Management of advanced cSCC is challenging, and many available systemic medications have modest efficacy. Cemiplimab has demonstrated efficacy in the treatment of advanced cSCC in clinical trials, but real-world data are still limited. With the objective of evaluating the efficacy of cemiplimab in a real-world clinical setting, we conducted a prospective observational study of 13 patients with advanced cSCC. Six patients (46%) had locally advanced disease, while 7 (54%) had metastatic disease. A total of 8 patients (62%) responded to cemiplimab. Five (38%) showed a partial response, while 3 (23%) showed a complete response. Four patients with an initial partial response presented subsequent disease progression during follow-up. Six patients (46%) developed AEs, most of which were mild (G1). PFS was 5.9 months, with a median follow-up was 9 months. In conclusion, cemiplimab demonstrated its utility in the treatment of advanced cSCC, with acceptable response rates, a remarkable number of complete responses, and a very good safety profile.
El manejo del carcinoma de células escamosas cutáneo (cSCC) avanzado es complicado, siendo modesta la eficacia de muchos de los fármacos sistémicos disponibles. Cemiplimab ha demostrado su eficacia en el tratamiento del cSCC avanzado en ensayos clínicos, pero los datos del mundo real siguen siendo limitados. Con el objetivo de evaluar la eficacia de cemiplimab en un entorno clínico del mundo real, realizamos un estudio observacional prospectivo de 13 pacientes con cSCC avanzado. Seis pacientes (46%) tenían enfermedad localmente avanzada, mientras que 7 (54%) tenían enfermedad metastásica. Un total de 8 pacientes (62%) respondieron a cemiplimab, 5 (38%) mostraron una respuesta parcial y 3 (23%) mostraron una respuesta completa. Cuatro pacientes con respuesta parcial inicial presentaron una progresión de la enfermedad subsiguiente durante el seguimiento. Seis pacientes (46%) desarrollaron efectos secundarios, siendo leve la mayoría de los mismos (G1). La supervivencia libre de progresión fue de 5,9 meses, con un seguimiento medio de 9 meses. En conclusión, cemiplimab demostró su utilidad en el tratamiento del cSCC avanzado, con unas tasas de respuesta aceptables, un número destacable de respuestas completas y un perfil de seguridad muy bueno.
Cutaneous squamous cell carcinoma (cSCC) is the second most common cutaneous neoplasm, and its incidence has increased in the last decades.1,2 Most cases of cSCC carry a good prognosis and can be treated with complete surgical excision.2,3 However, a small percentage of cases fall within the category of advanced cSCC, which is defined as a tumor that is surgically unresectable, that is not susceptible to curative radiation therapy, and/or that has developed nodal or visceral metastases.1,4 Management of patients with advanced cSCC is often challenging, and until recently the choice of systemic medications was limited and of modest efficacy.3–6 Of late, however, there is increasing evidence on the efficacy of immunotherapy in cSCC. Cemiplimab (an anti-PD1 monoclonal antibody) is the first immunotherapeutic agent approved for the treatment of metastatic or locally advanced cSCC, and it has demonstrated substantial antitumor activity with an acceptable safety profile in clinical trials.7–9 Evidence from real-world studies with cemiplimab is still scarce, and consists mainly on single case reports, with only two recent retrospective studies of patients treated with several PD-1 inhibitors (including cemiplimab).10,11 In the present study we report the clinical outcomes of 13 patients with advanced cSCC treated with cemiplimab. The main outcome measurement was treatment response. Secondary objectives were to evaluate treatment-related adverse events (AEs) and progression-free survival (PFS).
