One of the most clinically important aspects of recent advances in our understanding of psoriasis has been the detection of an association between this disease and an increased prevalence of cardiovascular risk factors. This increase in prevalence is, in turn, linked to a greater risk of morbidity and mortality related to acute myocardial infarction, cerebrovascular accident, and peripheral arterial disease. The chronic systemic inflammation present in psoriasis could explain why moderate to severe psoriasis is an independent risk factor for cardiovascular disease. The introduction of biologic therapies has greatly improved the expectations of treatment as well as the long-term control of psoriasis, and there is epidemiological evidence that these therapies may lower cardiovascular risk in psoriasis as they do in rheumatoid arthritis. Caution should, however, be exercised when prescribing biologic drugs in this setting, because adverse effects have been reported in association with the use of tumor necrosis factor inhibitors in patients with advanced congestive heart failure. Furthermore, a numerical imbalance (without statistical significance) between the groups receiving the biologic drug and the placebo groups was recently observed in the incidence of major cardiovascular events (nonfatal myocardial infarction and cerebrovascular accident and cardiovascular death) during the controlled periods of clinical trials of briakinumab and ustekinumab, 2 monoclonal antibodies that target the p40 subunit shared by IL-12 and IL-23. We review the current scientific evidence on this topic.
Uno de los aspectos clínicamente más relevantes de los recientes avances en el conocimiento de la psoriasis es su asociación con un aumento en la prevalencia de factores de riesgo cardiovascular, que determina un mayor riesgo de morbimortalidad relacionada con infarto agudo de miocardio, accidente cerebrovascular y arteriopatía periférica. La inflamación sistémica crónica asociada podría explicar en gran medida que la psoriasis moderada-grave sea un factor de riesgo independiente de enfermedad cardiovascular. La introducción de la terapia biológica ha mejorado en gran medida nuestras expectativas terapéuticas y el control a largo plazo de la enfermedad, y existen evidencias epidemiológicas de que puede mejorar también el riesgo cardiovascular, como ocurre en los pacientes con artritis reumatoide. Sin embargo, se han descrito algunos efectos adversos del tratamiento con agentes bloqueadores del factor de necrosis tumoral alfa en pacientes con insuficiencia cardíaca congestiva avanzada que obligan a tener especial precaución con su empleo en estos pacientes. Por otra parte, recientemente se ha observado un desequilibrio (aunque no estadísticamente significativo) en el número de acontecimientos adversos cardiovasculares mayores, que incluyen infarto de miocardio no letal, accidente cerebrovascular no letal y muertes de causa cardiovascular, en la fase controlada con placebo de los ensayos clínicos con briakinumab y ustekinumab, 2 anticuerpos monoclonales dirigidos contra p40, la subunidad común a IL-12 e IL-23. En el presente artículo se revisa la evidencia científica disponible en este campo.
The present article reviews the association between psoriasis and both cardiovascular risk factors and cardiovascular disease, paying special attention to the implications of this association for treatment, particularly with biologic therapies.
Certain conventional systemic treatments have effects that are counterproductive when viewed from the standpoint of the comorbidities associated with psoriasis. It is well known that ciclosporin can potentially cause or exacerbate hypertension, diabetes mellitus, and dyslipidemia, and that dyslipidemia is a common side effect of acitretin. The fact that biologic drugs lack these contraindications would tend to justify their use in patients with these and other comorbidities. However, there is also evidence to suggest that the treatment of psoriasis with biologic agents may, in some cases, be counterproductive from the point of view of cardiovascular risk.
The possibility that tumor necrosis factor (TNF) inhibitors may exacerbate congestive heart failure (CHF) obliges us to exercise particular caution when prescribing them in these patients, as stated in the Summaries of Product Characteristics for these drugs. Furthermore, the possibility has recently been raised that certain biologic agents may increase cardiovascular morbidity and mortality: an analysis of the clinical trials of monoclonal anti-p40 antibodies has shown a statistically nonsignificant numerical imbalance compared to the placebo group in the occurrence of major adverse cardiac events (MACE), especially at the start of treatment.1 MACE are defined as nonlethal myocardial infarction, nonlethal stroke, and death from cardiovascular causes.
Psoriasis and Cardiovascular RiskThere is abundant scientific evidence supporting the association of moderate to severe psoriasis with an increased prevalence of cardiovascular risk factors and we will not discuss this topic in depth because it has already been the subject of excellent review articles.
A number of multivariate models for calculating cardiovascular risk in asymptomatic and apparently healthy individuals have been developed in recent years. The Framingham charts were designed to estimate the 10-year risk of myocardial infarction or death from cardiovascular disease. The risk factors initially used included age, sex, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, blood pressure, diabetes, and smoking.2 The Framingham score identifies 3 categories of risk: low (≤ 10% risk of myocardial infarction), intermediate (10% to 20% risk), and high (>20% risk). The Framingham criteria were developed from the study of a cohort of residents in Framingham, Massachusetts and are not strictly applicable to other populations, and the risk estimate does not include stroke and other manifestations of cardiovascular disease. Despite extensive usage elsewhere, the Framingham risk score and other similar scales (REGICOR, Reynolds, SCORE) have been little used in the routine assessment of cardiovascular risk in patients with moderate to severe psoriasis.
