The etiology of chronic ulcers in kidney transplant recipients includes infection, tumors, and drugs. There is another, much rarer cause that should be taken into account in order to ensure a correct diagnosis and therapeutic management. A 62-year-old woman with chronic kidney failure secondary to vascular nephropathy in a single kidney, was on dialysis for 12 years using a right humerocephalic arteriovenous fistula. In 2001 she received a cadaveric kidney transplant and had maintained good renal function since that time. She had been on treatment with prednisone, ciclosporin, and mycophenolate, but after 10 years the ciclosporin was changed to rapamycin because of a squamous cell carcinoma on her left leg. She was seen in the dermatology department for a painful ulcer that had arisen on the dorsum of her right hand 9 months earlier, a year after starting treatment with rapamycin. The ulcer had started as an erosion, though here was no history of trauma, and had showed a progressive clinical course. Histology of a biopsy was nonspecific and culture was positive for Staphylococcus aureus. Treatment was therefore started according to the specific antibiogram, and 2 skin grafts were performed in the plastic surgery department, but were unsuccessful.
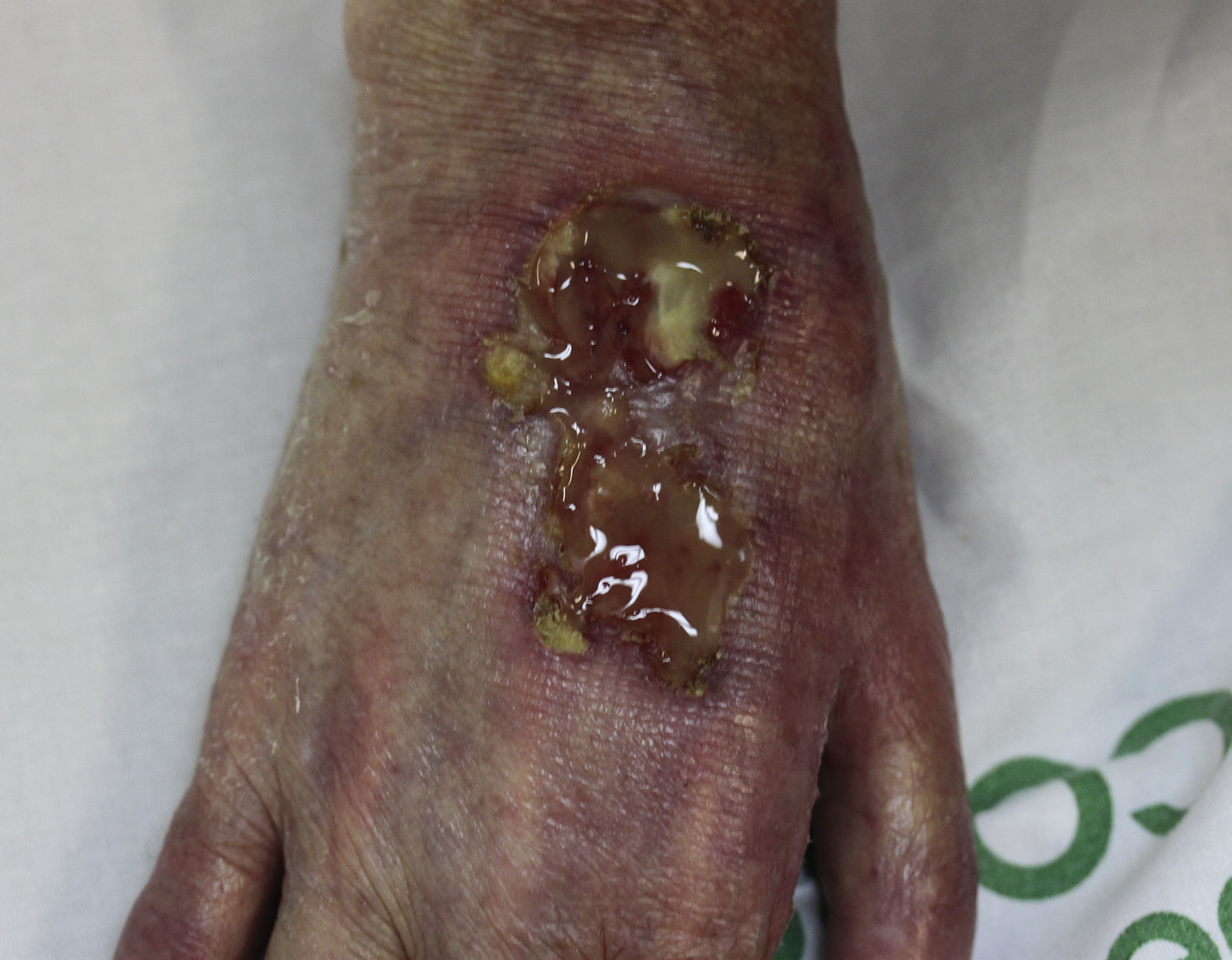
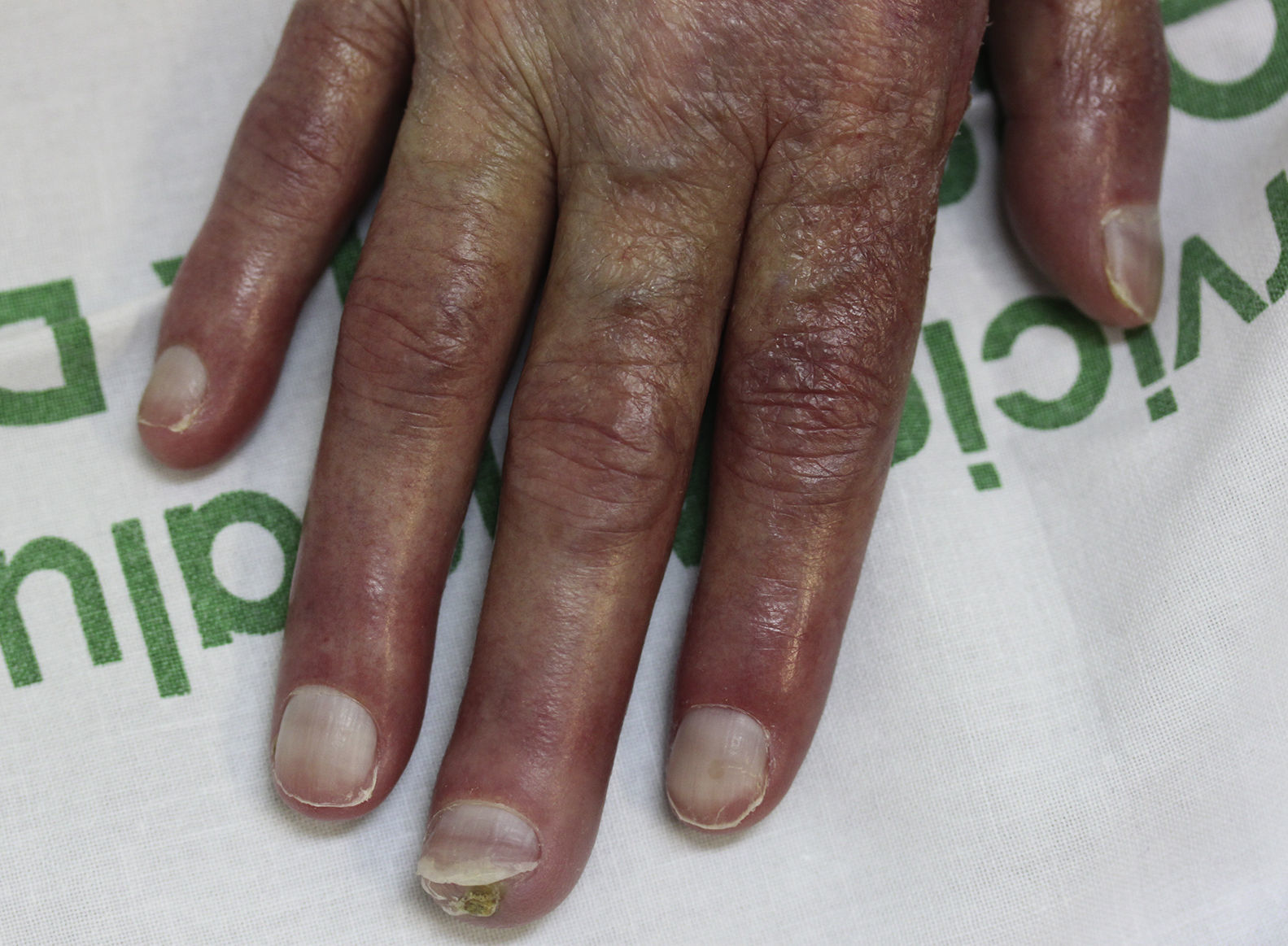
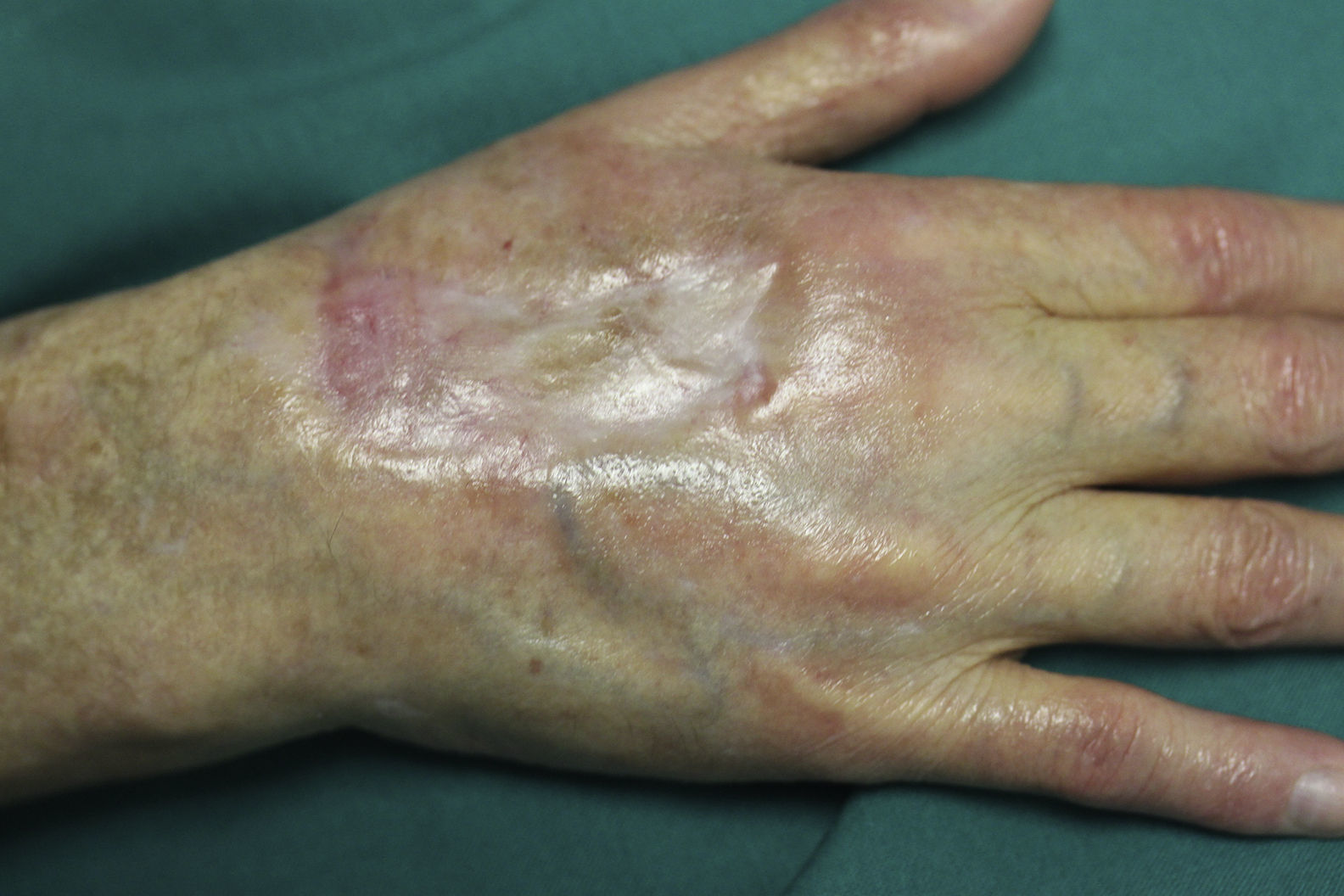
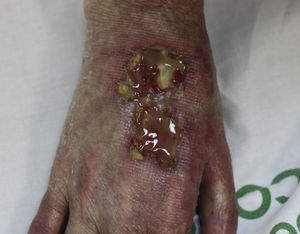
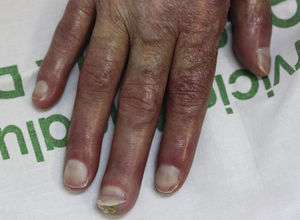
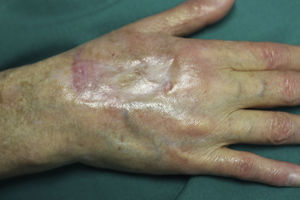