MethodsA prospective observational study was performed, including 13 patients treated with cemiplimab at Fundación Instituto Valenciano de Oncología between April 2019 and November 2020 (6 with locally advanced disease, 7 with nodal or visceral metastatic disease). Treatment was administered intravenously at the approved dose of 350mg every 3 weeks. A partial response (PR) was defined as a reduction of ≥20% of the clinical and/or radiological diameter, and a complete response (CR) as the complete clinical and radiological disappearance of the tumor. Progression was defined as an increase of the tumoral bulk of ≥10%. Stable disease did not meet criteria for either progression or complete or partial responses. Patients with disease progression received up to 6 cemiplimab cycles before treatment discontinuation. All patients were followed jointly both by the Oncology and Dermatology departments, and underwent a thorough physical exam and clinical interview prior to each infusion, as well as control laboratory tests including a complete blood cell count and a basic biochemistry panel (electrolytes, liver and kidney function and lactate dehydrogenase levels). Control clinical pictures were carried out every 6-9 weeks by the Dermatology department. Control imaging studies were performed with variable periodicity: to confirm clinical treatment responses, to assess disease progression, and to verify response maintenance. PDL-1 expression was studied in histological samples of 11 cases. A descriptive statistical analysis was performed with the program IBM-SPSS 25.0. This study was approved by the institutional review board of Fundación Instituto Valenciano de Oncología, and informed consent was obtained from patients or their families following the procedures established by the ethics committee at our hospital.
ResultsA total of 13 patients were included in this study. All but one patient were men, and most of them elderly (median age 81, range 56–91). The main clinical characteristics and treatment outcomes are depicted in Table 1. Patients received a median of 6 cycles of cemiplimab (range 1–23). Overall, 8 cases (62%) responded to cemiplimab after a median of 2 cycles (range 2-4). Three patients (23%) showed a complete response and 5 (38%) a PR. Responding patients received a median of 9 treatment cycles. All but one patient with an initial PR presented subsequent disease progression, leading to drug discontinuation. One of the patients with a CR (case 2) died of unrelated causes while still receiving cemiplimab. In case 8, cemiplimab was suspended after 11 cycles (5 cycles after achieving a complete radiological response), with no progression to date. Finally, the last patient with a CR (case 4) is still receiving cemiplimab (23 cycles to date): because of the high-risk location of the tumor, treatment discontinuation seemed too risky. PFS for responding patients was 5.9 months (range 1.9-15.5). Five patients showed no response to treatment (one of whom was lost to follow-up after receiving only one treatment cycle). The median follow-up was 9 months (range 3.5-16). Regarding treatment-related toxicity, 6 patients (46%) developed AEs. Most of them were mild (G1), and none of the patients in our series presented a serious or lethal adverse reaction. No patients discontinued treatment due to AEs. The most common side effects were fatigue, dizziness and diarrhea (Table 1). Finally, PD1-L expression was measured in 11 cases, with a median value of 10% (range 1–90%). PD1-L expression did not appear to be related to treatment response.
Main clinical characteristics and treatment ouctomes.
| Age | Sex | Primary tumor location | Metastases | PDL-1 expression | Best response | Number of cycles | Inmunorelated toxicity | Treatment discontinuation | Cause of discontinuation | |
|---|---|---|---|---|---|---|---|---|---|---|
| Patient 1 | 83 | M | Scalp | No | Weak positive (10%) | Progressive disease | 3 | Grade2 Rash | YES | Tumor progression |
| Patient 2 | 82 | M | Left cheek | No | Weak positive (5%) | Partial response | 14 | Grade1 Asthenia | YES | Died from an ischemic stroke (patient with history of a myocardial infarction and a previous ischemic stroke) |
| Patient 3 | 73 | M | Scalp | Regional lymph nodes | Weak positive (10%) | Partial response | 10 | No reported toxicity | YES | Tumor progression |