The authors of a case-control study found that the 10-year cardiovascular risk in patients with psoriasis calculated using the Framingham score was intermediate (mean 11.2), but nonetheless significantly higher than in a control cohort matched for age and sex.3 In a clinical trial enrolling patients with moderate to severe psoriasis and a Psoriasis Area and Severity Index (PASI) greater than 10, while the average Framingham Risk score at 10 years was 5.63, 41% of the patients had metabolic syndrome4; the presence of elevated levels of C-reactive protein (CRP) was indicative of increased risk of cardiovascular disease in this population.5
A recently published cohort study based on the UK General Practice Research Database (GPRD) indicates that severe psoriasis (defined by the use of systemic therapy) confers an increased risk of MACE (hazard ratio [HR] 1.53; 95% CI, 1.26-1.85) after adjustment for age, sex, presence of diabetes, hypertension, smoking, and hyperlipidemia6; the estimated absolute increase in risk of MACE at 10 years is 6.2%.
The UK GPRD has provided extensive data, but questions have been raised concerning how psoriasis was diagnosed in these patients, the definition of severe disease as disease requiring systemic treatment, and the applicability of the findings to other geographical areas. In 2006, Gelfand et al.7 published a prospective, population-based cohort study based on data from the UK GPRD in patients with psoriasis aged 20 to 90 years. They compared the incidence of myocardial infarction in patients with and without a diagnosis of psoriasis. Psoriasis was classified as severe if the patient had ever received systemic therapy. The data had been collected by general practitioners between 1987 and 2002 and mean follow-up was 5.4 years. Adjustments were made for hypertension, diabetes, history of myocardial infarction, hyperlipidemia, age, sex, smoking, and body mass index. Up to 5 controls without psoriasis were selected per case of psoriasis. In total, 556 995 controls, 127 139 patients with mild psoriasis, and 3837 patients with severe psoriasis were studied. The authors of that study found evidence that psoriasis was a risk factor independent of the risk factors for myocardial infarction listed above and reported that relative risk (RR) was age-dependent. For a 30-year old patient, RR was 1.29 (95% CI, 1.14-1.46) for mild and 3.10 (95% CI, 1.98-4.86) for severe psoriasis, while the corresponding RRs for a 60-year old patient were 1.08 (95% CI, 1.03-1.13) for mild and 1.36 (95% CI, 1.13-1.64) for severe disease. Another study confirmed the increased risk of myocardial infarction (HR 1.21; 95% CI, 1.10-1.32), angina (HR, 1.20; 95% CI, 1.12-1.29, atherosclerosis; (HR, 1.28; 95% CI, 1.10-1.48), peripheral vascular disease (HR, 1.29; 95% CI, 1.13-1.47), and stroke (HR, 1.12; 95% CI, 1.00-1.25) in 44 164 patients with a first-time diagnosis of psoriasis.8 Another study based on data from the GPRD also reported an increased risk of stroke in patients with psoriasis, both moderate (HR 1.06; 95% CI, 1.0-1.1) and severe (HR, 1.43; 95% CI, 1.1-1.9).9 Severe psoriasis was also found to be an independent risk factor (after adjusting for age, sex, smoking, diabetes, hypertension, and hyperlipidemia) for cardiovascular mortality (HR, 1.57; 95% CI, 1.26-1.96).10 The relative risk is higher in young patients: 2.69 (95% CI, 1.45-4.99) for a 40-year-old and 1.92 (95% CI, 1.41-2.62) for a 60-year-old.11
The following are descriptions of recently published studies with contrasting results. When the incidence of cardiovascular disease was analyzed following diagnosis of psoriasis (in the studies by the Gelfand group the diagnosis of psoriasis was prevalent, not incident), a trend was observed towards an increased risk of myocardial infarction (odds ratio [OR] 1.66; 95% CI, 1.03-2.66), but only in patients aged under 60 years and predominantly in those with severe psoriasis.11 However, at 0.51/1000 patient-years, the absolute risk was low. The risk of myocardial infarction was not increased when the entire cohort was considered, nor was that of stroke or transient ischemic attack. In another study of a very large cohort of patients in the Netherlands, no association between psoriasis (regardless of severity) and increased risk of hospitalization for acute ischemic heart disease was found after adjustment for age and sex.12 Nor was any association found between psoriasis and an increased risk of myocardial infarction or stroke in a database of German outpatients, although psoriasis was found to be associated with a higher prevalence of cardiovascular risk factors, such as obesity, diabetes, dyslipidemia, and hypertension.13 The risk of cardiovascular mortality may be increased only in patients whose psoriasis requires hospitalization and not in outpatients, and it may be higher in young patients.14 A study of hospitalized patients in Israel confirmed that psoriasis is an independent risk factor for diabetes, hypertension, obesity, and smoking; however, no increased risk of cardiovascular disease was observed when the analysis was corrected to take into account these risk factors.15
Apart from the above-noted exceptions, the findings of most studies are similar. A recent systematic review of the available evidence—including 90 studies—indicated that patients with psoriasis have a higher prevalence of cardiovascular risk factors and are at increased risk of ischemic heart disease, cerebrovascular disease, and peripheral arterial disease.16 Recently published evidence has confirmed these findings in Asian populations.17,18
This increase in cardiovascular morbidity could be independent of the shared risk factors and may contribute to the increase in all-cause mortality (in addition to that due to ischemic heart disease, cerebrovascular disease, and peripheral vascular disease) observed in patients with psoriasis in a study of 3236 cases and 2500 controls conducted in a large Veterans Administration Hospital.19
In a cohort study of the entire Danish adult population that included 34 371 patients with mild and 2621 with severe psoriasis (607 with psoriatic arthritis), an increase in the risk of all-cause mortality, cardiovascular mortality, myocardial infarction, coronary revascularization, stroke, and MACE was also observed.20 The adjusted rate ratios (equivalent to RRs) increased with disease severity and decreased with age of onset. The RRs for MACE were 1.20 (95% CI, 1.14-1.25) and 1.58 (95% CI 1.36-1.82) for patients with mild and severe psoriasis, respectively; the corresponding RRs for cardiovascular death were 1.14 (95% CI, 1.06-1.22) and 1.57 (95% CI, 1.27-1.94), respectively. Risk was similar in patients with severe psoriasis affecting only the skin and in those with psoriatic arthritis. In the same cohort, an increased risk of atrial fibrillation and ischemic stroke depending on the severity of psoriasis was also observed, with a higher RR in patients aged under 50 years (almost double that of the older individuals).21 Interestingly, in this Danish cohort, the prognosis in patients with a history of myocardial infarction was worse for those with psoriasis than for those without. The adjusted HR associated with psoriasis was 1.18 (95% CI, 0.97-1.43) for all-cause mortality and 1.26 (95% CI, 1.04-1.54) for MACE.22 In that study, the mean follow-up was 19.5 months for patients with psoriasis and 22 months for those without.
