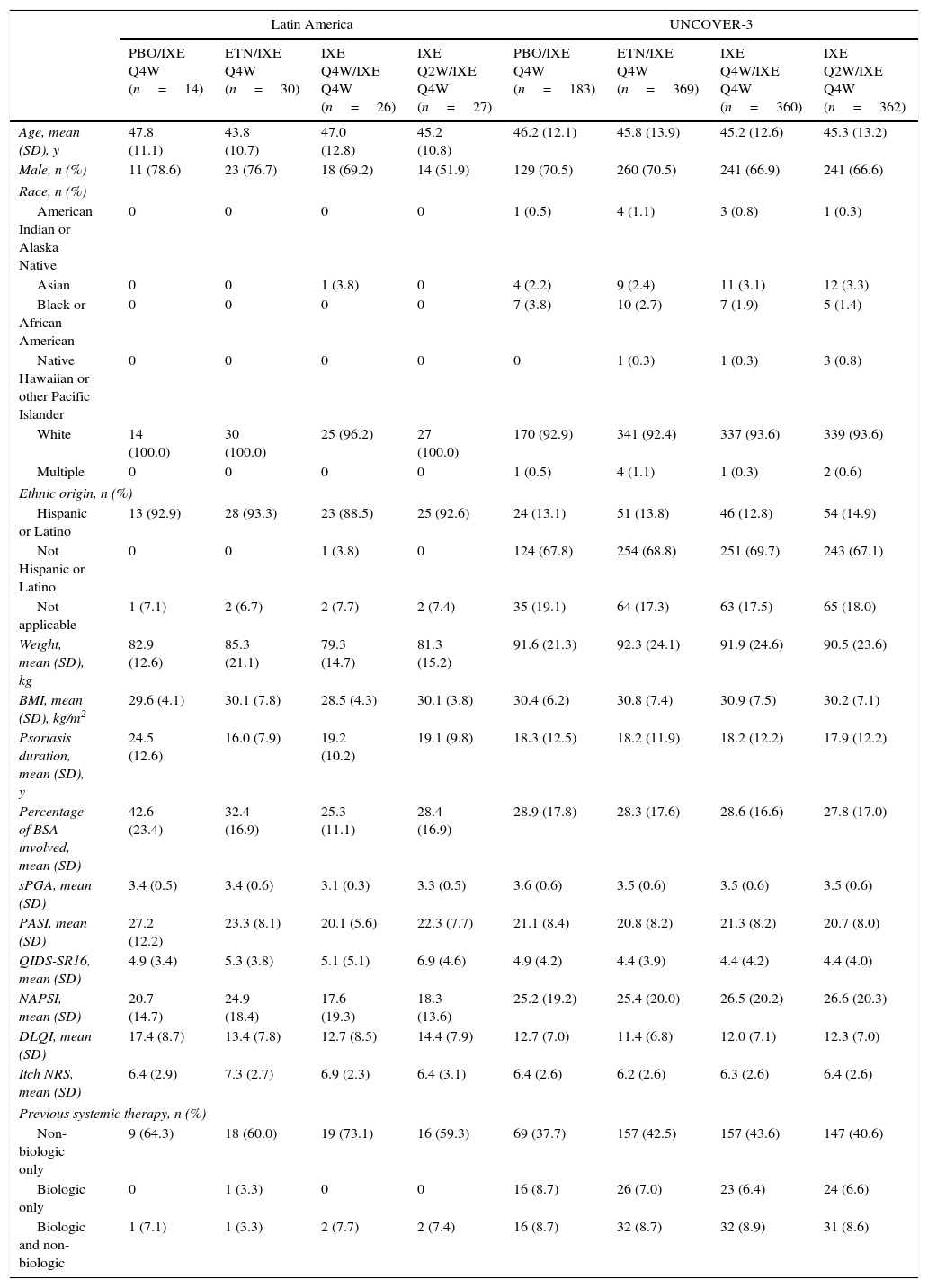
Baseline demographics and clinical characteristics of Latin American patients and the UNCOVER-3 ITT population were well balanced between treatment arms for week 12 (Table 1) and week 60 (Table 2). The Latin American subgroup was mainly white and of Hispanic or Latino ethnicity. This compared to the UNCOVER-3 ITT population that was mainly white and of non-Hispanic or Latino ethnicity (Tables 1 and 2).
Baseline demographics and clinical characteristics for the 12-week induction period.
| Latin America | UNCOVER-3 | |||||||
|---|---|---|---|---|---|---|---|---|
| PBO (n=14) | ETN (n=30) | IXE Q4W (n=29) | IXE Q2W (n=29) | PBO (n=193) | ETN (n=382) | IXE Q4W (n=386) | IXE Q2W (n=385) | |
| Age, mean (SD), y | 47.8 (11.1) | 43.8 (10.7) | 49.1 (13.6) | 45.3 (11.2) | 46.4 (12.1) | 45.8 (13.8) | 45.6 (12.8) | 45.6 (13.1) |
| Male, n (%) | 11 (78.6) | 23 (76.7) | 20 (69.0) | 15 (51.7) | 137 (71.0) | 269 (70.4) | 258 (66.8) | 254 (66.0) |
| Race, n (%) | ||||||||
| American Indian or Alaska Native | 0 | 0 | 0 | 0 | 1 (0.5) | 4 (1.0) | 4 (1.0) | 1 (0.3) |
| Asian | 0 | 0 | 1 (3.4) | 0 | 7 (3.6) | 11 (2.9) | 11 (2.8) | 12 (3.1) |
| Black or African American | 0 | 0 | 0 | 0 | 8 (4.1) | 10 (2.6) | 9 (2.3) | 5 (1.3) |
| Native Hawaiian or other Pacific Islander | 0 | 0 | 0 | 0 | 0 | 1 (0.3) | 1 (0.3) | 4 (1.0) |
| White | 14 (100.0) | 30 (100.0) | 28 (96.6) | 29 (100.0) | 176 (91.2) | 351 (91.9) | 360 (93.3) | 361 (93.8) |
| Multiple | 0 | 0 | 0 | 0 | 1 (0.5) | 5 (1.3) | 1 (0.3) | 2 (0.5) |
| Ethnic origin, n (%) | ||||||||
| Hispanic or Latino | 13 (92.9) | 28 (93.3) | 26 (89.7) | 27 (93.1) | 25 (13.0) | 52 (13.6) | 50 (13.0) | 57 (14.8) |
| Not Hispanic or Latino | 0 | 0 | 1 (3.4) | 0 | 133 (68.9) | 263 (68.8) | 269 (69.7) | 259 (67.3) |
| Not applicable | 1 (7.1) | 2 (6.7) | 2 (6.9) | 2 (6.9) | 35 (18.1) | 67 (17.5) | 67 (17.4) | 69 (17.9) |
| Weight, mean (SD), kg | 83.4 (13.8) | 84.7 (22.1) | 80.7 (15.3) | 81.4 (15.1) | 91.0 (21.5) | 92.2 (24.3) | 91.2 (23.9) | 90.4 (23.4) |
| BMI, mean (SD), kg/m2 | 29.8 (4.5) | 29.9 (8.2) | 29.1 (4.4) | 30.2 (3.6) | 30.2 (6.3) | 30.7 (7.6) | 30.7 (7.3) | 30.2 (7.1) |
| Psoriasis duration, mean (SD), y | 24.5 (12.6) | 16.0 (7.9) | 21.0 (12.6) | 18.5 (9.7) | 18.2 (12.5) | 18.1 (11.8) | 18.5 (12.5) | 17.8 (12.2) |
| Percentage of BSA involved, mean (SD) | 42.6 (23.4) | 32.4 (16.9) | 25.8 (11.4) | 27.7 (16.6) | 28.6 (17.5) | 28.3 (17.4) | 28.3 (16.5) | 28.0 (17.3) |
| sPGA, mean (SD) | 3.4 (0.5) | 3.4 (0.6) | 3.1 (0.4) | 3.3 (0.5) | 3.6 (0.6) | 3.5 (0.6) | 3.5 (0.6) | 3.5 (0.6) |