On examination, the ulcer occupied the dorsum of the right hand and measured 6×3cm. It was clean but had a bloodstained base (Fig. 1). The woman's hand was cold and immobile and she held it in a claw position. The skin was thin, dry, hairless, and of violaceous color, and the pulp of the middle finger had a hard, adherent keratotic papule (Fig. 2). The radial pulse was absent, but the humero-cephalic arteriovenous fistula, created 20 years earlier, was patent. A weak radial pulse was palpable when pressure was applied over the fistula. After echo-Doppler confirmation of the severely reduced flow in the distal ulnar and radial arteries, the patient underwent emergency intervention in the vascular surgery department, ligating the fistula. Two months later, the ulcer had healed with a sclerotic scar and although the hand remained atrophic and immobile, its skin color had improved (Fig. 3). The patient was referred to the rehabilitation department and her nephrologist reintroduced the rapamycin. Two months later, an ulcer developed on the scar as a result of the massages performed during rehabilitation; the rapamycin was definitively withdrawn and the lesion healed within a few weeks. The patient was followed up for 3 years with no recurrence of the lesion, but limb function and cosmetic appearance were not restored.
The cause of the ulcer was vascular steal syndrome caused by an arteriovenous fistula created more than 20 years earlier and that, as is usually the case, was not closed after the transplant. It is very likely that the introduction of rapamycin was an exacerbating factor