| Patient 4 | 80 | M | Right forehead (orbital invasion, clinical perineural invasion) | Regional lymph nodes | Strong Positive (90%) | Complete response | 23 | Grade 1 asthenia and dizziness | NO | |
| Patient 5 | 81 | M | Neck | Regional lymph nodes | Weak positive (5%) | Partial response | 6 | Grade 1 dizziness | YES | Tumor progression |
| Patient 6 | 86 | M | Nose | No | Weak positive (5%) | Progressive disease | 2 | No reported toxicity | YES | Tumor progression |
| Patient 7 | 59 | M | Left hand | Regional lymph nodes; pleural carcinomatosis. | Strong Positive (60%) | Progressive disease | 4 | No reported toxicity | YES | Tumor progression |
| Patient 8 | 77 | M | Left cheek | No | Weak Positive (20%) | Complete response | 11 | Grade 1 Diarrhea | YES | Sustained complete response |
| Patient 9 | 78 | M | Scalp | No | Weak positive (5%) | Progressive disease | 1 | No reported toxicity | YES | Lost to follow-up during the COVID-19 pandemic, subsequent tumor progression |
| Patient 10 | 56 | M | Left leg | Regional lymph nodes | Strong Positive (55%) | Partial response | 8 | Dermatologic toxicity (Grade 2 Psoriasis; Grade 1 bullous pemphigoid)Grade 1 Diarrhea | YES | Tumor progression |
| Patient 11 | 88 | M | Lip | Regional lymph nodes | Not studied | Partial response | 3 | No reported toxicity | YES | Died from COVID-19 infection |
| Patient 12 | 91 | F | Nose | No | Weak positive (1%) | Partial Response | 6 | No reported toxicity | NO | |
| Patient 13 | 88 | M | Left ear | Regional lymph nodes | No studied | Progressive disease | 6 | No reported toxicity | YES | Tumor progression |
Cemiplimab demonstrated substantial antitumor activity in the clinical trials that lead to its approval, with an objective response rate of almost 50% in the treatment of locally advanced and metastatic cSCC.7–9 In our series, we observed a remarkably high rate of CR (23%), with a higher overall response rate (RR) as compared to clinical trials (62%). Data from the previous two other real-world studies differ: Hanna et al.10 found a lower overall RR as compared to clinical trials (31.5%), while Salzmann et al.11 reported a higher RR (58.7%). Experience with pembrolizumab and nivolumab is more limited, but preliminary results show very similar RR than those of cemiplimab.10,11 It is still unclear why some patients show extraordinary responses to immunotherapy, and others do not respond at all. Focus has been placed in the role of PD-L1 expression and in the presence of TILs.11 Surprisingly, the available data suggests that treatment response does not seem to be related to PD-L1 expression.5 Our series further supports these findings, with patients with complete responses showing very variable levels pf PD-L1 expression. Optimal treatment duration is still unclear, and there is not enough evidence to determine how long therapy should be maintained after an objective response has been achieved.5,10,11 It seems, however, that patients responding to therapy induce a long-term response (especially those with complete responses), with few patients subsequently progressing.11 In our series, this was not the case for patients with partial responses, as all but one of them presented subsequent disease progression in spite of continuing therapy. In patients with a complete response, the decision to continue treatment was made based on their risk profile. In case 5, the high-risk location of the tumor and the fear to a potentially fatal disease recurrence prevented treatment discontinuation. In case 8, because of the tumor's low-risk location (potentially susceptible to surgical treatment in case of recurrence) we decided to discontinue treatment, with no signs of disease progression during follow-up. In this series of primarily elderly patients, cemiplimab demonstrated a very good safety profile, without any serious treatment-related AEs and without any treatment discontinuation due to intolerable AEs (Fig. 1). The most common AEs were similar to those reported in previous studies. Nevertheless, all patients receiving cemiplimab should be monitored for potential side effects, as these could be severe or even lethal. In summary, cemiplimab showed evident antitumor activity, albeit response rates were lower than previously reported in clinical trials. In this case series, the number of complete responses, and the drug's good tolerability profile were significant. The main limitations of this study are the small number of patients included and the relatively short follow-up (Fig. 2).