| PASI, mean (SD) | 27.2 (12.2) | 23.3 (8.1) | 20.3 (5.6) | 22.0 (7.6) | 21.1 (8.4) | 20.7 (8.2) | 21.2 (8.1) | 20.7 (8.2) |
| QIDS-SR16, mean (SD) | 4.9 (3.4) | 5.3 (3.8) | 5.4 (5.0) | 6.9 (4.4) | 5.1 (4.4) | 4.4 (3.9) | 4.5 (4.2) | 4.4 (4.0) |
| NAPSI, mean (SD) | 20.7 (14.7) | 24.9 (18.4) | 18.0 (18.9) | 17.9 (13.3) | 25.5 (19.6) | 25.1 (20.0) | 26.2 (20.2) | 26.1 (20.1) |
| DLQI, mean (SD) | 17.4 (8.7) | 13.4 (7.8) | 12.4 (8.2) | 14.4 (7.6) | 12.7 (7.0) | 11.5 (6.8) | 11.9 (7.0) | 12.4 (6.9) |
| Itch NRS, mean (SD) | 6.4 (2.9) | 7.3 (2.7) | 6.9 (2.3) | 6.5 (3.0) | 6.5 (2.6) | 6.2 (2.6) | 6.3 (2.6) | 6.4 (2.6) |
| Previous systemic therapy, n (%) | ||||||||
| Non-biologic only | 9 (64.3) | 18 (60.0) | 20 (69.0) | 17 (58.6) | 72 (37.3) | 162 (42.4) | 166 (43.0) | 157 (40.8) |
| Biologic only | 0 | 1 (3.3) | 0 | 0 | 16 (8.3) | 26 (6.8) | 23 (6.0) | 25 (6.5) |
| Biologic and non-biologic | 1 (7.1) | 1 (3.3) | 2 (6.9) | 2 (6.9) | 17 (8.8) | 34 (8.9) | 35 (9.1) | 33 (8.6) |
Abbreviations: BMI, body mass index; BSA, body surface area; DLQI, Dermatology Life Quality Index; ETN, etanercept; IXE Q2W, ixekizumab 80mg every 2 weeks; IXE Q4W, ixekizumab 80mg every 4 weeks; NAPSI, Nail Psoriasis Severity Index; NRS, numeric rating scale; PASI, Psoriasis Area and Severity Index; PBO, placebo; QIDS-SR16, Quick Inventory of Depressive Symptomatology-Self Report (16 items); sPGA, static Physician's Global Assessment.
Baseline demographics and clinical characteristics for patients who entered the 60-week long-term extension period.
| Latin America | UNCOVER-3 | |||||||
|---|---|---|---|---|---|---|---|---|
| PBO/IXE Q4W (n=14) | ETN/IXE Q4W (n=30) | IXE Q4W/IXE Q4W (n=26) | IXE Q2W/IXE Q4W (n=27) | PBO/IXE Q4W (n=183) | ETN/IXE Q4W (n=369) | IXE Q4W/IXE Q4W (n=360) | IXE Q2W/IXE Q4W (n=362) | |
| Age, mean (SD), y | 47.8 (11.1) | 43.8 (10.7) | 47.0 (12.8) | 45.2 (10.8) | 46.2 (12.1) | 45.8 (13.9) | 45.2 (12.6) | 45.3 (13.2) |
| Male, n (%) | 11 (78.6) | 23 (76.7) | 18 (69.2) | 14 (51.9) | 129 (70.5) | 260 (70.5) | 241 (66.9) | 241 (66.6) |
| Race, n (%) | ||||||||
| American Indian or Alaska Native | 0 | 0 | 0 | 0 | 1 (0.5) | 4 (1.1) | 3 (0.8) | 1 (0.3) |
| Asian | 0 | 0 | 1 (3.8) | 0 | 4 (2.2) | 9 (2.4) | 11 (3.1) | 12 (3.3) |
| Black or African American | 0 | 0 | 0 | 0 | 7 (3.8) | 10 (2.7) | 7 (1.9) | 5 (1.4) |
| Native Hawaiian or other Pacific Islander | 0 | 0 | 0 | 0 | 0 | 1 (0.3) | 1 (0.3) | 3 (0.8) |
| White | 14 (100.0) | 30 (100.0) | 25 (96.2) | 27 (100.0) | 170 (92.9) | 341 (92.4) | 337 (93.6) | 339 (93.6) |
