There have been reports of paradoxical induction or worsening of psoriasis during treatment with tumor necrosis factor (TNF) α agents (infliximab, etanercept, adalimumab, and certolizumab). It has been hypothesized that an imbalance between TNF-α and interferon α might have a role in the etiology and pathogenesis of these reactions. Paradoxical psoriasiform reactions can be divided clinically into de novo psoriasis and exacerbation of preexisting psoriasis. The first, which is more common and more extensively described in the literature, occurs in patients without a history of psoriasis who are receiving TNF-α therapy for another inflammatory disorder. The second can occur with or without changes in the morphology of the lesions. In this article, we review the literature on the clinical and histologic features of paradoxical psoriasiform reactions, analyze their clinical course and treatment, and propose a clinical management model for use in routine practice.
Paradójicamente se han descrito casos de inducción o empeoramiento de una psoriasis durante el tratamiento con todos los agentes anti-factor de necrosis tumoral α (anti-TNFα) (infliximab, etanercept, adalimumab y certolizumab). Se ha postulado que la alteración del equilibrio entre el TNFα y el interferón α estaría implicada en su etiopatogenia. Clínicamente se distinguen varios patrones de reacciones psoriasiformes paradójicas: la psoriasis de novo en pacientes que no han presentado anteriormente esta enfermedad y que reciben este tratamiento por otra enfermedad inflamatoria, que es la más frecuente y la mejor descrita, y la exacerbación de una psoriasis preexistente durante la terapia anti-TNFα, que puede presentarse con o sin un cambio de morfología. En este trabajo realizamos una revisión de la literatura en relación con las características clínicas e histológicas de este tipo de reacciones, así como de su evolución y tratamiento, y planteamos un esquema de manejo en la práctica clínica.
Psoriasis, a chronic inflammatory disease of unknown etiology, affects approximately 2% to 3% of the population worldwide.1 It has been speculated that it could be the result of a combination of genetic and environmental factors; a genetic predisposition does exist, but it has not been possible to establish a classic Mendelian pattern of inheritance.
Numerous factors that trigger the onset of psoriasis or aggravate established psoriasis have been described, including infections, stress, and drugs (β-blockers, lithium, …).1,2
Several hypotheses and models have been proposed to try to explain the pathogenesis of psoriasis; these have led us to look not only at the skin, but also at the multiple associated comorbid conditions, such as psoriatic arthritis and cardiovascular disease.3,4 A unifying hypothesis is the model of the cytokine network, which states that both external stimuli, such as stress, and endogenous ones, such as viruses, neuropeptides, or drug ingestion, can act as triggers that activate a cytokine cascade. These cytokines include tumor necrosis factor (TNF) α, derived from antigen-presenting dendritic cells and keratinocytes, and interferon (IFN) γ produced by activated type 1 helper T cells.1
Around 90% of individuals with psoriasis have the most common form, the so-called plaque psoriasis or psoriasis vulgaris. In the majority of cases, the disease is mild and can be controlled with topical therapy. However, up to a third of patients develop moderate or severe psoriasis and require systemic therapy, such as phototherapy, acitretin, methotrexate, or ciclosporin.5 The toxicity of these drugs and the frequent lack of response to them has led to the appearance over the past 15 years of the so-called biologic therapies, which act at different levels of the inflammatory cascade that gives rise to the plaques of psoriasis.
The use of biologic therapy is increasing worldwide for the treatment not only of psoriasis, but also of other chronic immune-mediated inflammatory diseases, such as rheumatoid arthritis, ankylosing spondylitis, and inflammatory bowel disease. At the present time, the most widely employed drugs are those that inhibit TNF-α (infliximab, etanercept, and adalimumab), though a number of side effects have been reported during their use, including infections, reactivation of latent tuberculosis, demyelinating diseases, and congestive heart failure.6–8
It has been reported that the cutaneous side effects of anti-TNF-α therapy are more prevalent than was previously thought.9 These effects include reactions at the site of infusion or injection, skin infections, eczema, and even psoriasis or psoriasiform reactions.10 Though paradoxical (as these drugs are of demonstrated efficacy in the treatment of psoriasis11–17), case reports and some case series describe patients with exacerbations of their psoriasis or even the new onset of distinct subtypes of psoriasis during anti-TNF-α therapy.9,10,18–73 The most relevant articles and reviews are summarized in Tables 1 and 2.
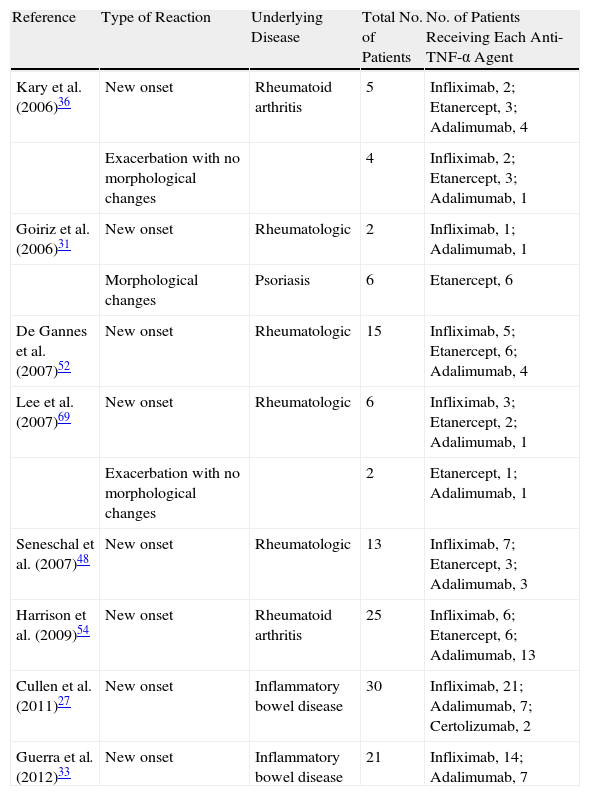
Review of the Literature: Articles With at Least 8 Patients who Developed Paradoxical Psoriasiform Reactions During Treatment With Tumor Necrosis Factor α Inhibitors.