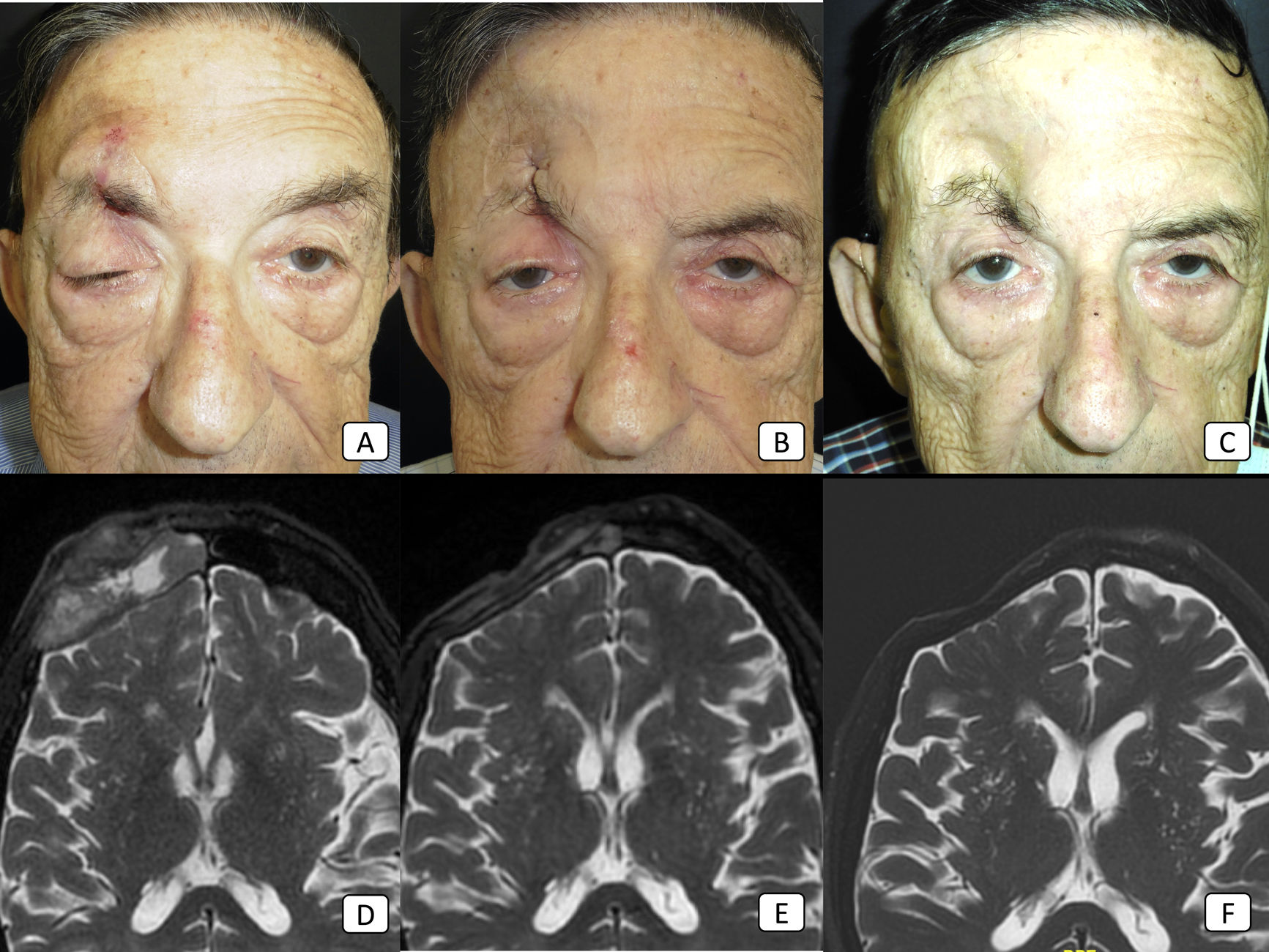
Case 8: advanced cSCC with orbital invasion, clinical perineural invasion, and regional nodal metastases. (A–C): clinical pictures of the patient before the start of therapy, after 2 cycles, and after 8 cycles, respectively. In (A), there is obvious ptosis of the right eyelid because of tumoral perineural invasion. (D–F): T2-weighted MRI images before the start of therapy, after 4 cycles, and after 18 cycles, respectively. The large, frontal mass seen in (D) has disappeared completely in (F), thus showing a complete treatment response.