The increased cardiovascular risk associated with psoriasis, which is independent of the traditional risk factors, could be partially explained by the presence of chronic inflammation, which may be reflected in the presence of elevated serum CRP, at least in some patients. Measurement of serum CRP levels can be useful in cardiovascular risk assessment in patients with intermediate risk as defined by the Framingham score.7 A significant correlation between CRP levels and the severity of psoriasis has been reported,23 with a significant percentage of patients having values above 3mg/L, an indication of high cardiovascular risk. Response to treatment with phototherapy is associated with a reduction in these levels, although mean levels in such patients are still higher than those of the control group.24
Other studies report ultrasound evidence of subclinical atherosclerosis24 and endothelial dysfunction in the brachial artery.25 These findings support the role of psoriasis-related chronic inflammation as an independent cardiovascular risk factor.
Effects of Treatment on Cardiovascular Risk in PsoriasisIf severe psoriasis or the inflammation associated with such disease confers an increased risk of cardiovascular disease independent of traditional risk factors, appropriate therapeutic control of the disease would contribute to a reduction in cardiovascular morbidity and mortality. This appears to be the case in patients with rheumatoid arthritis who are treated with methotrexate26 or TNF-α antagonists.27 Epidemiological and indirect evidence supporting this hypothesis in the case of psoriasis has recently been published; this is discussed below. While further epidemiological evidence will certainly be added to the information currently available, it is unlikely that evidence will be obtained from clinical trials since such studies would be impractical because of the duration and number of patients required.
Possible Beneficial EffectsThere is epidemiological evidence that treatment with methotrexate, especially at low doses and combined with folic acid supplementation, reduces the incidence of vascular disease in patients with psoriasis or rheumatoid arthritis.28 In a US insurance database cohort of 25 554 patients diagnosed with psoriasis (ICD-9 696.1), patients who had received systemic therapy were compared with a control group of patients who had been treated with phototherapy.29 This comparison revealed a trend towards an increase in the risk of myocardial infarction in the systemic treatment group (HR, 1.33; 95% CI, 0.90-1.96).29 Although the difference was not significant, the risk ratio in patients receiving systemic therapy was age dependent: in patients aged under 50 years the HR was 0.65 (95% CI, 0.32-1.34), and in those aged 50 to 70 years it was 1.37 (95% CI, 0.79-2.38).
With respect to biologic therapy, data has been published from a retrospective study of a cohort of 11 475 patients with psoriasis or psoriatic arthritis insured by Kaiser Permanente in South California.30 The results of that study suggest that treatment with TNF inhibitors is associated with a significant reduction (44%) in the incidence of myocardial infarction as compared to the group of patients with mild psoriasis who received topical therapy. Multivariate analysis identified the following protective factors: treatment with TNF inhibitors, female gender, age less than or equal to 65 years, and treatment with statins. In the same analysis, an increased risk of myocardial infarction was associated with type 2 diabetes, dyslipidemia, hypertension, and psoriatic arthritis.30
Other authors have reported a significant reduction in CRP levels at 12 weeks from start of treatment with etanercept in patients with psoriasis and psoriatic arthritis (independent of concomitant treatment with statins) and an association between baseline PASI and CRP independent of body mass index; however the improvement in CRP levels was smaller in obese patients.31 This difference could be related with the lower efficacy of fixed-dose biologics in the treatment of psoriasis in obese patients and in those weighing more than 90 to 100kg.32
The beneficial effect of effective treatment of psoriasis on the biological markers of cardiovascular risk has also been described in the case of phototherapy24 and systemic treatment in general.33
Possible Detrimental Effects of Anti-TNF Therapies in CHFThe direct and indirect effects of TNF on the myocardium are complex and difficult to understand: TNF can induce cardiomyocyte hypertrophy and indirectly affect the heart through chronic inflammation.34 Elevated TNF levels have been reported in a high percentage of patients with CHF and have been shown to be associated with a reduction in survival in this setting.35 Nevertheless, in patients with CHF, no therapeutic effect was demonstrated in 3 clinical trials with the TNF inhibitors etanercept and infliximab,36 and in an infliximab trial mortality was higher in the group treated with infliximab 10mg/kg (a higher dose than that used in psoriasis and rheumatoid arthritis) than in the controls.37 These findings constitute the evidence base for the contraindication against the use of anti-TNF antibodies in patients with CHF classified as grade III-IV by the New York Heart Association (NYHA) and for the recommendation that special caution must be exercised when prescribing etanercept—both specified in the corresponding Summaries of Product Characteristics.