| Reference | Type of Reaction | Underlying Disease | Total No. of Patients | No. of Patients Receiving Each Anti-TNF-α Agent |
| Kary et al. (2006)36 | New onset | Rheumatoid arthritis | 5 | Infliximab, 2; Etanercept, 3; Adalimumab, 4 |
| Exacerbation with no morphological changes | 4 | Infliximab, 2; Etanercept, 3; Adalimumab, 1 | ||
| Goiriz et al. (2006)31 | New onset | Rheumatologic | 2 | Infliximab, 1; Adalimumab, 1 |
| Morphological changes | Psoriasis | 6 | Etanercept, 6 | |
| De Gannes et al. (2007)52 | New onset | Rheumatologic | 15 | Infliximab, 5; Etanercept, 6; Adalimumab, 4 |
| Lee et al. (2007)69 | New onset | Rheumatologic | 6 | Infliximab, 3; Etanercept, 2; Adalimumab, 1 |
| Exacerbation with no morphological changes | 2 | Etanercept, 1; Adalimumab, 1 | ||
| Seneschal et al. (2007)48 | New onset | Rheumatologic | 13 | Infliximab, 7; Etanercept, 3; Adalimumab, 3 |
| Harrison et al. (2009)54 | New onset | Rheumatoid arthritis | 25 | Infliximab, 6; Etanercept, 6; Adalimumab, 13 |
| Cullen et al. (2011)27 | New onset | Inflammatory bowel disease | 30 | Infliximab, 21; Adalimumab, 7; Certolizumab, 2 |
| Guerra et al. (2012)33 | New onset | Inflammatory bowel disease | 21 | Infliximab, 14; Adalimumab, 7 |
Abbreviation: TNF, tumor necrosis factor.
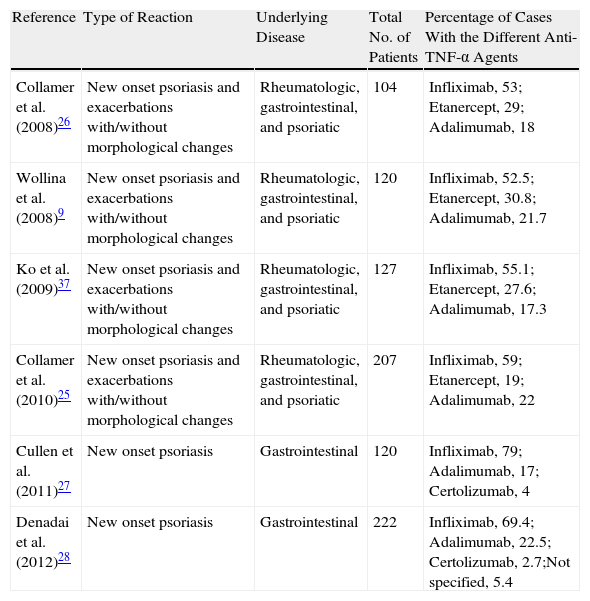
Most Relevant Revisions Published in the Literature.
| Reference | Type of Reaction | Underlying Disease | Total No. of Patients | Percentage of Cases With the Different Anti-TNF-α Agents |
| Collamer et al. (2008)26 | New onset psoriasis and exacerbations with/without morphological changes | Rheumatologic, gastrointestinal, and psoriatic | 104 | Infliximab, 53; Etanercept, 29; Adalimumab, 18 |
| Wollina et al. (2008)9 | New onset psoriasis and exacerbations with/without morphological changes | Rheumatologic, gastrointestinal, and psoriatic | 120 | Infliximab, 52.5; Etanercept, 30.8; Adalimumab, 21.7 |
| Ko et al. (2009)37 | New onset psoriasis and exacerbations with/without morphological changes | Rheumatologic, gastrointestinal, and psoriatic | 127 | Infliximab, 55.1; Etanercept, 27.6; Adalimumab, 17.3 |
| Collamer et al. (2010)25 | New onset psoriasis and exacerbations with/without morphological changes | Rheumatologic, gastrointestinal, and psoriatic | 207 | Infliximab, 59; Etanercept, 19; Adalimumab, 22 |
| Cullen et al. (2011)27 | New onset psoriasis | Gastrointestinal | 120 | Infliximab, 79; Adalimumab, 17; Certolizumab, 4 |
| Denadai et al. (2012)28 | New onset psoriasis | Gastrointestinal | 222 | Infliximab, 69.4; Adalimumab, 22.5; Certolizumab, 2.7;Not specified, 5.4 |
Abbreviation: TNF, tumor necrosis factor.
Moustou et al.10 published a literature review in which they established the strength of the association between published side effects and the use of 1 or more anti-TNF-α agents, classifying the association as poor, moderate, strong, or definitive. The authors used descriptions of the side effects in meta-analyses, randomized trials, retrospective or prospective studies, case series, and case reports. They analyzed the number of anti-TNF-α agents implicated, the number of distinct inflammatory diseases in which the side effect had occurred during treatment with anti-TNF-α drugs, and the clinical course after withdrawal and reintroduction of the drug. The authors not only described the clinical manifestations of these cutaneous reactions, but they also established that there was a strong relationship between anti-TNF-α therapy and new onset psoriasis or psoriasiform reactions.
