Monogenic autoinflammatory diseases are a heterogeneous emergent group of conditions that are currently under intensive study. We review the etiopathogenesis of these syndromes and their principal manifestations. Our aim is to propose a classification system based on the clinicopathologic features of typical skin lesions for routine clinical use in dermatology. Our focus is on diagnosis in pediatric practice given that this is the period when the signs and symptoms of these syndromes first appear. In Part 1 we discuss the course of urticaria-like syndromes, which include cryopyrin-associated periodic conditions and hereditary periodic fever syndromes. Pustular syndromes are also covered in this part. Finally, we review the range of therapies available as well as the genetic mutations associated with these autoinflammatory diseases.
Las enfermedades monogénicas autoinflamatorias son un grupo de enfermedades emergentes y heterogéneas en continuo estudio y desarrollo en la actualidad. Nuestro objetivo es revisar estas enfermedades desde el punto de vista de su etiopatogenia y principales manifestaciones, con el fin de proponer una clasificación, basada en las características clinicopatológicas de las lesiones cutáneas típicas, que resulte de utilidad en la práctica clínica habitual de los dermatólogos. El texto está enfocado en el diagnóstico de estos síndromes durante la edad pediátrica, ya que es el periodo habitual de aparición de los primeros síntomas y signos. La primera parte de la revisión se centrará en el desarrollo de los síndromes urticariformes, que incluyen a su vez las criopirinopatías y los síndromes hereditarios asociados a fiebres periódicas, y de los síndromes pustulosos, resumiendo al final del texto las alternativas terapéuticas de estos síndromes autoinflamatorios y sus mutaciones genéticas.
The autoinflammatory diseases are a group of diseases characterized largely by inborn errors of the innate immune system that lead to exaggerated, antigen-independent, inflammatory responses. In this respect, they differ from autoimmune diseases, where alterations in the adaptive immune system lead to an increase in antigen-dependent responses. The innate immunity system constitutes the first line of defense against pathogens and other noxious stimuli through the recognition of pathogen-associated molecular patterns (PAMPs) or damage-associated molecular patterns (DAMPs) and the subsequent activation of multiple inflammatory signaling cascades and the main effector cells of the innate immune system: macrophages, neutrophils, mast cells, and natural killer cells.1 Although all autoinflammatory syndromes have distinctive clinical features, the vast majority are characterized by early onset (in childhood or even during the neonatal period), recurrent episodes of fever, multisystemic inflammation, and a broad spectrum of cutaneous manifestations. Numerous classification systems exist in the literature for hereditary autoinflammatory diseases. In this review, we will focus on the clinicopathologic features of associated skin lesions to facilitate the recognition of these diseases in routine dermatology practice.
Urticaria-like SyndromesIn this first section, we will focus on 2 groups of autoinflammatory diseases: cryopyrin-associated periodic syndromes (CAPS) and hereditary periodic fever syndromes, which frequently manifest with urticaria-like lesions and erythematous and edematous plaques.