Referral to a cardiologist is recommended in the case of patients with NYHA grade I-II CHF (no symptoms or only slight limitation on intense or very intense exertion) before and during treatment with TNF inhibitors, especially if dyspnea on exertion is present. It is important to make sure that the patient's ejection fraction is above 45%-50% and to screen for objective signs of CHF (atrial fibrillation, ultrasound abnormalities or elevated brain natriuretic peptide levels) in these patients.38 Ustekinumab could arguably be considered the option of choice in patients with CHF because the Summary of Product Characteristics for that agent does not include any relevant contraindications or precautions.39
Biologic therapy is not contraindicated in patients with heart diseases other than CHF, and TNF inhibitors may indeed protect against the development of cardiovascular disease.31
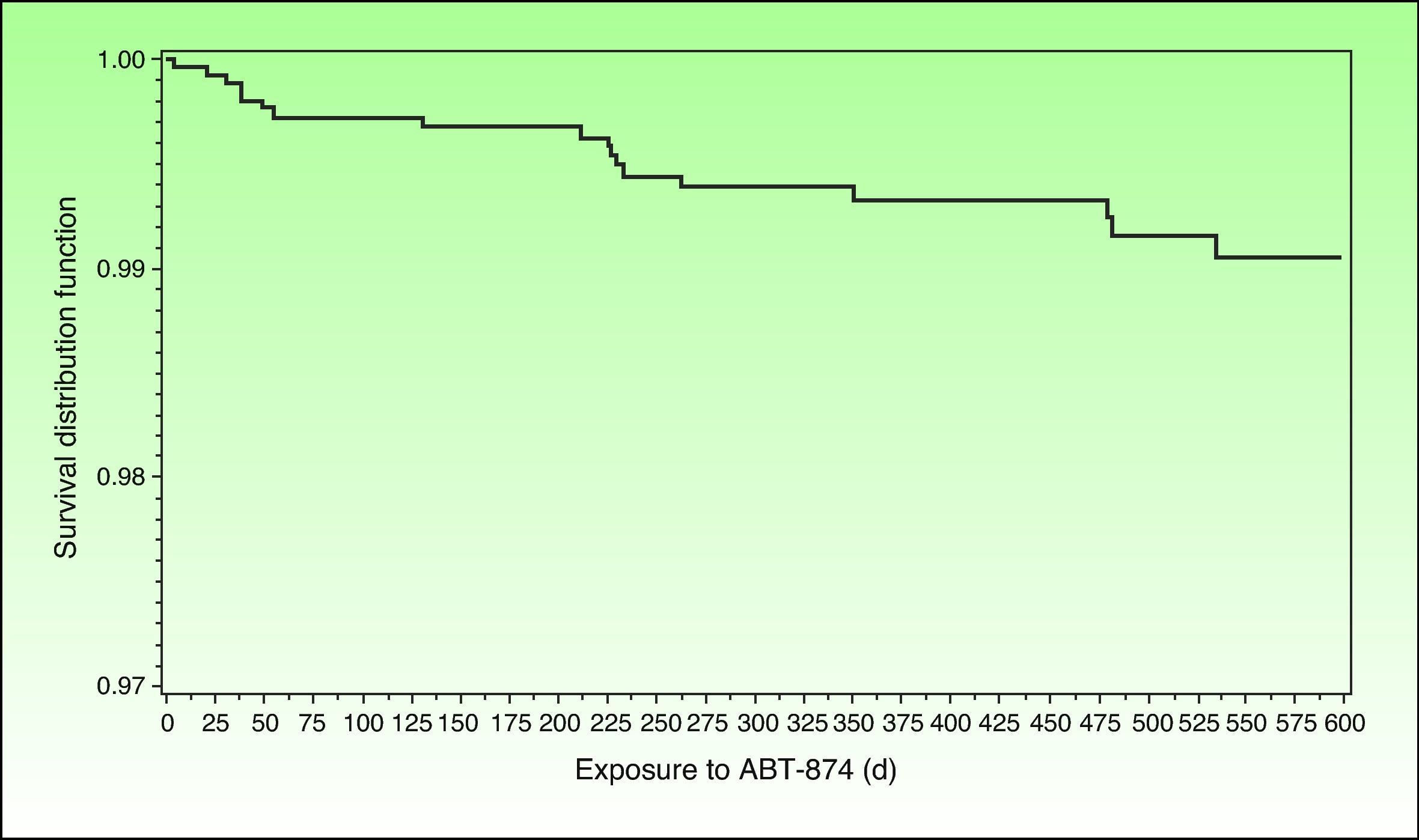
Incidence of MACE in Clinical Trials With Biologic AgentsIn 1 of the 4 pivotal studies of briakinumab, a monoclonal anti-p40 antibody, 18 MACE had been recorded (11 cases of nonfatal myocardial infarction, 3 of nonfatal stroke, and 4 cardiovascular deaths) by the end of November 2009. Five of these adverse cardiovascular events occurred during the first 12 weeks of treatment, which was placebo-controlled (there were no MACE in the control group), 2 occurred between weeks 12 and 52, and 11 took place during the open-label extension phase.40 The frequency of MACE was distributed in an apparently homogeneous manner over time, and no clear pattern emerged of greater frequency during any given period (Fig. 1). The corresponding incidence rates were 1.33/100 patient-years (95% CI, 0.43-3.10) in the placebo-controlled phase and 0.60 (95% CI, 0.35-0.94) taking into account all periods of treatment with briakinumab. The rate of MACE was higher (2.15 events/100 patient-years) in patients who had 2 or more cardiovascular risk factors (history of diabetes, obesity, uncontrolled hypertension [≥140/90], or a history of cardiovascular disease) than in patients with no such history (0.13 events/100 patient-years), a finding that led to an amendment of the study protocol.41 It is interesting to highlight that no MACE were reported during the controlled phase, (placebo or etanercept) of the other 2 published clinical trials.41–43 Nor were any MACE recorded in another trial in which briakinumab was compared with methotrexate with a 52-week follow up.44 However, rates of infections and malignancies adjusted to exposure were higher in the combined analysis of the 3 first trials42 and in the fourth trial.45 For reasons that are unexplained but possibly related to the cost of undertaking additional studies, the laboratory initially developing this drug withdrew its application for authorization to market briakinumab (Ozespa)45 and subsequently discontinued clinical development of the drug in July 2011.
