Since the introduction of omalizumab for the treatment of chronic spontaneous urticaria (CSU), it has been possible to control the disease in a high percentage of patients who had been refractory to treatment with high doses of antihistamines1. According to the most recent European guidelines2, treatment with ciclosporin is indicated in cases where omalizumab has already failed. In this indication, it is prescribed off-label, although it has traditionally been used for treatment of CSU3. Published data are scarce, and only 2 randomized clinical trials involving no more than 16 weeks4 and 8 weeks5 of treatment have been performed. Nevertheless, there are cohorts and case series of patients treated with this drug at low doses for longer periods (i.e., up to 10 years)6. We performed the present study to improve our knowledge of ciclosporin for treatment of CSU in patients whose therapy with omalizumab had already failed.
We designed an observational, longitudinal, prospective study of patients diagnosed with CSU and treated with ciclosporin at our center. Previous treatment with omalizumab had failed. We recorded epidemiological and clinical data and assessed the severity of urticaria using the 7-day Urticaria Activity Score (UAS7d). We also recorded the dose of ciclosporin at different points during treatment (baseline, maximum dose, end of treatment), duration of treatment and follow-up, and adverse effects observed.
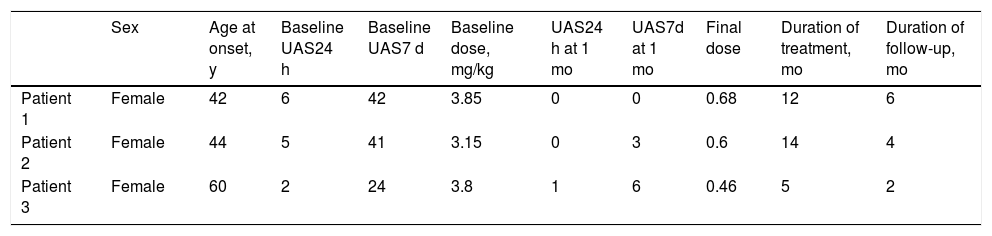
Of a total of 38 patients who had received treatment with omalizumab, 3 (7.9%) did not respond to the biologic and were switched to ciclosporin (Table 1). The UAS7d score at initiation of treatment with ciclosporin was 24, 41, and 42. Ciclosporin was started at 3.8, 3.15, and 3.85 mg/kg/d, respectively. The disease was controlled (UAS7d < 7) in all 3 patients at the first month of treatment, thus making it possible to gradually taper the dose of ciclosporin without flare-ups before suspending it altogether after 5, 14, and 12 months, with doses ranging from 0.46 and 0.68 mg/kg/d. We recorded no recurrences of urticaria in any of the patients. Similarly, no relevant adverse effects were reported during follow-up.
Results From Our Series
| Sex | Age at onset, y | Baseline UAS24 h | Baseline UAS7 d | Baseline dose, mg/kg | UAS24 h at 1 mo | UAS7d at 1 mo | Final dose | Duration of treatment, mo | Duration of follow-up, mo | |
|---|---|---|---|---|---|---|---|---|---|---|
| Patient 1 | Female | 42 | 6 | 42 | 3.85 | 0 | 0 | 0.68 | 12 | 6 |
| Patient 2 | Female | 44 | 5 | 41 | 3.15 | 0 | 3 | 0.6 | 14 | 4 |
| Patient 3 | Female | 60 | 2 | 24 | 3.8 | 1 | 6 | 0.46 | 5 | 2 |
While ciclosporin has traditionally been used for treatment of CSU, no definitive regimens have been established. A meta-analysis published in 20177 analyzed various studies from the literature, including 2 clinical trials4,5. The results indicate that ciclosporin is an effective treatment for CSU, although they are difficult to extrapolate to real life, since candidates for this approach have long-standing CSU that does not respond to omalizumab and often requires cycles of oral corticosteroids. We must go back to articles published before approval of omalizumab if we are to find regimens for affected patients in the medium term, without inducing the feared adverse effects of ciclosporin. Kessel and Toubi6 performed a prospective study of 20 patients with CSU that could not be controlled with ciclosporin at 3 mg/kg/d for 3 months who received the drug at 1-2 mg/kg/d continuously until control was achieved. In 8 cases, the drug could be discontinued after 8-14 months of treatment; the remaining 12 patients continued to receive ciclosporin for 60-120 months without adverse effects.
Boubouka et al.8 reported similar results from their prospective study, in which a cohort of 30 patients with CSU received treatment with ciclosporin at doses ranging from 1.5 to 2.5 mg/kg/d depending on symptoms for 5 months. The disease was controlled in 88% of cases with a final dose of 0.55 mg/kg/d.
Our experience indicates that ciclosporin is an option for treating CSU after failure of omalizumab. In our opinion, the most appropriate regimen (i.e., fewer adverse effects with the same efficacy) is an initial dose of 3-4 mg/kg/d until the disease is controlled, followed by gradual tapering to very low doses (<1 mg/kg/d).
Please cite this article as: Pinilla Martín B, Tous Romero F, Ortiz de Frutos FJ. Ciclosporina: una vieja amiga en el tratamiento de la urticaria crónica espontánea. Actas Dermosifiliogr. 2021. https://doi.org/10.1016/j.ad.2020.01.012