| Multiple | 0 | 0 | 0 | 0 | 1 (0.5) | 4 (1.1) | 1 (0.3) | 2 (0.6) |
| Ethnic origin, n (%) | ||||||||
| Hispanic or Latino | 13 (92.9) | 28 (93.3) | 23 (88.5) | 25 (92.6) | 24 (13.1) | 51 (13.8) | 46 (12.8) | 54 (14.9) |
| Not Hispanic or Latino | 0 | 0 | 1 (3.8) | 0 | 124 (67.8) | 254 (68.8) | 251 (69.7) | 243 (67.1) |
| Not applicable | 1 (7.1) | 2 (6.7) | 2 (7.7) | 2 (7.4) | 35 (19.1) | 64 (17.3) | 63 (17.5) | 65 (18.0) |
| Weight, mean (SD), kg | 82.9 (12.6) | 85.3 (21.1) | 79.3 (14.7) | 81.3 (15.2) | 91.6 (21.3) | 92.3 (24.1) | 91.9 (24.6) | 90.5 (23.6) |
| BMI, mean (SD), kg/m2 | 29.6 (4.1) | 30.1 (7.8) | 28.5 (4.3) | 30.1 (3.8) | 30.4 (6.2) | 30.8 (7.4) | 30.9 (7.5) | 30.2 (7.1) |
| Psoriasis duration, mean (SD), y | 24.5 (12.6) | 16.0 (7.9) | 19.2 (10.2) | 19.1 (9.8) | 18.3 (12.5) | 18.2 (11.9) | 18.2 (12.2) | 17.9 (12.2) |
| Percentage of BSA involved, mean (SD) | 42.6 (23.4) | 32.4 (16.9) | 25.3 (11.1) | 28.4 (16.9) | 28.9 (17.8) | 28.3 (17.6) | 28.6 (16.6) | 27.8 (17.0) |
| sPGA, mean (SD) | 3.4 (0.5) | 3.4 (0.6) | 3.1 (0.3) | 3.3 (0.5) | 3.6 (0.6) | 3.5 (0.6) | 3.5 (0.6) | 3.5 (0.6) |
| PASI, mean (SD) | 27.2 (12.2) | 23.3 (8.1) | 20.1 (5.6) | 22.3 (7.7) | 21.1 (8.4) | 20.8 (8.2) | 21.3 (8.2) | 20.7 (8.0) |
| QIDS-SR16, mean (SD) | 4.9 (3.4) | 5.3 (3.8) | 5.1 (5.1) | 6.9 (4.6) | 4.9 (4.2) | 4.4 (3.9) | 4.4 (4.2) | 4.4 (4.0) |
| NAPSI, mean (SD) | 20.7 (14.7) | 24.9 (18.4) | 17.6 (19.3) | 18.3 (13.6) | 25.2 (19.2) | 25.4 (20.0) | 26.5 (20.2) | 26.6 (20.3) |
| DLQI, mean (SD) | 17.4 (8.7) | 13.4 (7.8) | 12.7 (8.5) | 14.4 (7.9) | 12.7 (7.0) | 11.4 (6.8) | 12.0 (7.1) | 12.3 (7.0) |
| Itch NRS, mean (SD) | 6.4 (2.9) | 7.3 (2.7) | 6.9 (2.3) | 6.4 (3.1) | 6.4 (2.6) | 6.2 (2.6) | 6.3 (2.6) | 6.4 (2.6) |
| Previous systemic therapy, n (%) | ||||||||
| Non-biologic only | 9 (64.3) | 18 (60.0) | 19 (73.1) | 16 (59.3) | 69 (37.7) | 157 (42.5) | 157 (43.6) | 147 (40.6) |
| Biologic only | 0 | 1 (3.3) | 0 | 0 | 16 (8.7) | 26 (7.0) | 23 (6.4) | 24 (6.6) |
| Biologic and non-biologic | 1 (7.1) | 1 (3.3) | 2 (7.7) | 2 (7.4) | 16 (8.7) | 32 (8.7) | 32 (8.9) | 31 (8.6) |
Abbreviations: BMI, body mass index; BSA, body surface area; DLQI, Dermatology Life Quality Index; ETN, etanercept; IXE Q2W, ixekizumab 80mg every 2 weeks; IXE Q4W, ixekizumab 80mg every 4 weeks; NAPSI, Nail Psoriasis Severity Index; NRS, numeric rating scale; PASI, Psoriasis Area and Severity Index; PBO, placebo; QIDS-SR16, Quick Inventory of Depressive Symptomatology-Self Report (16 items); sPGA, static Physician's Global Assessment.