Survival distribution function showing the time to onset of MACE occurring between start of treatment with briakinumab and 101 days after the final dose administered in the briakinumab (ABT-874) studies M05-736, M06-890, M10-114, M10-315, M10-255, and M10-016 (interim analysis on November 26, 2009). Source: Langley R, et al.41
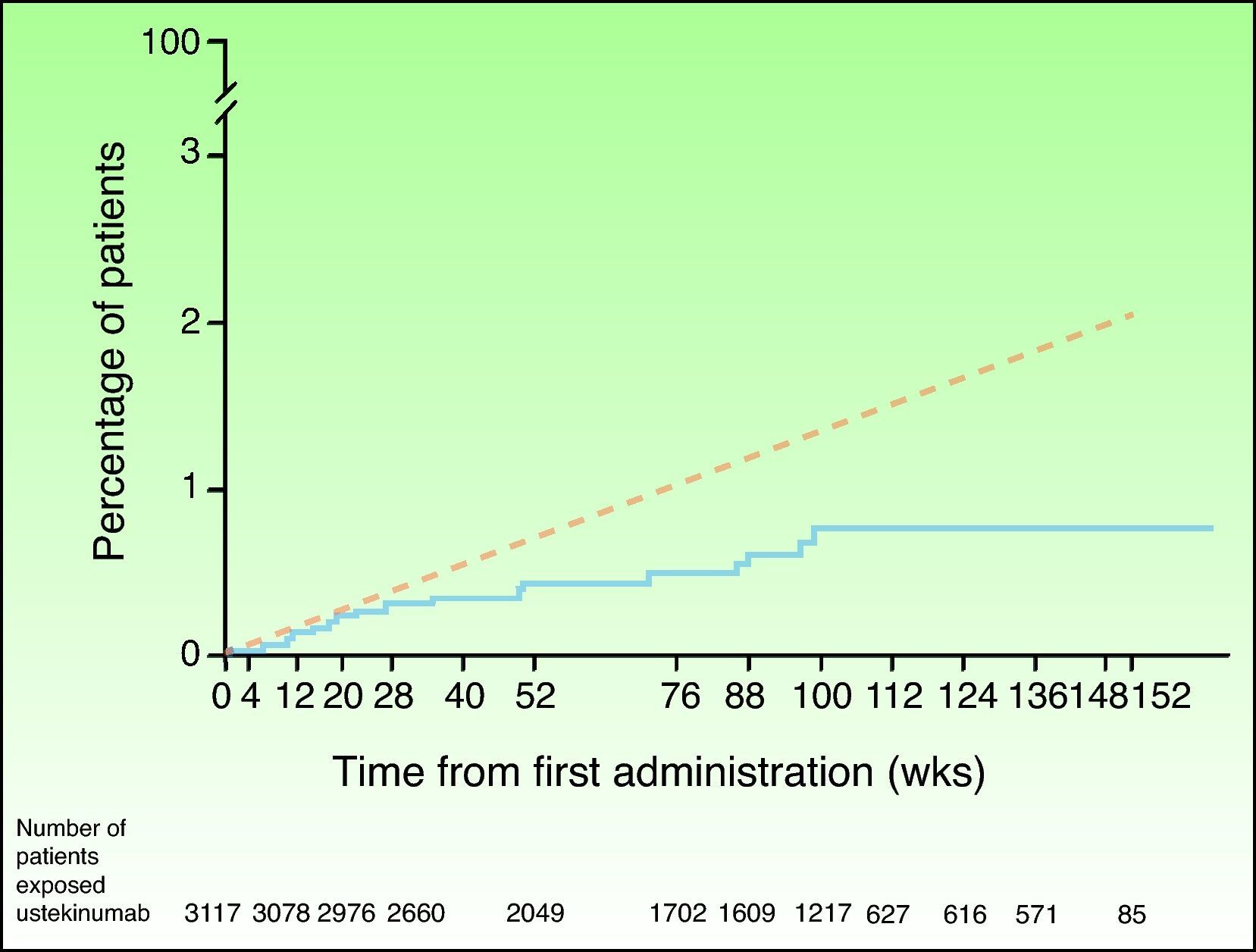
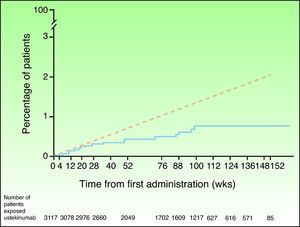
In view of the suspicion that this numerical imbalance in the incidence of MACE in the placebo-controlled phase of one of the clinical trials with briakinumab might be an indicator of a possible adverse effect of this class of drugs rather than a product of chance in a population at high risk for cardiovascular disease, a safety analysis was carried out of the clinical trials with ustekinumab in the treatment of psoriasis and other indications.46 In the placebo-controlled phase, 5 MACE were reported in the 1582 psoriasis patients treated with ustekinumab in phase II and III clinical trials (0.3%; 95% CI, 0.1%-0.7%), and none were observed in the 732 patients who received placebo (0.0%; 95% CI, 0.0%-0.5%); the risk difference was not statistically significant in any of the studies.47 MACE rates were stable throughout both the controlled and uncontrolled phases of the study, with 19 of the 3117 patients treated with ustekinumab experiencing MACE during a 3-year follow-up period; the combined event rate per 100 patient-years was 0.44 (95% CI, 0.27-0.67). Of the patients who experienced MACE, 95% had 2 or more cardiovascular risk factors compared with 55% in the group of patients who did not experience MACE. The cumulative rates of myocardial infarction and stroke were similar to expected rates based on the findings of the US Framingham Heart Study and the UK GPRD until week 28 and lower thereafter (Fig. 2). The standardized incidence rate of myocardial infarction and stroke in phase II and III clinical trials of ustekinumab in psoriasis ranged between 0.34 and 0.52, a finding that suggests that the risk of these 2 events occurring in the population treated with ustekinumab in clinical trials may be lower than that of the general population in the USA or the GPRD population with psoriasis.47
Cumulative rates of myocardial infarction and stroke over time based on Kaplan-Meier estimates of time to first event in patients treated for psoriasis with ustekinumab in phase II and phase III trials (continuous blue line) and expected rate over time based on a predictive model developed using data from the Framingham Heart Study and adjusted for baseline risk factors (dashed red line). Source: http://www.nelm.nhs.uk/en/NeLM-Area/News/2011---January/17/Marketing-authorisation-application-for-briakinumab-Ozespa-withdrawn- (last accessed January 30, 2012).45