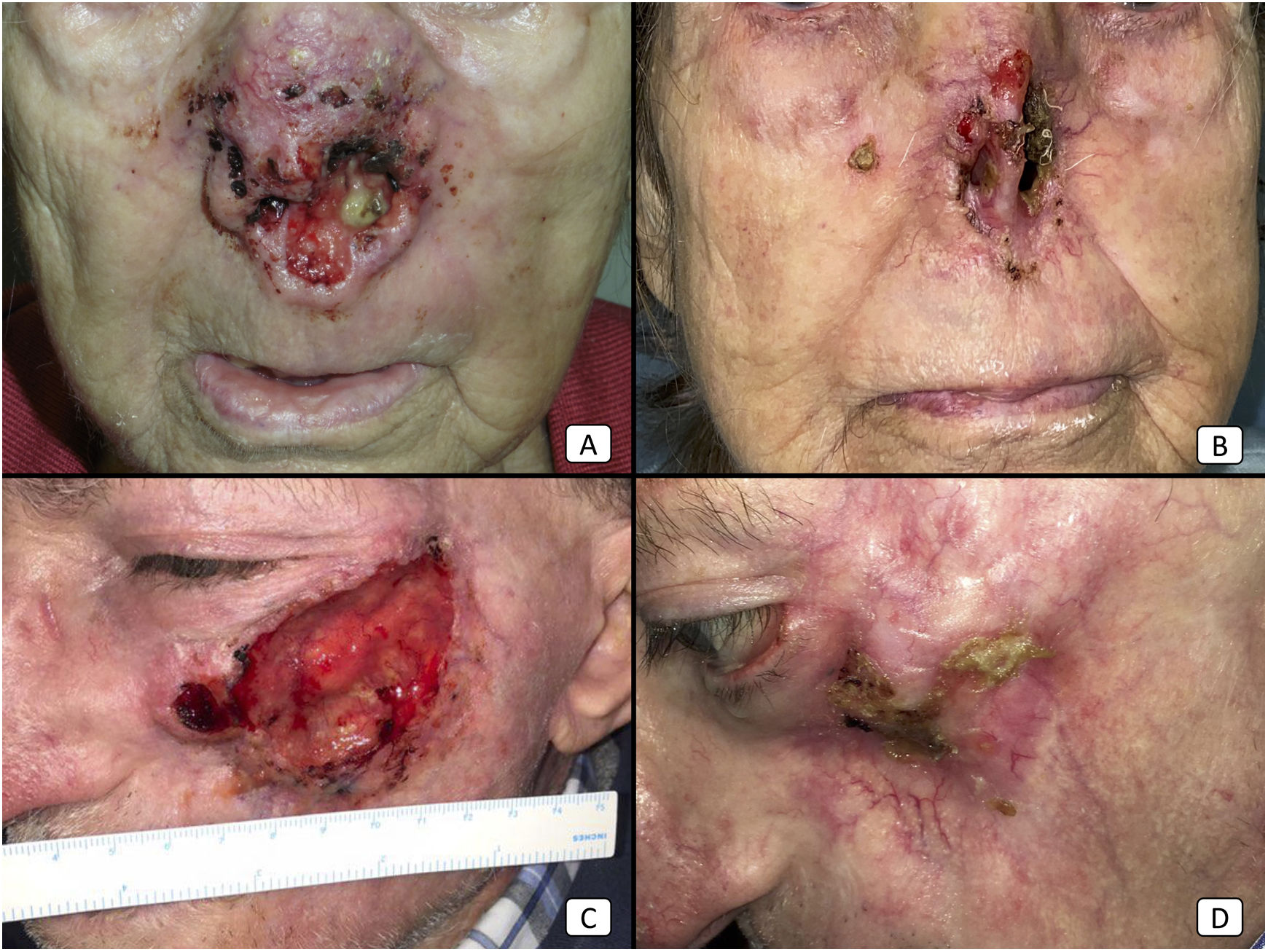
Cases 12 (A, B) and 2 (C, D). (A, B): Locally advanced cSCC in the nose of an elderly woman. There is a dramatical reduction of the tumor bulk after only 2 cycles of cemiplimab (B). (C, D): Locally advanced cSCC of the left cheek in an elderly man. Before starting therapy, the patient presented a large, ulcerated tumor (C). After 10 cycles, the patient showed a complete treatment response, with complete clearance of the tumoral mass and secondary maxillary bone exposure.
Cemiplimab has proved to be effective in the treatment of advanced cSCC in real-world clinical practice. Although preliminary data have yielded lower response rates than previously reported in clinical trials, its significant antitumor activity and its good tolerability place it as one of the first-line therapies for advanced cSCC. Further investigation is needed to define additional treatment approaches (such as adjuvant and neo-adjuvant immunotherapy) and to determine patient profiles that can predict the probability of treatment response.
Conflict of interestsThe authors declare that they have no conflict of interest.