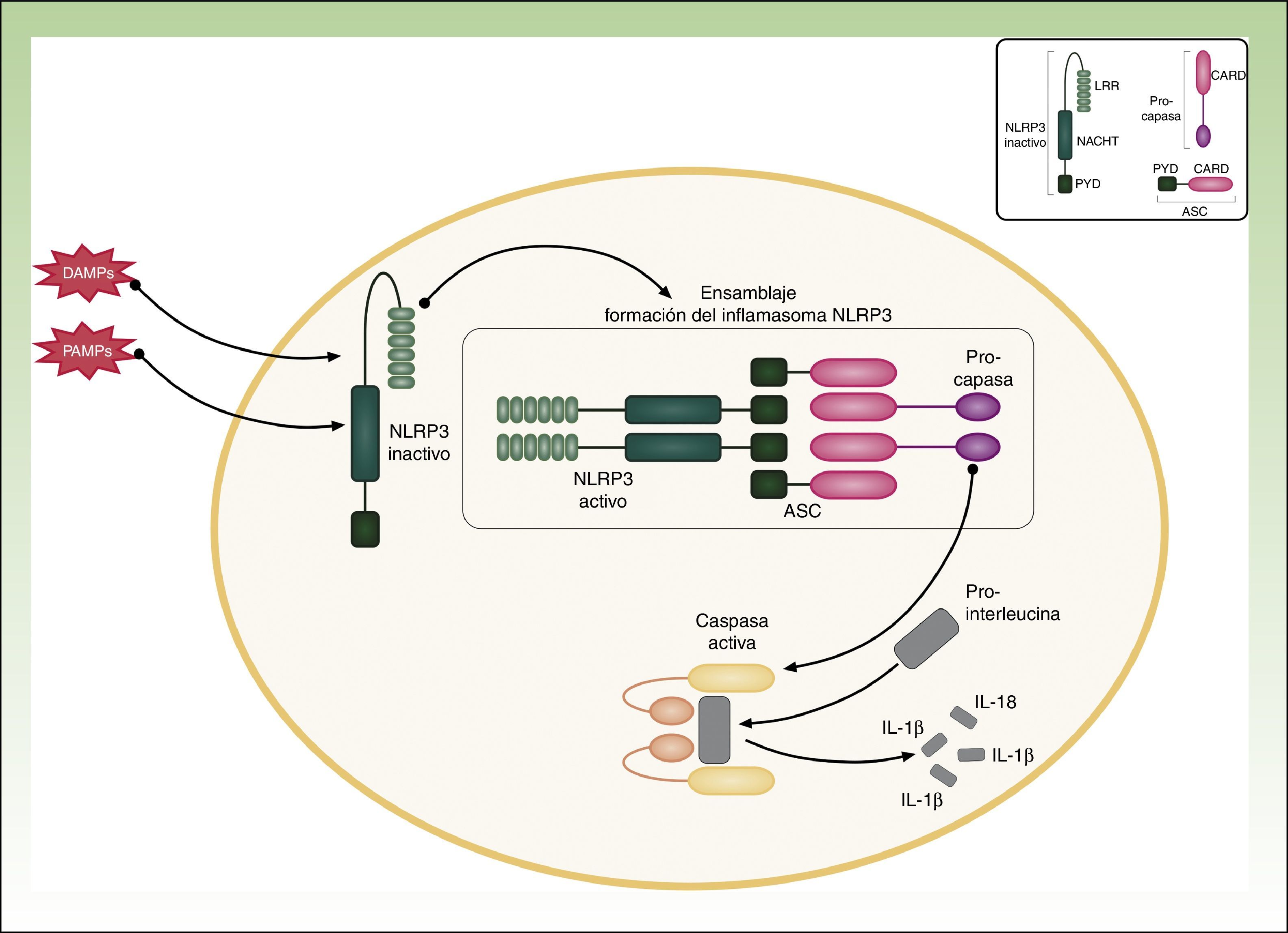
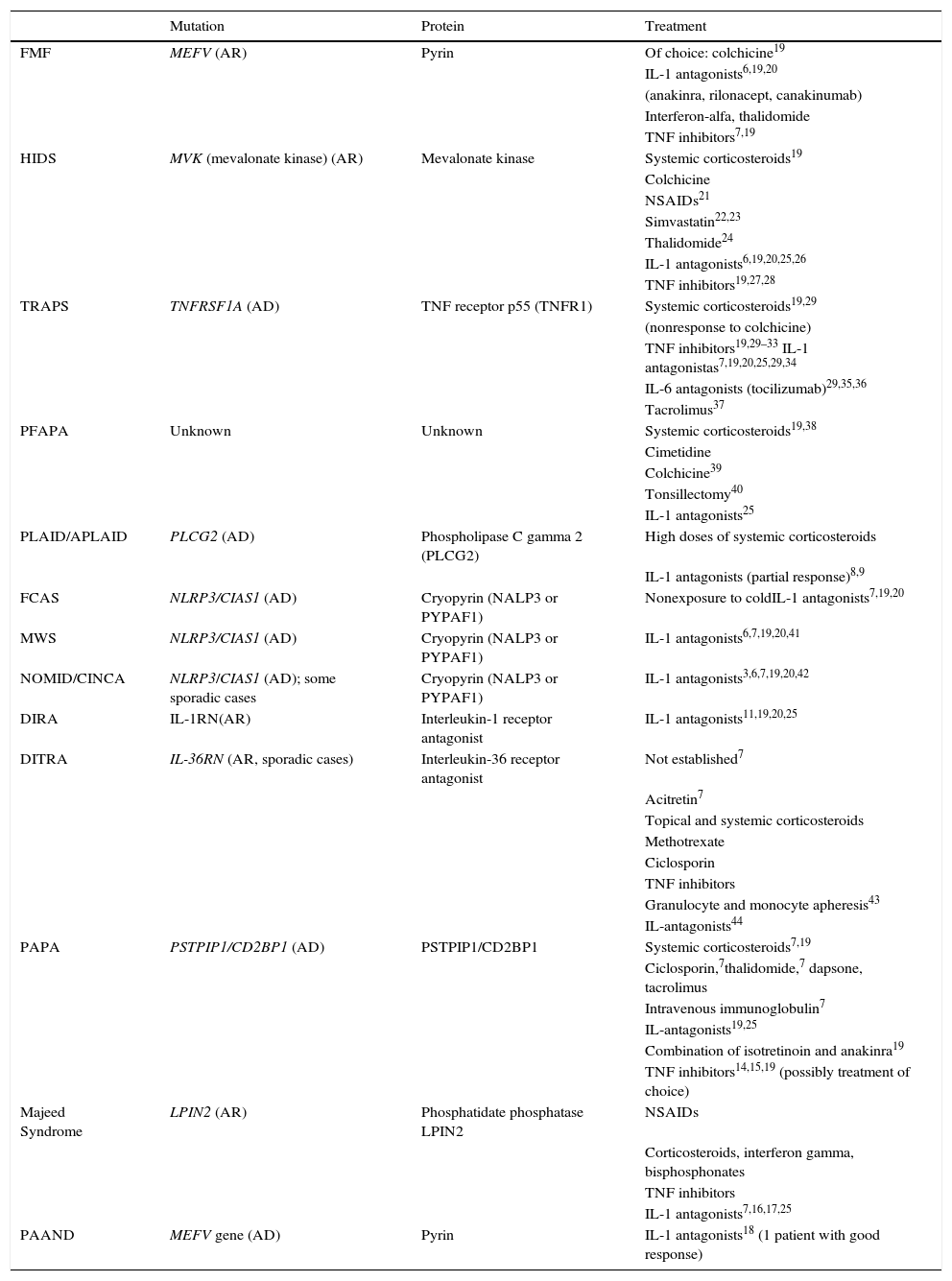
Cryopyrin-associated Periodic SyndromesCAPS encompass a group of 3 allelic disorders: familial cold autoinflammatory syndrome (FCAS), Muckle-Wells syndrome, and CINCA (chronic infantile neurological cutaneous articular syndrome) or NOMID (neonatal-onset multisystem inflammatory disease). The 3 disorders are inherited in an autosomal dominant manner, have variable penetrance, and are all caused by gain-of-function mutations in the NLRP3 (CIAS1) gene. NLRP3 codes for the protein cryopyrin (NALP3 or PYPAF1), which is an essential component of the inflammasome NLRP3. The inflammasome is a protein complex that responds to multiple stimuli by activating a series of intracellular interactions through caspase 1 that end with the production of powerful proinflammatory cytokines, such as interleukin (IL) 1β and IL-18 (Fig. 1). The activation of cryopyrin is triggered by the recognition of PAMPs or DAMPs. NLRP3 mutations that have been implicated in CAPS to date are gain-of-function mutations that constitutively activate the NLRP3 inflammasome, leading to an increased production of proinflammatory cytokines (Table 1).