The data with respect to TNF inhibitors are incomplete. Only 1 relevant study of etanercept has been published. In a recent study of 506 Canadian patients with a 48-month follow-up, a MACE rate of 1.7 per 100 patient-years was recorded in a trial that had a substantial drop-out rate and a follow-up equivalent to 1305 patient-years.47 A review has been published of cumulative safety data for all those treated with adalimumab for moderate to severe psoriasis in clinical trials.48 During the placebo-controlled phases of these 3 registered trials, the authors reported 1 MACE in 294 patient-years of exposure to adalimumab (incidence rate 0.34 per 100 patient-years [95% CI, 0.01-1.90]) and 1 MACE in 148 patient-years of exposure to placebo (incidence rate of 0.68, 95% CI, 0.02 to 3.76).48 In the 7 registered adalimumab trials there were 15 MACE (6 nonfatal myocardial infarction, 4 nonfatal stroke, and 5 cardiovascular deaths) in 4185 patient-years of exposure to adalimumab, giving an incidence rate of 0.36 per 100 patient-years (95% CI, 0.20-0.59).48
A recent meta-analysis reviewed 22 placebo-controlled clinical trials comprising 10 183 patients receiving anti-TNF (adalimumab, etanercept, and infliximab) and anti-p40 (briakinumab and ustekinumab) biologic agents to evaluate the absolute difference in MACE risk during the placebo-controlled phase of these trials.1 During the controlled phases of the trials with anti-p40, 10 out of 3179 patients experienced MACE compared to none of the 1474 patients receiving placebo. The Mantel-Haenszel risk difference was 0.012 per person-year (95% CI, –0.001 to 0.026, P=.12). In the anti-TNF trials, MACE were recorded in only 1 of the 3838 patients receiving the biologic agent and 1 of the 1812 patients receiving placebo. The corresponding risk difference was –0.0005 events per person-year (95% CI, –0.010 to 0.009, P=0.94).
Since the risk difference was not significant in either case, these findings do not confirm the hypothesis that biologic therapy of any type is associated with an increased risk of MACE during the placebo-controlled phase of these trials. However, neither do they rule out the possibility that a statistically significant difference might be found in a larger patient population. Clinical trials are not an ideal tool for identifying signs of infrequent risk; registry studies are more appropriate. The methodology of another recently published meta-analysis of anti-p40 trials excluded trials in which no MACE were reported.49 That meta-analysis detected a significant increase in the risk of MACE in patients treated with anti-p40 antibodies compared to the placebo groups (OR = 4.23; 95% CI, 1.07-16.75; P = 04); the difference was not statistically significant when the trials for each drug were analyzed separately.