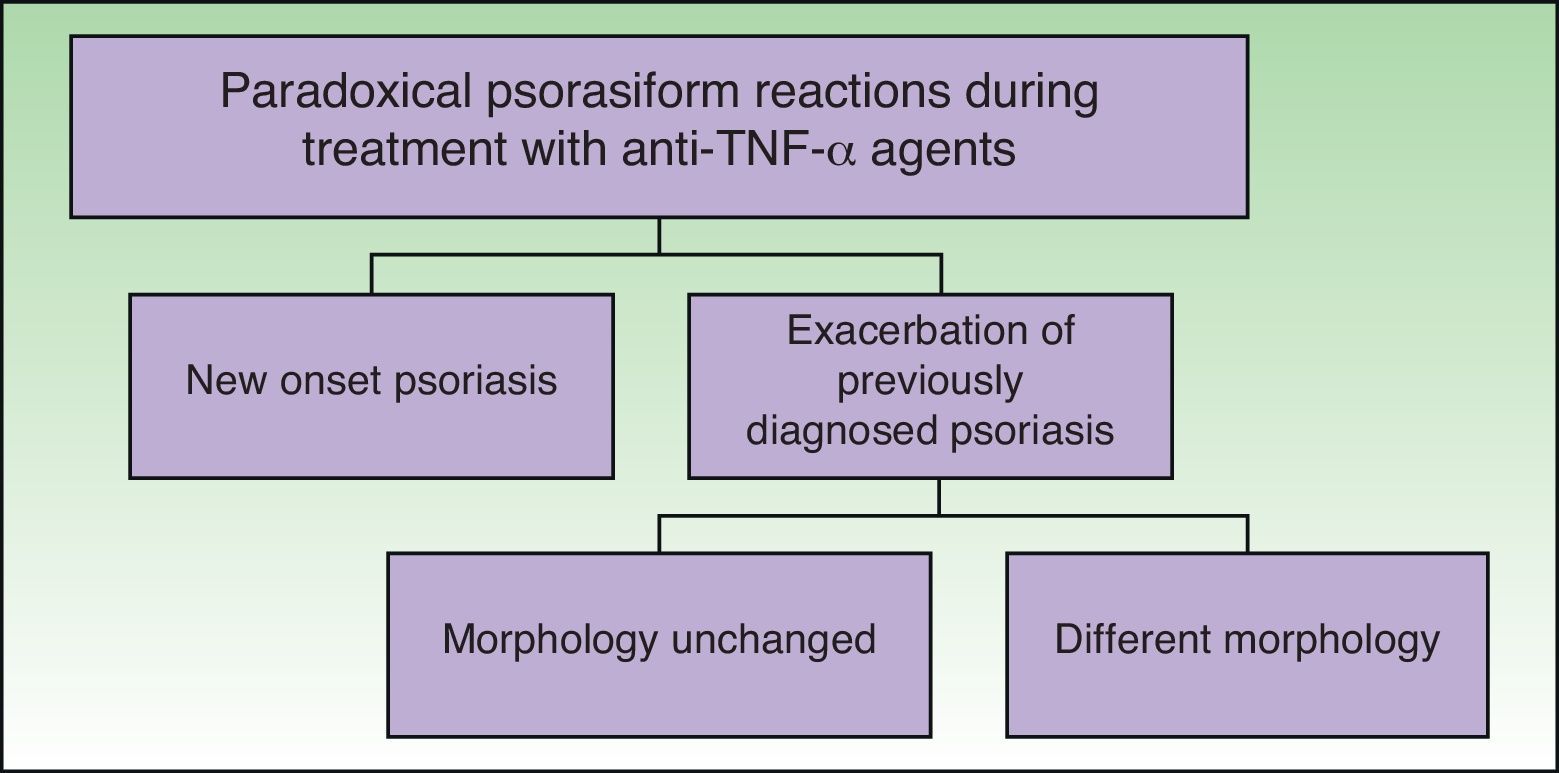
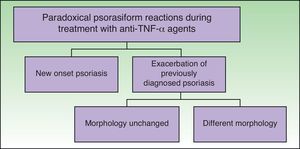
ClassificationDistinct patterns of paradoxical psoriasiform reactions can be distinguished during treatment with TNF-α-inhibitors (Fig. 1):
- -
The induction of new onset psoriasis, which is the appearance of psoriasis lesions in patients who have not previously been diagnosed with this disease and who are receiving anti-TNF-α treatment for another inflammatory disease.
- -
An exacerbation of pre-existing psoriasis, with or without morphologic differences, during anti-TNF-α therapy.
The pathophysiology of the induction or exacerbation of psoriasis during treatment with TNF-α inhibitors is still unknown. A number of theories have been proposed, such as a disruption of the balance between TNF-α and IFN-α, activation of self-reactive T lymphocytes, wrong diagnosis, natural course of the primary disease, or infections that trigger such reactions.9,10,25,37 In their article on the pathogenesis of psoriasiform reactions, Collamer et al.25 explained that a disruption of cytokine balance could lead to increased IFN-α production by dendritic cells in genetically predisposed individuals, and that genetic polymorphisms could play a role in this paradoxical reaction secondary to TNF-α blockade.
De Gannes et al.,52 in an article published in 2007, demonstrated that patients who developed new onset psoriasis during treatment with anti-TNF-α agents presented elevated expression of antimyxovirus-resistance protein A (MxA), an adenosine triphosphatase that is selectively induced in response to type 1 IFN and could be used as a surrogate marker of lesional type 1 IFN activity. In that study, staining for MxA in biopsies from patients receiving treatment with anti-TNF was more intense than in the biopsies from patients with psoriasis vulgaris not associated with anti-TNF-α therapy. Such an increase would favor the formation of psoriasis lesions in predisposed individuals. Subsequently, Seneschal et al.48 described 13 patients who developed distinct subtypes of psoriasis; biopsies from those patients revealed increased production of MxA protein compared with biopsies from healthy skin or from noninduced psoriasis lesions. Those authors concluded that increased IFN-α levels associated with cytokine imbalance (both situations caused by TNF-α inhibition) could play an important role in the appearance of this type of reaction, which they considered to be a drug reaction rather than true psoriasis.