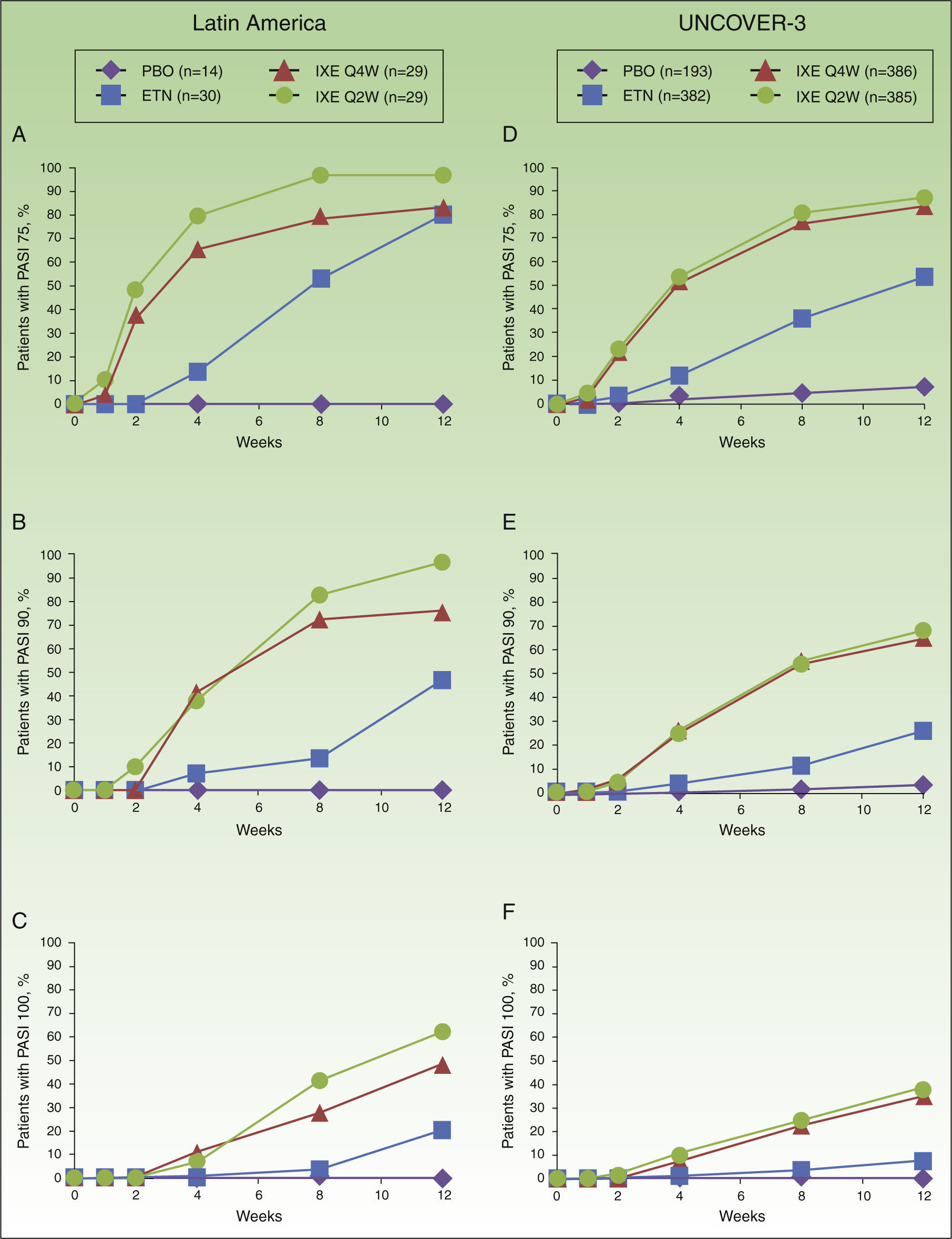
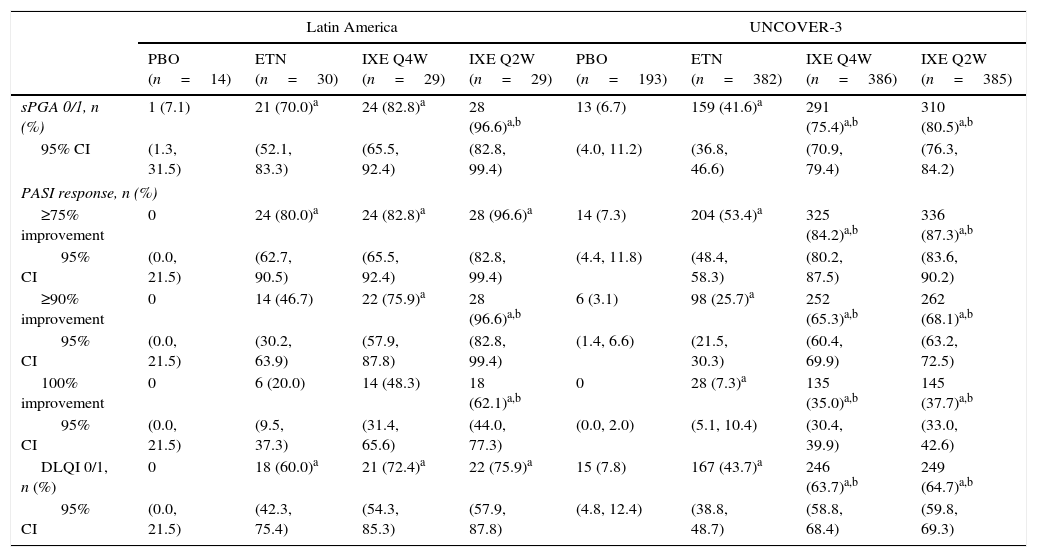
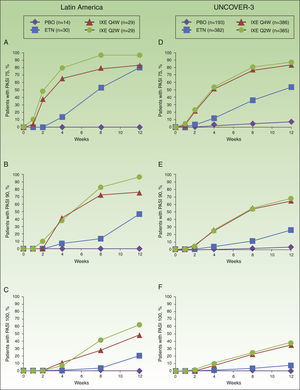
In Latin American patients, both dose regimens of ixekizumab showed greater efficacy than placebo and etanercept at week 12 with respect to the coprimary endpoints of the Psoriasis Area and Severity Index (PASI) 75 and static Physician's Global Assessment (sPGA) 0/1 (Table 3 and Fig. 2). Statistically significantly greater proportions of Latin American patients achieved PASI 75 as early as week 2 for both ixekizumab groups compared with etanercept (p<0.001 vs etanercept). By week 2, 48.3% of Latin American patients given ixekizumab Q2W and 37.9% of patients given ixekizumab Q4W achieved PASI 75 (Fig. 2). These results are generally aligned with the efficacy of ixekizumab in the UNCOVER-3 ITT population (Table 3 and Fig. 2).
Clinical responses at week 12.
| Latin America | UNCOVER-3 | |||||||
|---|---|---|---|---|---|---|---|---|
| PBO (n=14) | ETN (n=30) | IXE Q4W (n=29) | IXE Q2W (n=29) | PBO (n=193) | ETN (n=382) | IXE Q4W (n=386) | IXE Q2W (n=385) | |
| sPGA 0/1, n (%) | 1 (7.1) | 21 (70.0)a | 24 (82.8)a | 28 (96.6)a,b | 13 (6.7) | 159 (41.6)a | 291 (75.4)a,b | 310 (80.5)a,b |
| 95% CI | (1.3, 31.5) | (52.1, 83.3) | (65.5, 92.4) | (82.8, 99.4) | (4.0, 11.2) | (36.8, 46.6) | (70.9, 79.4) | (76.3, 84.2) |
| PASI response, n (%) | ||||||||
| ≥75% improvement | 0 | 24 (80.0)a | 24 (82.8)a | 28 (96.6)a | 14 (7.3) | 204 (53.4)a | 325 (84.2)a,b | 336 (87.3)a,b |
| 95% CI | (0.0, 21.5) | (62.7, 90.5) | (65.5, 92.4) | (82.8, 99.4) | (4.4, 11.8) | (48.4, 58.3) | (80.2, 87.5) | (83.6, 90.2) |
| ≥90% improvement | 0 | 14 (46.7) | 22 (75.9)a | 28 (96.6)a,b | 6 (3.1) | 98 (25.7)a | 252 (65.3)a,b | 262 (68.1)a,b |
| 95% CI | (0.0, 21.5) | (30.2, 63.9) | (57.9, 87.8) | (82.8, 99.4) | (1.4, 6.6) | (21.5, 30.3) | (60.4, 69.9) | (63.2, 72.5) |
| 100% improvement | 0 | 6 (20.0) | 14 (48.3) | 18 (62.1)a,b | 0 | 28 (7.3)a | 135 (35.0)a,b | 145 (37.7)a,b |
| 95% CI | (0.0, 21.5) | (9.5, 37.3) | (31.4, 65.6) | (44.0, 77.3) | (0.0, 2.0) | (5.1, 10.4) | (30.4, 39.9) | (33.0, 42.6) |
| DLQI 0/1, n (%) | 0 | 18 (60.0)a | 21 (72.4)a | 22 (75.9)a | 15 (7.8) | 167 (43.7)a | 246 (63.7)a,b | 249 (64.7)a,b |
| 95% CI | (0.0, 21.5) | (42.3, 75.4) | (54.3, 85.3) | (57.9, 87.8) | (4.8, 12.4) | (38.8, 48.7) | (58.8, 68.4) | (59.8, 69.3) |
p<0.01 compared with etanercept. Data were analyzed with Cochran-Mantel-Haenszel test and are NRI. The Wilson Score method without continuity correction was used to construct the 95% CI.