Genetic Mutations and Treatment Alternatives.
| Mutation | Protein | Treatment | |
|---|---|---|---|
| FMF | MEFV (AR) | Pyrin | Of choice: colchicine19 |
| IL-1 antagonists6,19,20 | |||
| (anakinra, rilonacept, canakinumab) | |||
| Interferon-alfa, thalidomide | |||
| TNF inhibitors7,19 | |||
| HIDS | MVK (mevalonate kinase) (AR) | Mevalonate kinase | Systemic corticosteroids19 |
| Colchicine | |||
| NSAIDs21 | |||
| Simvastatin22,23 | |||
| Thalidomide24 | |||
| IL-1 antagonists6,19,20,25,26 | |||
| TNF inhibitors19,27,28 | |||
| TRAPS | TNFRSF1A (AD) | TNF receptor p55 (TNFR1) | Systemic corticosteroids19,29 |
| (nonresponse to colchicine) | |||
| TNF inhibitors19,29–33 IL-1 antagonistas7,19,20,25,29,34 | |||
| IL-6 antagonists (tocilizumab)29,35,36 | |||
| Tacrolimus37 | |||
| PFAPA | Unknown | Unknown | Systemic corticosteroids19,38 |
| Cimetidine | |||
| Colchicine39 | |||
| Tonsillectomy40 | |||
| IL-1 antagonists25 | |||
| PLAID/APLAID | PLCG2 (AD) | Phospholipase C gamma 2 (PLCG2) | High doses of systemic corticosteroids |
| IL-1 antagonists (partial response)8,9 | |||
| FCAS | NLRP3/CIAS1 (AD) | Cryopyrin (NALP3 or PYPAF1) | Nonexposure to coldIL-1 antagonists7,19,20 |
| MWS | NLRP3/CIAS1 (AD) | Cryopyrin (NALP3 or PYPAF1) | IL-1 antagonists6,7,19,20,41 |
| NOMID/CINCA | NLRP3/CIAS1 (AD); some sporadic cases | Cryopyrin (NALP3 or PYPAF1) | IL-1 antagonists3,6,7,19,20,42 |
| DIRA | IL-1RN(AR) | Interleukin-1 receptor antagonist | IL-1 antagonists11,19,20,25 |
| DITRA | IL-36RN (AR, sporadic cases) | Interleukin-36 receptor antagonist | Not established7 |
| Acitretin7 | |||
| Topical and systemic corticosteroids | |||
| Methotrexate | |||
| Ciclosporin | |||
| TNF inhibitors | |||
| Granulocyte and monocyte apheresis43 | |||
| IL-antagonists44 | |||
| PAPA | PSTPIP1/CD2BP1 (AD) | PSTPIP1/CD2BP1 | Systemic corticosteroids7,19 |
| Ciclosporin,7thalidomide,7 dapsone, tacrolimus | |||
| Intravenous immunoglobulin7 | |||
| IL-antagonists19,25 | |||
| Combination of isotretinoin and anakinra19 | |||
| TNF inhibitors14,15,19 (possibly treatment of choice) | |||
| Majeed Syndrome | LPIN2 (AR) | Phosphatidate phosphatase LPIN2 | NSAIDs |
| Corticosteroids, interferon gamma, bisphosphonates | |||
| TNF inhibitors | |||
| IL-1 antagonists7,16,17,25 | |||
| PAAND | MEFV gene (AD) | Pyrin | IL-1 antagonists18 (1 patient with good response) |
Abbreviations: AD, autosomal dominant inheritance; APLAID, autoinflammation PLCG2-associated antibody deficiency and immune dysregulation syndrome; AR, autosomal recessive inheritance; CINCA, chronic infantile neurological cutaneous articular syndrome; DIRA, deficiency of the interleukin-1-receptor antagonist DITRA, deficiency of the IL-36 receptor antagonist; HIDS, hyperimmunoglobulinemia D syndrome; IL, interleukin; FMF, familial Mediterranean fever; MWS, Muckle-Wells syndrome; NOMID, neonatal-onset multisystem inflammatory disease; NSAIDs, nonsteroidal anti-inflammatory drugs; PAPA, pyogenic sterile arthritis, pyoderma gangrenosum, and acne; PFAPA, periodic fever, aphthous stomatitis, pharyngitis, and cervical adenitis; PLAID, PLCG2-associated antibody deficiency and immune dysregulation syndrome; TRAPS, tumor necrosis factor receptor-associated syndrome; TNF, tumor necrosis factor.