in the initially poor clinical course, but after its withdrawal, the grafts were unsuccessful due to chronic ischemia caused by the vascular steal. The diagnosis was clinical: limb appearance, skin texture and color, and distal digital ischemia that prompted examination of the pulses and temperature, both of which were clearly diminished. Treatment by closure of the fistula led to revascularization and healing of the ulcer within few weeks. Reintroduction of the rapamycin was counterproductive and caused appearance of a new ulcer on minimal trauma; the ulcer did not heal until the rapamycin was withdrawn. In this patient, the diagnosis of vascular steal syndrome was delayed by 9 months and left permanent sequelae.
Arteriovenous fistulas created for hemodialysis lead to vascular steal in 70% of patients, but only become symptomatic in 10% as the steal is compensated by collateral revascularization.1 Patients on long-term hemodialysis have a higher incidence of calciphylaxis and of vascular risk factors, such as diabetes and systemic hypertension and, similar to patients with systemic lupus erythematosus, are more likely to develop vascular steal syndrome.2 Ischaemic symptoms develop during the first month, and a late presentation is more difficult to recognize. Clinical manifestations develop gradually and are associated with pain and paresthesias. The alterations can progress to ischemic necrosis with the consequent associated morbidity.3,4 Few reports have been published on vascular steal syndrome in transplant recipients, and only 1 