However, another study found no differences between induced and noninduced psoriasis on immunohistochemical analysis with staining for the mRNA of IFN and TNF-α and for vascular endothelial growth factor.53
Some authors believe that there must be a genetic predisposition, as the majority of patients receiving anti-TNF-α therapy for a wide range of diseases do not develop psoriasis. Some environmental factor may also be exerting an effect, as the latency between the initiation of treatment and the appearance of psoriasis is very variable (from days to years).25,27,37
This wide variability makes it difficult to establish a causal relationship in some cases. It is therefore important to exclude any other factor that could trigger the appearance of psoriasis, such as infection, trauma, stress, or new drugs.44
According to some authors, paradoxical psoriasiform reactions are the result of a class effect, as cases have been reported with all 3 classic TNF-α inhibitors (infliximab, etanercept, and adalimumab), in frequencies proportional to the prevalence of their use.37 More recently, the phenomenon has been described in patients treated with certolizumab, a newer anti-TNF-α agent. In the literature, we have found a review article describing 6 cases related to this drug in patients with inflammatory bowel disease, in addition to another case from our own records.27 Recurrence after the administration of a distinct anti-TNF-α drug supports the class effect theory.25,27,33,36,37,55
Reports have also been published of cases of new onset psoriasis or exacerbations during treatment with psoriasis drugs other than the TNF-α inhibitors, including drugs such as efalizumab, ciclosporin, and anthralin, as well as after phototherapy or glucocorticoid withdrawal.55 Furthermore, other biologic agents have been implicated, such as abatacept in a patient who developed guttate psoriasis during treatment with etanercept and, 2 years later, a further episode with the same morphology during treatment with abatacept.70 For Rallis et al.44 this suggests a gap in our understanding of the pathophysiology of psoriasis and of the exact mechanism by which some of these drugs act.44
New Onset PsoriasisThe large majority of published cases refer to new onset psoriasis, particularly in patients with rheumatologic diseases. Based on published studies, it has been established that the prevalence of psoriasis during anti-TNF-α therapy is between 0.6% and 5.3%, depending on differences between the populations studied and treated.27,54 In a recent article on psoriasis induced during treatment with anti-TNF-α drugs for inflammatory bowel disease, the reported prevalence was 1.62%.33
Data coming from the larger series and from literature reviews are not particularly homogeneous. This, and the fact that not all cases will have been published, could lead to biased conclusions. In a recent review article that included 207 cases (after excluding patients with any known trigger), it was reported that 85% of patients had developed new onset psoriasis.25
There is a female predominance (2 women to 1 man), due to cases associated with rheumatoid arthritis and inflammatory bowel disease.25,37 Series of patients with rheumatoid arthritis show the greatest difference, up to 5.3 women to 1 man.54 When all cases are included, the mean age at onset is 44.9 years (range, 13-78 years)25; however, the mean age among patients with gastrointestinal disease is lower (30 years).27
Looking at all cases that were fully reviewed, the drug most commonly implicated was infliximab, accounting for 55.1% to 59% cases.25,37 In their review of the literature, Ko et al.37 identified 127 cases reported up to September 2007. Those authors stated that the second most common drug was etanercept (27.6%) and, finally, adalimumab (17.3%). However, in a more recent series by Collamer et al.,25 22% of all cases were on treatment with adalimumab and 17% with etanercept.
Cullen et al.27 performed a review of 120 cases, including 30 cases of their own, with the additional inclusion criterion of a history of inflammatory bowel disease. Their results indicated that 79% had received infliximab, 17% adalimumab, and 4% the new drug, certolizumab; it must be taken into account that etanercept is not approved for use in inflammatory bowel disease.
Those percentages were proportional to the use of each one of the drugs. In contrast, in a series of patients from the British Society for Rheumatology Biologics Register, with a description of 25 cases of new onset psoriasis in a cohort of 9826 patients with AR, 13 were on treatment with adalimumab.54 That group of 25 patients was compared with another group formed of patients receiving treatment with disease-modifying antirheumatic drugs; none of the patients in the latter group developed psoriasis after a follow-up of 2.81 years.
The latency is very variable, from days to 80 months, with a mean of 10.5 months. In the review by Ko et al.,37 the latency was shorter in patients treated with etanercept, although 42.9% were exacerbations of previously known psoriasis rather than new onset psoriasis. Other authors, such as Chen et al.,24 who published a review of cases arising with etanercept, have also reported a shorter latency with this drug. In the 25 patients included in the review by those authors, the mean latency was 3.5 months after the initiation of treatment.