The Role of Interleukin-17 in AtherogenesisIt has recently been proposed that interleukin (IL) 17 could play a stabilizing role in atheromatous plaque,50,51 a finding that might explain the possible increase in the incidence of MACE at the beginning of treatment, not only in the case of anti-p40 antibodies but also in the new class of IL-17 antagonists currently under development.52
The evidence is, however, contradictory. One study demonstrated that IL-17 and interferon-γ are present in coronary atherosclerotic plaques, which contain T cells that express both cytokines, and that they act synergistically to produce proinflammatory cytokines in cultured human vascular smooth muscle cells.53 Another study identified a significant increase in the number of T helper (TH)17 cells and in levels of TH17-related cytokines (IL-17, IL-6, and IL-23) in the peripheral blood of patients with acute coronary syndrome together with decreased levels of the cytokines related to regulatory T cells (Treg) (IL-10 and TGF-β1) as compared to patients with stable angina and controls.54 It has recently been suggested that this increase in the number and activation of these cells may be limited to TH1 cells and TH17/TH1 cells, a recently described subset, but does not affect the overall TH17 cell population.55
In 2009, Taleb and colleagues49 published a study showing that the deletion of the suppressor of cytokine signaling 3 (SOCS3) gene in the T cells of mice genetically predisposed to atherosclerosis by double deletion of the low-density lipoprotein receptor gene (LDLr–/–) induced the production of IL-17 and IL-10 and led to an IL-17-dependent reduction in lesion development and vascular inflammation. In vivo administration of IL-17 reduced the expression of endothelial vascular cell adhesion molecule-1 and vascular T cell infiltration, and limited the development of atherosclerotic lesions. By contrast, overexpression of SOCS3 in T cells reduced IL-17 levels and accelerated atherosclerosis. In human samples from patients undergoing carotid endarterectomy, the authors detected increased expression of IL-17 in the plaques with a stable (fibrous) phenotype compared to those having an unstable (atheromatous) phenotype.51
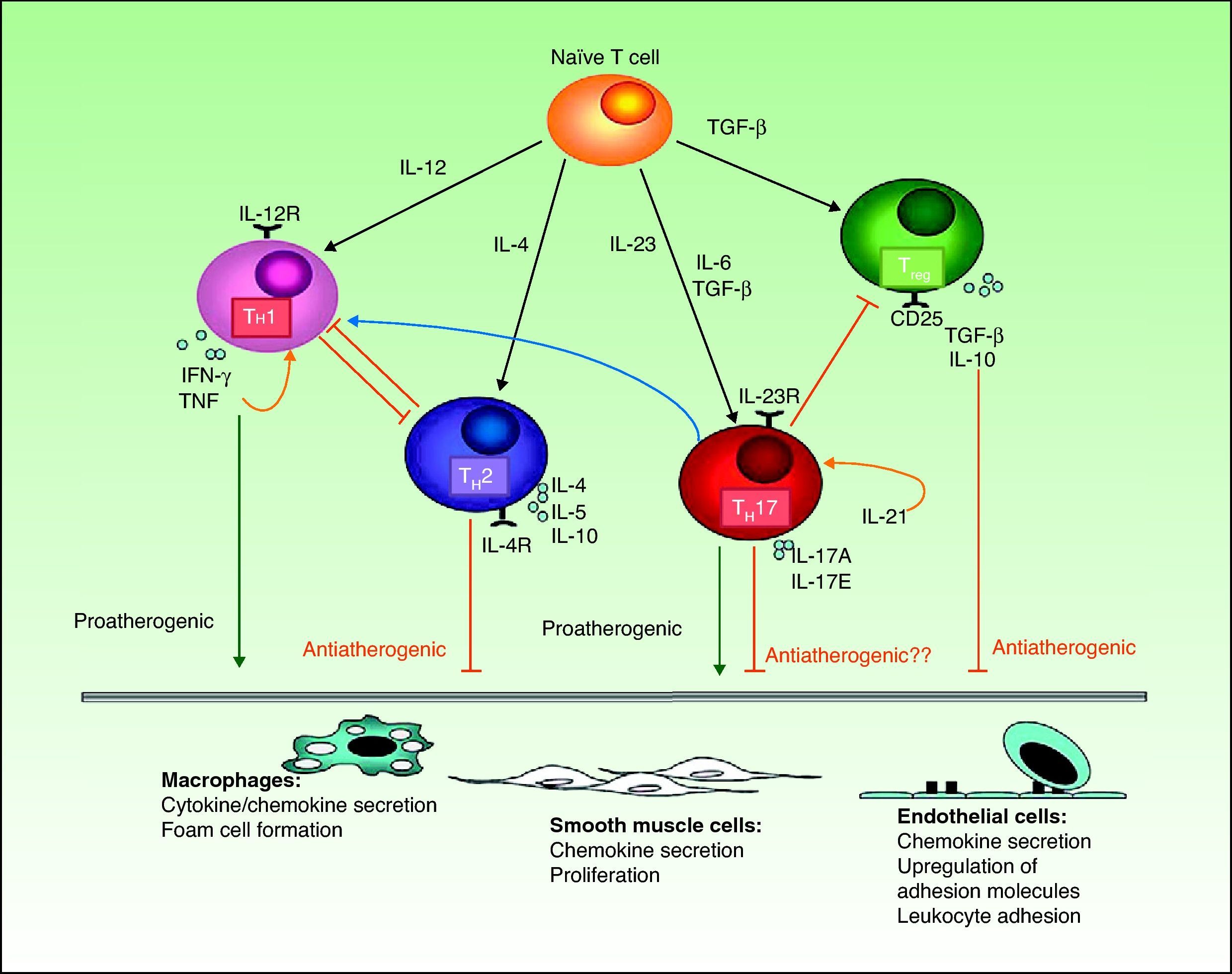
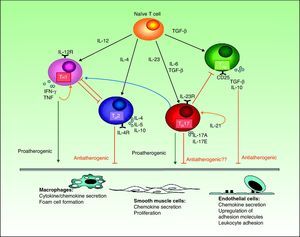
However, most published studies indicate that both TH1 and TH17 cytokines have a proinflammatory effect that favors the development of atheromatous plaques (Fig. 3). The activation of the TH1 response by IL-12 is well known in both psoriasis and atherosclerosis. In atherosclerosis, this mechanism plays an essential role not only in plaque formation but also in maintaining inflammation and developing the unstable phenotype that makes the plaques prone to rupture.56 TH17 lymphocytes produce IL-17, IL-6, IL-21, IL-22, and TNF, and have a profound synergistic effect on atherogenesis. Although TH1 cells predominate over TH17 cells in the proportion of 10 to 1 in atherosclerotic plaques,54 the TH17 cells act synergistically. Most of the experimental evidence and biomarker studies indicate a link between IL-17 and instability in atherosclerotic plaques, a finding that may partially explain the increased risk of myocardial infarction in patients with psoriasis.57
Interactions between different T cells and cytokines implicated in atherosclerosis showing their functional significance in the main cell types present in the plaque.IFN indicates interferon; IL, interleukin; TGF, transforming growth factor; TH, T helper cells; TNF, tumor necrosis factor; TREG, regulatory T cells.