case has been published of a transplant recipient with a fistula present for 20 years who developed an ulcer of traumatic origin on the dorsum of the hand. In that case, the ulcer healed slowly by second intention but the pain, loss of movement, and paresthesias persisted until the fistula was closed.5
The implication of rapamycin in the onset and persistence of the ulcer is also interesting. This drug acts by binding to the FKBP12 proteins in the cytosol, inhibiting the mTOR pathway. It has antiproliferative, antiangiogenic, and immunosuppressant effects. Its use in solid organ and hematologic transplant has increased, despite reports of side effects such as dyslipidemia, peripheral edema, cytopenia, acne, proteinuria, and oral ulcers in 98% of patients, leading to the need for drug withdrawal in 46% of cases.6,7 There is a clear relationship between rapamycin and the poor healing of ulcers, and its use is therefore not recommended during the first 6 months after transplant because of the poor clinical course of the surgical wound.8 It is more effective if introduced later, but if a chronic ulcer develops, it may need to be withdrawn to allow healing.9,10
In conclusion, as arteriovenous fistulas are typically left in place in transplant recipients, it is important to examine their function before starting treatment with rapamycin.
Conflicts of InterestThe author declares that she has no conflicts of interest.
Please cite this article as: Ruiz AP. Úlcera crónica en paciente con trasplante renal. Actas Dermosifiliogr. 2017;108:589–590.