More than half of published cases have occurred in patients with rheumatologic diseases, particularly rheumatoid arthritis, which accounts for 42.5% to 50.4% of cases, depending on the study.25,37
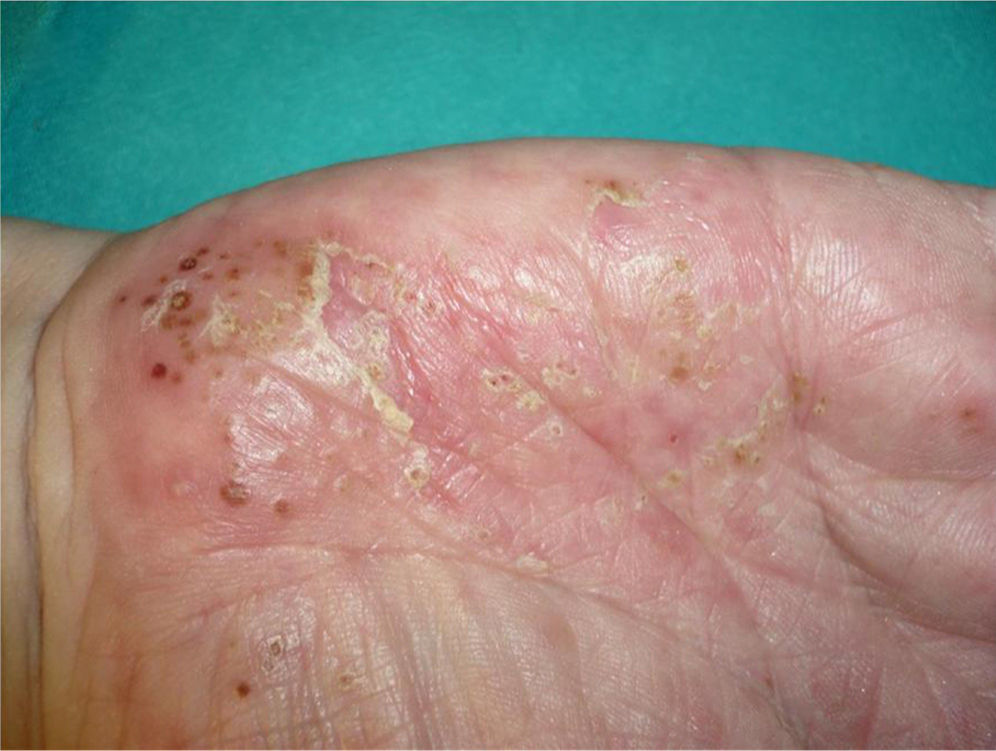
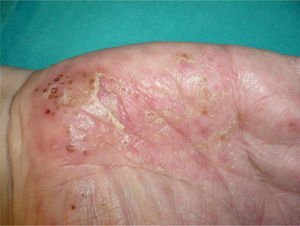
Overall, the cases reported have been of any type of psoriasis and at any site: vulgaris, guttatate, inverse, or generalized or palmoplantar pustulosis (Fig. 2); the latter subtype was the most common form in the larger series and reviews.9,25,37 Adalimumab has been reported to be the drug most frequently implicated in palmoplantar pustulosis, and more than half of the paradoxical reactions described with this drug are of this subtype. However, in the article by Ko et al.,37 patients treated with etanercept were more likely to develop plaque psoriasis, though this finding is probably not evaluable because, as commented above, the events in that study were exacerbations in almost half of cases.
The fact that the incidence of palmoplantar pustulosis in cases of psoriasis induced by TNF-α inhibitors is twice the incidence in the general population with psoriasis could suggest greater TNF-α expression in the eccrine glands of susceptible individuals.67
In a review that only included patients with inflammatory bowel disease, the authors stated that these patients most commonly presented involvement of the palms and soles (43%) and of the scalp (42%).27 However, in a recent series from Spain including 21 patients with inflammatory bowel disease, the most common form was plaque psoriasis, predominantly affecting the limbs (62%), though the trunk and the scalp were also affected.33
Between 36% and 100% of patients, depending on the series, were receiving other psoriasis treatments concomitantly, including methotrexate and azathioprine, which have thus not been shown to be capable of preventing this type of reaction.25,27,33
The treatment most frequently used for this side effect has been topical therapy with corticosteroids, employed in almost 40% of cases according to the review by Ko et al.,37 with no other changes in treatment. Resolution of the flare of psoriasis was reported in a quarter of patients. Topical therapy associated with withdrawal of the implicated anti-TNF-α agent was employed in a further 50%. The use of systemic psoriasis treatments, with or without interruption of the implicated agent, was more successful than the topical treatments (44% achieved resolution of the lesions). Only 15% of the patients who were changed to an alternative anti-TNF-α agent achieved a satisfactory response, while this figure rose to 64% among those who received treatments with a different mechanism of action. Those authors therefore considered that the essential action was to discontinue the anti-TNF-α agent. In mild or moderate cases, they recommended topical treatments or phototherapy in monotherapy or in combination with acitretin, depending on the response, whereas ciclosporin and methotrexate could be of benefit in acute or serious cases. In those cases in which the skin lesions did not resolve, no differences were found with regard to severity of the episode or type or distribution of the psoriasis compared with patients in whom the lesions resolved.
In the review by Cullen et al.27 of patients with inflammatory bowel disease, approximately 50% responded to topical therapy, as also found in the study by Collamer et al.,25 in which the majority of patients responded to conservative treatment, without withdrawal of the implicated drug. In some patients with rheumatoid arthritis who developed psoriasis, whether of new onset or an exacerbation, interruption or dose reduction of the implicated drug caused the skin lesions to improve or disappear.36
The more recent incorporation of other drugs with distinct mechanisms of action, such as ustekinumab, an anti-p40 agent, broadens the spectrum of treatments for this type of reaction. Spanish authors described a patient with Crohn disease who developed psoriasis with 2 anti-TNF-α drugs, infliximab and adalimumab. Ustekinumab, at a dose of 90mg every 8 weeks, achieved remission of the skin lesions, while control of the gastrointestinal disease was maintained with azathioprine and mesalazine.47 In contrast, there has also been a case report in which, despite this treatment, an episode of palmoplantar pustulosis did not resolve or even got worse.46 More recently, Puig et al.43 reported their experience in another patient with psoriatic arthritis and performed a review of cases treated with ustekinumab. Those authors concluded that this drug could be a good therapeutic option in patients who develop paradoxical reactions with TNF-α inhibitors.