Abbreviations: CI, confidence interval; DLQI, Dermatology Life Quality Index; ETN, etanercept 50mg twice weekly; IXE Q2W, ixekizumab 80mg every 2 weeks; IXE Q4W, ixekizumab 80mg every 4 weeks; NRI, non-responder imputation; PASI, Psoriasis Area and Severity Index; PBO, placebo; sPGA, static Physician's Global Assessment.
Proportion of patients achieving PASI 75 (a and d), PASI 90 (b and e) and PASI 100 (c and f) from baseline through to week 12 in Latin American (a, b, and c) and UNCOVER-3 (d, e, and f) patients (non-responder imputation). Abbreviations: ETN, etanercept 50mg twice weekly; IXE Q2W, ixekizumab 80mg every 2 weeks; IXE Q4W, ixekizumab 80mg every 4 weeks; PASI, Psoriasis Area and Severity Index; PBO, placebo.
Among Latin American patients, ixekizumab Q2W showed statistically significantly greater efficacy than etanercept and placebo for PASI 90 (p<0.001 vs etanercept; p<0.001 vs placebo) and PASI 100 (p=0.001 vs etanercept; p<0.001 vs placebo) at week 12; ixekizumab Q4W showed higher response rates than placebo and etanercept for PASI 90 (p=0.023 vs etanercept; p<0.001 vs placebo) and PASI 100 (p=0.023 vs etanercept; p=0.002 vs placebo) (Table 3). At week 12, PASI 90 and PASI 100 were achieved, respectively, by 96.6% and 62.1% of patients given ixekizumab Q2W and 75.9% and 48.3% given ixekizumab Q4W, vs 0% and 0% of patients given placebo, and 46.7% and 20.0% of patients given etanercept (Table 3 and Fig. 2).
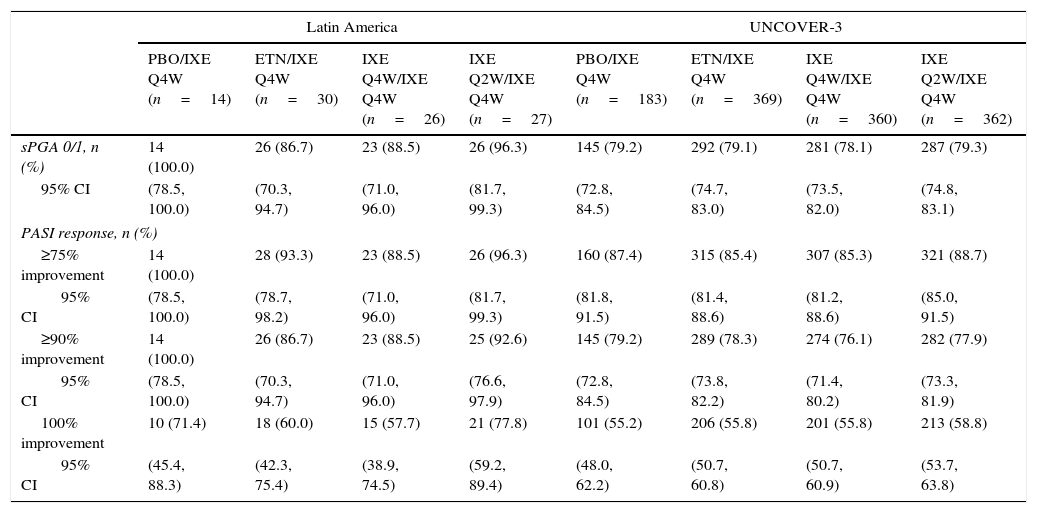
Long-term efficacyAmong Latin American patients who continued in the long-term extension period up to week 60 with ixekizumab Q4W, sPGA 0/1 and PASI 75 were maintained or achieved, respectively, by 86.7–100.0% and 88.5–100.0% among the Latin American patients and 78.1–79.3% and 85.3–88.7% among the UNCOVER-3 ITT population (Table 4).
Clinical responses at week 60.
| Latin America | UNCOVER-3 | |||||||
|---|---|---|---|---|---|---|---|---|
| PBO/IXE Q4W (n=14) | ETN/IXE Q4W (n=30) | IXE Q4W/IXE Q4W (n=26) | IXE Q2W/IXE Q4W (n=27) | PBO/IXE Q4W (n=183) | ETN/IXE Q4W (n=369) | IXE Q4W/IXE Q4W (n=360) | IXE Q2W/IXE Q4W (n=362) | |
| sPGA 0/1, n (%) | 14 (100.0) | 26 (86.7) | 23 (88.5) | 26 (96.3) | 145 (79.2) | 292 (79.1) | 281 (78.1) | 287 (79.3) |
| 95% CI | (78.5, 100.0) | (70.3, 94.7) | (71.0, 96.0) | (81.7, 99.3) | (72.8, 84.5) | (74.7, 83.0) | (73.5, 82.0) | (74.8, 83.1) |
| PASI response, n (%) | ||||||||
| ≥75% improvement | 14 (100.0) | 28 (93.3) | 23 (88.5) | 26 (96.3) | 160 (87.4) | 315 (85.4) | 307 (85.3) | 321 (88.7) |
| 95% CI | (78.5, 100.0) | (78.7, 98.2) | (71.0, 96.0) | (81.7, 99.3) | (81.8, 91.5) | (81.4, 88.6) | (81.2, 88.6) | (85.0, 91.5) |
| ≥90% improvement | 14 (100.0) | 26 (86.7) | 23 (88.5) | 25 (92.6) | 145 (79.2) | 289 (78.3) | 274 (76.1) | 282 (77.9) |
| 95% CI | (78.5, 100.0) | (70.3, 94.7) | (71.0, 96.0) | (76.6, 97.9) | (72.8, 84.5) | (73.8, 82.2) | (71.4, 80.2) | (73.3, 81.9) |
| 100% improvement | 10 (71.4) | 18 (60.0) | 15 (57.7) | 21 (77.8) | 101 (55.2) | 206 (55.8) | 201 (55.8) | 213 (58.8) |
| 95% CI | (45.4, 88.3) | (42.3, 75.4) | (38.9, 74.5) | (59.2, 89.4) | (48.0, 62.2) | (50.7, 60.8) | (50.7, 60.9) | (53.7, 63.8) |
Data are NRI. The Wilson Score method without continuity correction was used to construct the 95% CI. Statistical analyses were not performed for week 60 data.
Abbreviations: CI, confidence interval; ETN, etanercept 50mg twice weekly; IXE Q2W, ixekizumab 80mg every 2 weeks; IXE Q4W, ixekizumab 80mg every 4 weeks; NRI, non-responder imputation; PASI, Psoriasis Area and Severity Index; PBO, placebo; sPGA, static Physician's Global Assessment.