The most direct evidence of the proatherogenic role of IL-17 comes from very recent studies.57 When Van Es et al.58 transplanted LDLr-/- mice with bone marrow from IL-17-receptor deficient mice they observed a reduction in lesions induced by a western-type diet in the aorta of the transplanted mice. Xie et al.59 demonstrated that apoE-/- mice exhibit increased levels of TH17 cytokines (IL-17, IL-6) and a decrease in the number of Treg cells and the corresponding cytosine TGF-β1 compared to age-matched C57BL/6J mice (without deletion). In the same apoE-deficient, atherosclerosis-prone mouse model the administration of an IL-17A antibody produced in rats decreased atherosclerotic lesion development and reduced plaque vulnerability, cellular infiltration, endothelial activation, and cytokine secretion.60 The administration of a rat anti-mouse IL-17A antibody in another study also reduced plaque size in apoE–/– mice, although no reduction in IL-17A levels was observed.61 By contrast, administration of an anti-mouse IL-17A antibody had no effect on the size of the lesions despite a reduction in IL-17A levels.62 In any case, IL-17A blockade does not appear to inhibit the action of other IL-17 isoforms, nor that of other TH17 cell-derived cytokines, whose role remains poorly understood..62 The authors of a recent study reported that blockade of IL-17A with a soluble IL-17 receptor resulted in an increase in the number of IL-17A+ T cells in the aorta of aged apoE–/– mice and reduced the development of atherosclerotic lesions induced by a western diet.63
In another study, C57BL/6 mice with IL-17A–/– deletion developed smaller atherosclerosis lesions with less lipid deposition than mice with no deletion after 12 weeks on a high-fat diet.58 Although the precise role of IL-17 in atherosclerosis remains a subject of debate, the most recent studies have provided quite direct evidence that it is predominantly proatherogenic. In any case it is probably very complex and dependent both on local levels of IL-17 during the different stages of plaque formation and the different isomorphs of the cytokine.62,63 In humans, IL-17A expression appears to be associated with increased inflammation and more vulnerable atherosclerotic plaques.64 Constitutive expression of IL-17E by cells in atherosclerotic plaques and, furthermore, the presence of IL-17E+ B cells and IL-17A/F+ neutrophils in the advanced and complicated plaques are indications that the contribution of the IL-17 cytokines is complex and dependent on disease stage and activity.65
In any case, based on the currently available scientific evidence, we cannot assert that the administration of any biologic agent is associated with an increased risk of MACE in patients with moderate to severe psoriasis. Moreover, there is a growing body of epidemiologic evidence that appropriate treatment of psoriasis and associated inflammation can reduce the risk of cardiovascular morbidity and mortality, which is already elevated in these patients owing to the increased prevalence of traditional risk factors in this setting. Furthermore, there is abundant epidemiological evidence suggesting that moderate to severe psoriasis is an independent risk factor for cardiovascular disease and death, especially in younger patients.
It is therefore essential to properly assess cardiovascular risk in these patients before treatment and periodically during treatment and to treat traditional risk factors according to current therapeutic algorithms. Patients with moderate to severe psoriasis, like those with rheumatoid arthritis, have increased cardiovascular risk due to the inflammatory component of the disease. Consequently, it is probably appropriate to multiply by 1.5 the score obtained using the Framingham or SCORE charts, as has been proposed in patients with rheumatoid arthritis.66
Further studies are needed to establish the role in the evaluation of cardiovascular risk in these patients of the presence of psoriatic arthritis and high levels of CRP or other markers of inflammation and to determine the actual effect of proper control of psoriasis and the type of treatment prescribed.
Conflicts of InterestDr Lluis Puig has participated in clinical trials sponsored by Abbott, Janssen, Merck, and Pfizer, and has received remunerations as a consultant or speaker from Abbott, Janssen, Merck, and Pfizer.
Please cite this article as: Puig L. Riesgo cardiovascular y psoriasis: papel de la terapia biológica. Actas Dermosifiliogr. 2012;103:853–62.