Exacerbation of Previous PsoriasisAlthough exacerbations of previous psoriasis, with or without changes of morphology, have been more common in the daily clinical practice of the authors, exacerbations only accounted for 15% of cases included in the review of 207 patients by Collamer et al.25 It is thus difficult to establish the characteristics of this type of reaction. Comparison of these patients with those with new onset psoriasis shows no significant difference according to sex; the agent most frequently implicated has been etanercept (62%), followed by the monoclonal antibodies (infliximab [23%] and adalimumab [15%]). Further studies are needed to clarify whether this difference is significant or arises because etanercept is the most widely used agent in dermatology. Collamer et al. considered that patients with psoriasis and psoriatic arthritis tended to develop lesions that differed from their previous psoriasis, most commonly presenting as guttate psoriasis. In 38% of such patients, the lesions resolved after discontinuation of the implicated agent and complete or partial remission was achieved in 53% while maintaining the implicated agent and adding other psoriasis treatments. The data on recurrence with other anti-TNF-α drugs are not evaluable jointly due to the heterogeneity of the patients and the short follow-up in some cases, and we will therefore discuss each subtype separately.
Exacerbation of Previous Psoriasis With No Change in MorphologyIn 2010, Mourao et al.55 published a review of 5 previously reported cases and added a further 3 cases to describe the exacerbation of previous psoriasis during treatment with an anti-TNF-α agent. In 1 of the patients, etanercept lost efficacy after 41 months and the skin disease subsequently worsened during treatment with adalimumab and infliximab. The latency period of these exacerbations has been very variable, with reports of 2 weeks to 32months after the start of exposure. There were no clear triggering factors.
The possibility of reappearance of the skin lesions has also been described in patients with latent psoriasis who receive treatment with anti-TNF-α therapy to control rheumatoid arthritis. Kary et al.36 described 3 patients, of whom 2 developed an episode of pustular psoriasis and the other of plaque psoriasis. In the opinion of those authors, the clinical improvement after withdrawal or dose reduction of the implicated drug supports the relationship with the drug.
In some patients with psoriatic arthritis, the skin lesions have deteriorated while the arthritis has responded adequately to treatment.55
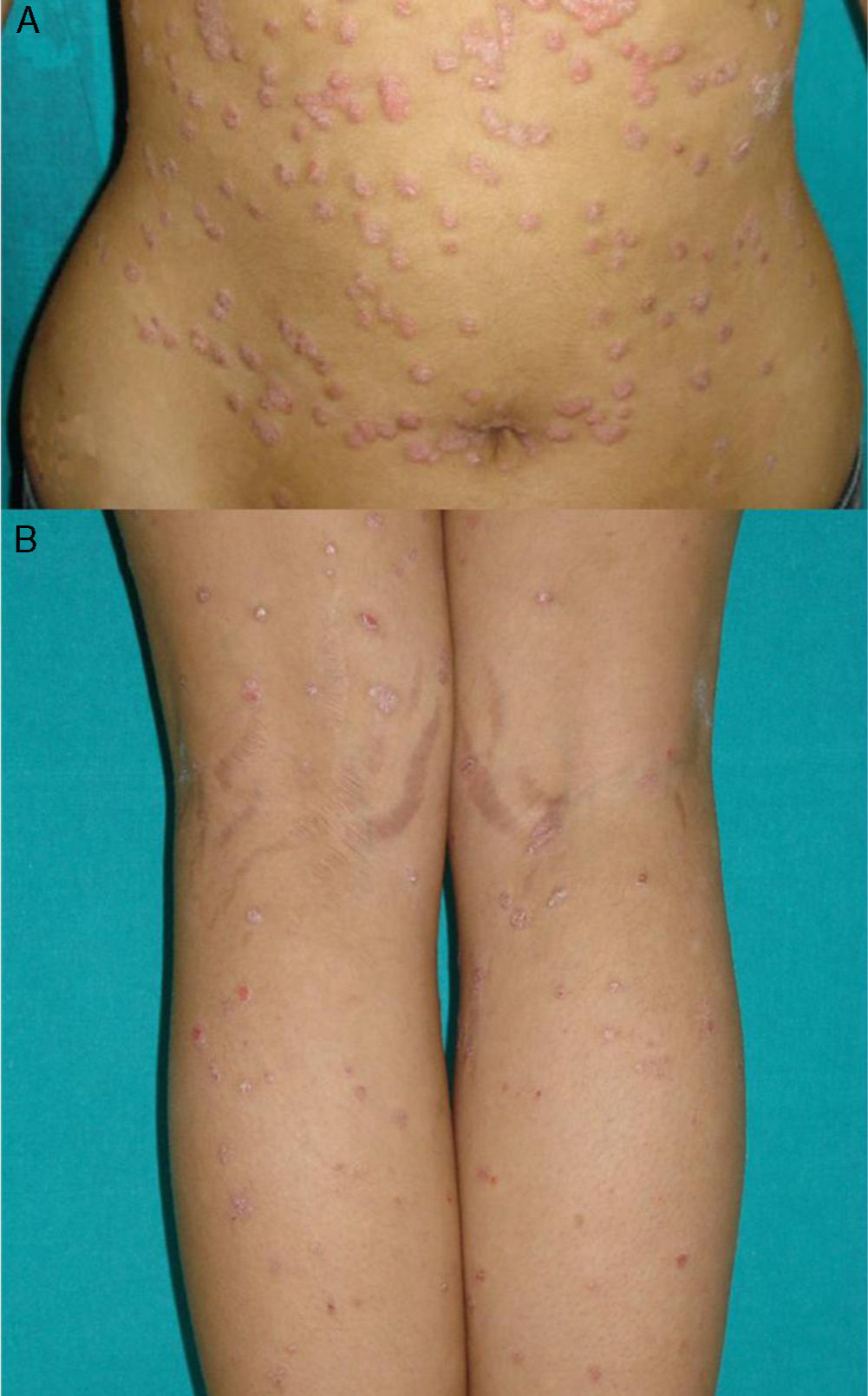
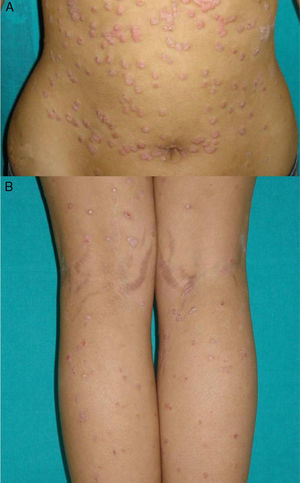
Exacerbation of Previous Psoriasis With a Change in MorphologyFew data are available in the literature on exacerbations in which the morphology of the psoriasis changes during treatment with biologic agents. Our experience suggests that this type of reaction is more common than the literature to date would indicate. In 2007, a paper published by our department described 8 cases of new onset or exacerbation of psoriasis.31 Six of those patients developed guttate psoriasis between 15 days and 18 months after starting treatment with etanercept for severe plaque psoriasis. None of the patients presented other triggers. Substitution of the biologic agent by ciclosporin was necessary to control the psoriasis in 1 of the patients due to a lack of response to topical corticosteroids. This type of paradoxical reaction is characterized by the sudden onset of small drop-like lesions predominantly in areas not previously affected by psoriasis, while the original plaques remain in remission (Fig. 3). These outbreaks should not be confused with a flare or exacerbation of the psoriasis of a different origin, not induced by the biologic agent; in these cases the new lesions are similar to the previous psoriasis lesions and appear in the same areas. Nor should they be confused with a flare-up of psoriasis, which is typically defined as a deterioration of the psoriasis of more than 125% compared to the baseline situation, or a change in morphology induced by the biological agent, but after its interruption. The changes in morphology to which we are referring in this section occur without discontinuation of the drug.