For all efficacy endpoints during the induction period and long-term extension period up to week 60 with Q4W ixekizumab, Q2W during the induction dosing period provided greater efficacy than Q4W induction dosing (Tables 3 and 4).
Quality of lifeFor Latin American patients, quality of life, as measured by the Dermatology Life Quality Index (DLQI), was improved at week 12 with greater proportions of patients given ixekizumab Q2W and Q4W achieving DLQI 0/1 compared with placebo (Table 3). For the UNCOVER-3 ITT population, quality of life was also improved at week 12 with greater proportions of patients given ixekizumab Q2W and Q4W achieving DLQI 0/1 compared with placebo and etanercept (Table 3).
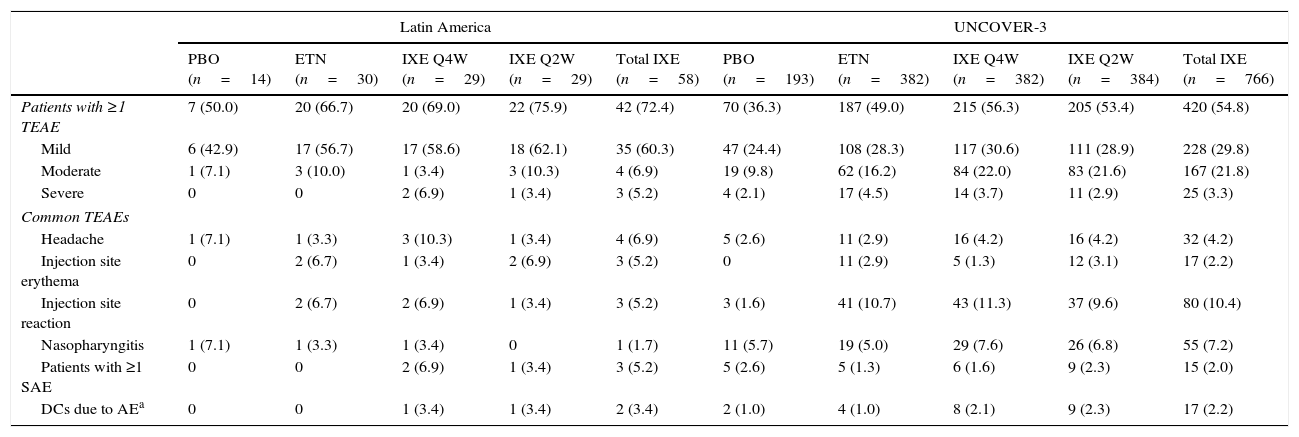
SafetyDuring the induction period, the incidence of serious adverse events for Latin American patients was greater with ixekizumab Q4W treatment compared with ixekizumab Q2W, etanercept, or placebo (Table 5). At week 60, the incidence of serious adverse events was greatest in Latin Americans who received ixekizumab Q2W during the induction dosing period and ixekizumab Q4W during the long-term extension period (Table 6). The incidence of serious adverse events was similar across treatment arms for the UNCOVER-3 safety population during both the induction and long-term extension periods (Tables 5 and 6). Treatment-emergent adverse events (TEAEs) reported for ≥5% of Latin American patients with ixekizumab exposure (N=58) up to week 12 were headache, injection site erythema, and injection site reaction (Table 5). TEAEs reported for ≥5% of the UNCOVER-3 safety population with ixekizumab exposure (N=766) up to week 12 were injection site reaction and nasopharyngitis (Table 5).
Adverse events for the 12-week induction period.
| Latin America | UNCOVER-3 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PBO (n=14) | ETN (n=30) | IXE Q4W (n=29) | IXE Q2W (n=29) | Total IXE (n=58) | PBO (n=193) | ETN (n=382) | IXE Q4W (n=382) | IXE Q2W (n=384) | Total IXE (n=766) | |
| Patients with ≥1 TEAE | 7 (50.0) | 20 (66.7) | 20 (69.0) | 22 (75.9) | 42 (72.4) | 70 (36.3) | 187 (49.0) | 215 (56.3) | 205 (53.4) | 420 (54.8) |
| Mild | 6 (42.9) | 17 (56.7) | 17 (58.6) | 18 (62.1) | 35 (60.3) | 47 (24.4) | 108 (28.3) | 117 (30.6) | 111 (28.9) | 228 (29.8) |
| Moderate | 1 (7.1) | 3 (10.0) | 1 (3.4) | 3 (10.3) | 4 (6.9) | 19 (9.8) | 62 (16.2) | 84 (22.0) | 83 (21.6) | 167 (21.8) |
| Severe | 0 | 0 | 2 (6.9) | 1 (3.4) | 3 (5.2) | 4 (2.1) | 17 (4.5) | 14 (3.7) | 11 (2.9) | 25 (3.3) |
| Common TEAEs | ||||||||||
| Headache | 1 (7.1) | 1 (3.3) | 3 (10.3) | 1 (3.4) | 4 (6.9) | 5 (2.6) | 11 (2.9) | 16 (4.2) | 16 (4.2) | 32 (4.2) |
| Injection site erythema | 0 | 2 (6.7) | 1 (3.4) | 2 (6.9) | 3 (5.2) | 0 | 11 (2.9) | 5 (1.3) | 12 (3.1) | 17 (2.2) |
| Injection site reaction | 0 | 2 (6.7) | 2 (6.9) | 1 (3.4) | 3 (5.2) | 3 (1.6) | 41 (10.7) | 43 (11.3) | 37 (9.6) | 80 (10.4) |
| Nasopharyngitis | 1 (7.1) | 1 (3.3) | 1 (3.4) | 0 | 1 (1.7) | 11 (5.7) | 19 (5.0) | 29 (7.6) | 26 (6.8) | 55 (7.2) |
| Patients with ≥1 SAE | 0 | 0 | 2 (6.9) | 1 (3.4) | 3 (5.2) | 5 (2.6) | 5 (1.3) | 6 (1.6) | 9 (2.3) | 15 (2.0) |
| DCs due to AEa | 0 | 0 | 1 (3.4) | 1 (3.4) | 2 (3.4) | 2 (1.0) | 4 (1.0) | 8 (2.1) | 9 (2.3) | 17 (2.2) |
Data is n (%).