Mössner et al.39 published a series of 5 patients (3 men and 2 women) who developed palmoplantar pustulosis during or after treatment with infliximab for plaque psoriasis. In 1 of their patients, the palmoplantar changes were associated with widespread pustular lesions. In 3 of the cases, there was a simultaneous exacerbation of the plaques. None of them had previously presented generalized or palmoplantar pustular psoriasis. The latency period varied between 3 and 40 weeks. Risk factors included infection in 1 patient and abrupt withdrawal of the infliximab in another. Lesion control by the addition of topical corticosteroids without the withdrawal of infliximab was only achieved in 1 patient.
Spanish authors have reported the case of a patient on treatment with etanercept for plaque psoriasis. The patient developed pustular psoriasis 24hours after undergoing the tuberculin test, which could have been the trigger.73
An erythrodermic form of psoriasis appearing after starting the drug, with a shorter latency period than other types of psoriasiform reaction, has occasionally been reported, and we have also observed this in our clinical practice. Santos-Juanes et al.57 described 2 patients with plaque psoriasis who had previously received numerous psoriasis treatments with suboptimal responses. Those patients developed erythroderma during treatment with etanercept, but were subsequently stable during treatment with infliximab and adalimumab. However, both patients showed an improvement of more than 80% in the PASI (Psoriasis Area Severity Index) after 4 weeks of treatment with ustekinumab.57
Histopathologic CharacteristicsHistological confirmation was only available for 39.4% of the 127 cases reviewed by Ko et al.37 Some authors consider that these reactions present a histology compatible with palmoplantar pustulosis or psoriasis, indistinguishable from cases unrelated to anti-TNF-α therapy.36
However, Seneschal et al.,48 who described in detail the biopsies of their patients with psoriasiform reactions during anti-TNF-α therapy, did find some differences. Skin biopsy had been performed in 11 of their 13 cases. Clinically the lesions were typical small areas of plaque psoriasis, associated with palmoplantar pustulosis or keratoderma in 3 of them. In 5 patients, the authors observed a psoriasiform pattern with parakeratosis, hyperkeratosis, and acanthosis. Three of those 5 cases also presented a lichenoid infiltrate, and another 3 biopsies also showed a focal lichenoid pattern. Keratinocyte necrosis was detected in 3 patients. Seven of the samples had signs of spongiosis with epidermal edema, and a unilocular subcorneal pustule was observed in another case.
Recently, Laga et al.68 evaluated the histological spectrum of psoriasiform reactions associated with anti-TNF-α therapy in 16 biopsies from 9 patients. Those authors reported different histologic patterns, including lichen planus-type dermatitis, sterile pustular folliculitis, and a pattern similar to psoriasis. They concluded that correlation with the clinical findings was crucial to be able to make a diagnosis in this type of reaction. In our opinion, reactions that do not correspond clinically or histologically to palmoplantar pustulosis or psoriasis should not be included in the group of psoriasiform reactions induced by or associated with anti-TNF-α drugs.
In favor of this opinion, other authors consider that the histologic findings of psoriasiform reactions are identical or very similar to the patient's psoriasis prior to anti-TNF-α therapy and differ from pustular drug reactions. They include epidermal hyperplasia, parakeratosis, epidermal lymphocyte infiltrates, dilated capillaries, and intraepidermal pustulosis.53
Initial ManagementThe steps detailed below and in Tables 3 and 4 should be followed for the management of any patient who develops a paradoxical psoriasiform reaction during treatment with any anti-TNF-α agent:
- •
A detailed medical history should be obtained, including the following information:
- ∘
Sociodemographic details.
- ∘
Personal and family history of psoriasis.
- ∘
Personal medical and surgical history, including a drug history, paying particular attention to the duration of drug treatments.
- ∘
Details of the duration of the underlying disease that required the biologic therapy and concomitant or previous therapies administered.
- ∘
Details of the duration of the current biologic treatment and the dose at the time of onset of the reaction.
- ∘
Clinical course of the episode of psoriasis, the type or types of lesion, and history of similar rashes.
- ∘
Information sufficient to exclude possible triggering factors, such as infection, stress, or the initiation of treatment with new drugs.