Adverse events for the long-term extension period up to week 60.
| Latin America | UNCOVER-3 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PBO/IXE Q4W (n=14) | ETN/IXE Q4W (n=30) | IXE Q4W/IXE Q4W (n=26) | IXE Q2W/IXE Q4W (n=27) | Total (n=97) | PBO/IXE Q4W (n=183) | ETN/IXE Q4W (n=369) | IXE Q4W/IXE Q4W (n=360) | IXE Q2W/IXE Q4W (n=362) | Total (n=1274) | |
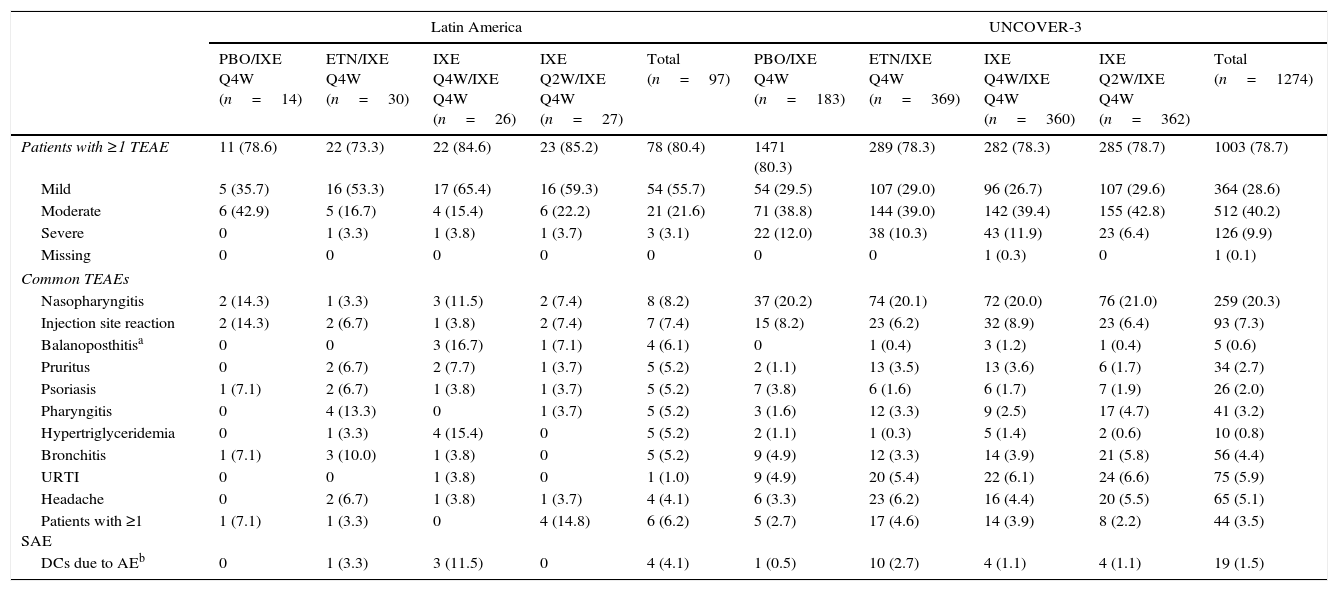
| Patients with ≥1 TEAE | 11 (78.6) | 22 (73.3) | 22 (84.6) | 23 (85.2) | 78 (80.4) | 1471 (80.3) | 289 (78.3) | 282 (78.3) | 285 (78.7) | 1003 (78.7) |
| Mild | 5 (35.7) | 16 (53.3) | 17 (65.4) | 16 (59.3) | 54 (55.7) | 54 (29.5) | 107 (29.0) | 96 (26.7) | 107 (29.6) | 364 (28.6) |
| Moderate | 6 (42.9) | 5 (16.7) | 4 (15.4) | 6 (22.2) | 21 (21.6) | 71 (38.8) | 144 (39.0) | 142 (39.4) | 155 (42.8) | 512 (40.2) |
| Severe | 0 | 1 (3.3) | 1 (3.8) | 1 (3.7) | 3 (3.1) | 22 (12.0) | 38 (10.3) | 43 (11.9) | 23 (6.4) | 126 (9.9) |
| Missing | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (0.3) | 0 | 1 (0.1) |
| Common TEAEs | ||||||||||
| Nasopharyngitis | 2 (14.3) | 1 (3.3) | 3 (11.5) | 2 (7.4) | 8 (8.2) | 37 (20.2) | 74 (20.1) | 72 (20.0) | 76 (21.0) | 259 (20.3) |
| Injection site reaction | 2 (14.3) | 2 (6.7) | 1 (3.8) | 2 (7.4) | 7 (7.4) | 15 (8.2) | 23 (6.2) | 32 (8.9) | 23 (6.4) | 93 (7.3) |
| Balanoposthitisa | 0 | 0 | 3 (16.7) | 1 (7.1) | 4 (6.1) | 0 | 1 (0.4) | 3 (1.2) | 1 (0.4) | 5 (0.6) |
| Pruritus | 0 | 2 (6.7) | 2 (7.7) | 1 (3.7) | 5 (5.2) | 2 (1.1) | 13 (3.5) | 13 (3.6) | 6 (1.7) | 34 (2.7) |
| Psoriasis | 1 (7.1) | 2 (6.7) | 1 (3.8) | 1 (3.7) | 5 (5.2) | 7 (3.8) | 6 (1.6) | 6 (1.7) | 7 (1.9) | 26 (2.0) |
| Pharyngitis | 0 | 4 (13.3) | 0 | 1 (3.7) | 5 (5.2) | 3 (1.6) | 12 (3.3) | 9 (2.5) | 17 (4.7) | 41 (3.2) |
| Hypertriglyceridemia | 0 | 1 (3.3) | 4 (15.4) | 0 | 5 (5.2) | 2 (1.1) | 1 (0.3) | 5 (1.4) | 2 (0.6) | 10 (0.8) |
| Bronchitis | 1 (7.1) | 3 (10.0) | 1 (3.8) | 0 | 5 (5.2) | 9 (4.9) | 12 (3.3) | 14 (3.9) | 21 (5.8) | 56 (4.4) |
| URTI | 0 | 0 | 1 (3.8) | 0 | 1 (1.0) | 9 (4.9) | 20 (5.4) | 22 (6.1) | 24 (6.6) | 75 (5.9) |
| Headache | 0 | 2 (6.7) | 1 (3.8) | 1 (3.7) | 4 (4.1) | 6 (3.3) | 23 (6.2) | 16 (4.4) | 20 (5.5) | 65 (5.1) |
| Patients with ≥1 SAE | 1 (7.1) | 1 (3.3) | 0 | 4 (14.8) | 6 (6.2) | 5 (2.7) | 17 (4.6) | 14 (3.9) | 8 (2.2) | 44 (3.5) |
| DCs due to AEb | 0 | 1 (3.3) | 3 (11.5) | 0 | 4 (4.1) | 1 (0.5) | 10 (2.7) | 4 (1.1) | 4 (1.1) | 19 (1.5) |
Data is n (%).