- ∘
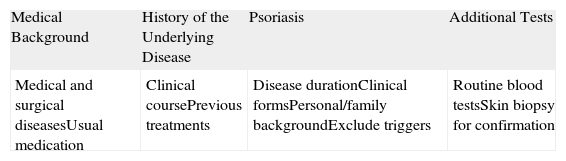
Initial Management of Patients With New Onset Psoriasis During Treatment With Tumor Necrosis Factor α Inhibitors.
| Medical Background | History of the Underlying Disease | Psoriasis | Additional Tests |
| Medical and surgical diseasesUsual medication | Clinical coursePrevious treatments | Disease durationClinical formsPersonal/family backgroundExclude triggers | Routine blood testsSkin biopsy for confirmation |
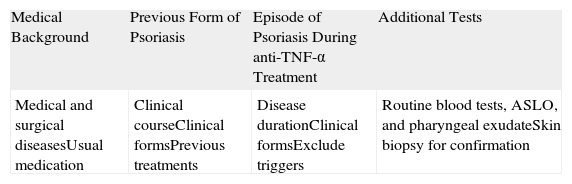
Initial Management of Patients With an Exacerbation of Previously Diagnosed Psoriasis During Treatment With Tumor Necrosis Factor α Inhibitors.
| Medical Background | Previous Form of Psoriasis | Episode of Psoriasis During anti-TNF-α Treatment | Additional Tests |
| Medical and surgical diseasesUsual medication | Clinical courseClinical formsPrevious treatments | Disease durationClinical formsExclude triggers | Routine blood tests, ASLO, and pharyngeal exudateSkin biopsy for confirmation |
Abbreviations: ASLO, antistreptolysin O antibody; TNF, tumor necrosis factor.
The management of these patients is not well established. The following recommendations are based on our experience and on published reviews:
- ∘
Laboratory tests including complete blood count, liver and kidney function tests, antistreptolysin O antibody, and pharyngeal exudate at the start of the episode.
- ∘
Except in clinically characteristic cases, 1 or several biopsies of the skin lesions. This is useful for diagnostic confirmation because, as some authors have stated, not all reactions described as psoriasiform are true psoriasis.
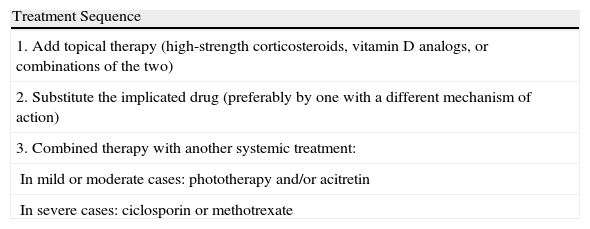
The general treatment of paradoxical psoriasiform reactions is summarized in Table 5. However, based on our experience and on our review of the literature, we consider it useful to detail the treatment of each one of the subtypes:
- •
In patients with new onset psoriasis during treatment with TNF-α inhibitors, the initial use of topical therapy with high-strength corticosteroids, vitamin D analogs, or combinations of the 2 is recommended. If this does not control the episode, 2 situations may arise. When it is necessary to continue the anti-TNF-α drug responsible for inducing the psoriasis because it adequately controls the patient's underlying nondermatologic disease without serious side effects, partial control of the skin lesions with topical therapy may be acceptable, with the possible addition of other therapies (phototherapy or systemic therapy). If it is not essential to continue the anti-TNF-α agent, it is best to substitute the implicated drug. Because of the possible class effect of the anti-TNF-α drugs, we recommend that the new therapy should act via a pathway that does not inhibit TNF-α in order to avoid a deterioration or recurrence of the skin disease.
- •
In case of erythrodermic psoriasis, the first step in all patients should be to substitute the drug by another with a rapid response, such as ciclosporin or ustekinumab, to treat the episode.
- •
In patients with a change in the morphology of the psoriasis, which is not serious in the majority of cases, the most widely used initial option is to continue using the same drug at the same dose and to prescribe topical therapy. If this is insufficient to control the episode, we recommend substitution of the first agent by another drug, preferably one with a different mechanism of action, as in cases of new onset psoriasis. Though possible, the option to add another systemic drug is, in our experience, less effective. This delays the substitution, which will be necessary in the large majority of patients in the end. However, therapeutic modalities such as phototherapy in monotherapy or in combination with acitretin in mild or moderate cases, or drugs such as ciclosporin or methotrexate in more serious cases, have been shown to be useful in some patients, as mentioned above in the text.
General Treatment of Paradoxical Psoriasiform Reactions.
| Treatment Sequence |
| 1. Add topical therapy (high-strength corticosteroids, vitamin D analogs, or combinations of the two) |
| 2. Substitute the implicated drug (preferably by one with a different mechanism of action) |
| 3. Combined therapy with another systemic treatment: |
| In mild or moderate cases: phototherapy and/or acitretin |
| In severe cases: ciclosporin or methotrexate |
The authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of dataThe authors declare that they followed their hospital's regulations regarding the publication of patient information and that written informed consent for voluntary participation was obtained for all patients.
Right to privacy and informed consentThe authors declare that no private patient data are disclosed in this article.
Conflicts of InterestDr E. Daudén undertakes or has undertaken the following activities: member of Advisory Boards, consultant, reception of grants, research support, participation in clinical trials and fees for lectures with the following pharmaceutical companies: Abbott, Astellas, Biogen, Centocor Ortho Biotech Inc, Galderma, Glaxo, Janssen-Cilag, Leo Pharma, MSD, Pfizer, Novartis, Stiefel, Wyeth Pharmaceuticals, 3M and Celgene.
Dr. R. Navarro declares that she has no conflicts of interest.
Please cite this article as: Navarro R, Daudén E. Reacciones psoriasiformes paradójicas durante el tratamiento con terapia anti-factor de necrosis tumoral. Manejo clínico. Actas Dermosifiliogr. 2014;105:752–761.