Including death.
Abbreviations: AE, adverse event; DCs, discontinuations; ETN, etanercept 50mg twice weekly; IXE Q2W, ixekizumab 80mg every 2 weeks; IXE Q4W, ixekizumab 80mg every 4 weeks; PBO, placebo; SAE, serious adverse event; TEAE, treatment-emergent adverse event; URTI, upper respiratory tract infection.
For Latin American patients, 2 patients discontinued due to adverse events during the induction period with ixekizumab treatment (Table 5). During the long-term extension period, discontinuations due to adverse events were greater for individuals who received ixekizumab Q4W during the induction period compared with ixekizumab Q2W, etanercept, or placebo treatment (Table 6). In the UNCOVER-3 safety population, discontinuations due to adverse events were similar with ixekizumab Q2W and Q4W treatment, which were greater than those for placebo and etanercept treatment (Table 5). During the long-term extension period, discontinuations due to adverse events were fewer with ixekizumab treatment during the induction period compared with etanercept treatment (Table 6). There were no deaths reported during the induction period. One death was reported during the long-term extension period with ixekizumab Q2W/Q4W treatment. The event was independently adjudicated by external consultants, who concluded that the cause of death was a cardiovascular cerebrovascular event and was not related to ixekizumab.
Among Latin American patients who continued in the long-term extension period up to week 60 (N=97), the total TEAEs reported by ≥5% of the patients were nasopharyngitis, injection site reaction, balanoposthitis, pruritus, psoriasis, pharyngitis, hypertriglyceridemia, and bronchitis (Table 6). Among UNCOVER-3 patients (N=1274), the total TEAEs reported by ≥5% of the patients included nasopharyngitis, injection site reaction, upper respiratory tract infection, and headache (Table 6).
There were no cases of active tuberculosis for Latin American patients or the UNCOVER-3 population during the induction or long-term extension period up to week 60. For Latin American patients, there were no adverse events of hepatitis in the induction period. For the long-term extension period, 1 hepatitis event was reported which was mild in severity and resolved within a month.
DiscussionIn this subgroup analysis of the phase 3 UNCOVER-3 study, ixekizumab Q2W and Q4W, compared with placebo and etanercept, provided Latin American patients with moderate-to-severe psoriasis significant resolution of plaque psoriasis. At week 12, ixekizumab Q2W demonstrated higher rates than etanercept for achieving PASI 90 and PASI 100. Rapid onset efficacy with ixekizumab was noted: Latin American patients given ixekizumab Q2W or Q4W had significant differences in PASI 75 compared with those given etanercept as early as week 2. Long-term treatment with ixekizumab Q4W for up to 60 weeks continued to provide high resolution of plaques. The safety profile of ixekizumab was comparable in Latin American patients and the UNCOVER-3 safety population. Furthermore, there was no reactivation of tuberculosis, which is a particular safety concern within Latin America.
The results of this subgroup analysis support the notion, reported by other studies, that the general efficacy profile of anti-psoriasis treatment in Latin American patients is comparable to that of the general global psoriasis population. A subgroup analysis of an open-label, single-arm study confirmed the efficacy and safety of efalizumab in a Latin American population with moderate-to-severe psoriasis.25 In another subgroup analysis, the randomized, double-blind PRISTINE study showed that response rates to etanercept for patients with moderate-to-severe psoriasis from Asia, Central Europe, and Latin America were similar to those observed in the overall study population.26 Subgroup analysis in the longitudinal prospective international observational PSOLAR study showed that living in Latin America or Europe (vs North America) was significantly associated with achieving a Physician's Global Assessment score of 0/1 and improving DLQI with biological psoriasis therapy.27 Collectively, these studies suggest that, although genetic factors may contribute to the development of psoriasis in different populations, Latin American patients seem to respond similarly to treatment compared to general psoriasis study populations.
The observed safety profile for ixekizumab in Latin American patients during the induction period was consistent with its mechanism of action and the previously demonstrated safety profile for ixekizumab.16,23,24 The overall safety profile in Latin American patients was comparable to that in the UNCOVER-1, -2, and -3 populations.23,24 Similarly to the UNCOVER-2 and -3 populations,23 overall rates of TEAEs and infections for Latin American patients were more frequent in patients given ixekizumab than in those given etanercept or placebo. Although injection site reactions were among the most frequently reported adverse reactions among Latin American patients receiving ixekizumab at week 12, occurrences were similar for patients receiving etanercept and the majority were mild or moderate in severity and did not lead to discontinuation. Among Latin American patients who continued treatment with ixekizumab Q4W during the long-term extension period, nasopharyngitis and injection site reaction were the most common any-grade TEAEs reported, although overall they were mild to moderate, treatable, and did not lead to discontinuation of study drug. No cases of active tuberculosis occurred during the study.
The limitations of this subgroup analysis are that the study was neither designed nor powered to show significance in subgroups, such as ethnicity subgroups, and subgroups comprised relatively small sample sizes, thereby making accurate inferences challenging. Hence, these results should be interpreted with caution. Despite these limitations, this subgroup analysis suggests that both ixekizumab dosing regimens were effective for treating moderate-to-severe psoriasis in Latin American patients and that long-term treatment with ixekizumab Q4W results in high clearance of moderate-to-severe psoriasis with a tolerable safety profile.
FundingThis study was supported by Eli Lilly and Company.
Conflicts of interestsFernando Valenzuela was principal investigator for the UNCOVER-3 trial and has participated in advisory boards for AbbVie, Novartis, and Pfizer. Claudia de la Cruz Fernandez was principal investigator for the UNCOVER-3 trial and has participated in advisory boards for AbbVie, Janssen, Novartis, and Pfizer and received research support from AbbVie, Eli Lilly and Company, and Pfizer. Ricardo Luis Galimberti is a senior researcher at Novartis, Eli Lilly and Company, and AbbVie. Livia Goncalves is a full-time employee of Eli Lilly and Company. Sirel Gürbüz is a full-time employee of Eli Lilly and Company and is a minor owner of Lilly stocks and stock options. Ricardo Romiti has participated in advisory boards or as a consultant or speaker for, AbbVie, Galderma, Janssen, LEO Pharma, Eli Lilly and Company, MSD, Novartis, Pfizer and UCB Pharma. Missy McKean-Matthews has no conflict of interest.
Ethical responsibilitiesProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
We thank the patients, their families, the study sites, and the study personnel who participated in this clinical trial. This study was sponsored by Eli Lilly and Company.
Medical writing support was provided by Andrew Sakko and Prue Stanford, and editorial support was provided by Teri Tucker, of inVentiv Health Clinical, and funded by Eli Lilly and Company.