FCAS is the least aggressive of the 3 CAPS. Clinical manifestations begin in the first year of life but are often noticeable in the neonatal period. They typically appear within 2 to 7hours of exposure to cold and resolve after approximately 12hours.2 Lesions are not triggered by contact with cold objects.
Cutaneous ManifestationsPatients with FCAS tend to develop erythematous edematous papules and plaques similar to those seen in acute urticaria, but with greater symmetry. Skin biopsy shows a predominantly neutrophilic, perivascular dermal infiltrate, contrasting with the predominantly lymphocytic, eosinophilic infiltrate without vasculitis seen in idiopathic urticaria. It should be noted, however, that both FCAS and urticaria exhibit a variable degree of dermal edema and the dominant component of the infiltrate may also vary. Biopsy thus is a very useful diagnostic aid but it is not pathognomic.
Associated Clinical ManifestationsIn addition to skin lesions, patients with FCAS typically experience episodes of fever, chills, conjunctival injection, sweating, dizziness, arthromyalgia, fatigue, and headache. Secondary amyloidosis is very rare and is observed in just 2% of patients. Patients may have an elevated peripheral white blood cell count during flares.
Muckle-Wells syndromeMuckle-Wells syndrome, also known as urticaria-deafness-amyloidosis syndrome, is similar to FCAS, but it has more severe clinical outcomes. Onset is also common in childhood, but the age of presentation is more variable. Multiple triggers have been described for Muckle-Wells syndrome, the most common of which are heat and cold.
Cutaneous ManifestationsThe clinicopathologic features of Muckle-Wells syndrome are similar to those of FCAS. A characteristic finding is urticaria with a predominantly neutrophilic inflammatory infiltrate without edema in the dermis.
Associated Clinical ManifestationsAssociated clinical manifestations include headache, aseptic meningitis, conjunctival injection, papillary edema, arthralgia, arthritis, and episodes of fever, which last longer than in FCAS (up to 36hours) and are more intermittent. Patients may also develop progressive sensorineural deafness, which starts in childhood and can progress to complete hearing loss. Between 25% and 33% of patients develop secondary amyloidosis with frequent involvement of the kidneys.2 Laboratory studies during flares typically show elevated erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP), thrombocytosis, anemia, and neutrophilic leukocytosis.
Neonatal-onset Multisystem Inflammatory Disease/Chronic Infantile Neurological Cutaneous Articular SyndromeNOMID, or CINCAS, is the severest of the CAPS. The first symptoms tend to appear shortly after birth. Neonatal onset has been reported for 70% of patients and almost 100% of patients show symptoms by the age of 6 months.
Many cases are sporadic and the germline NLRP3 mutation is present only in 55% to 60% of patients, suggesting the existence of considerable genetic heterogeneity with a wide variety of somatic mutations.3,4 Because of this considerable heterogeneity, some authors have suggested that patients with suspected CAPS should undergo initial evaluation before being referred for genetic testing. Proposed criteria include a history of at least 3 recurrent episodes of moderate fever and urticaria, an age of under 20 years of age at disease onset, and elevated CRP levels.4
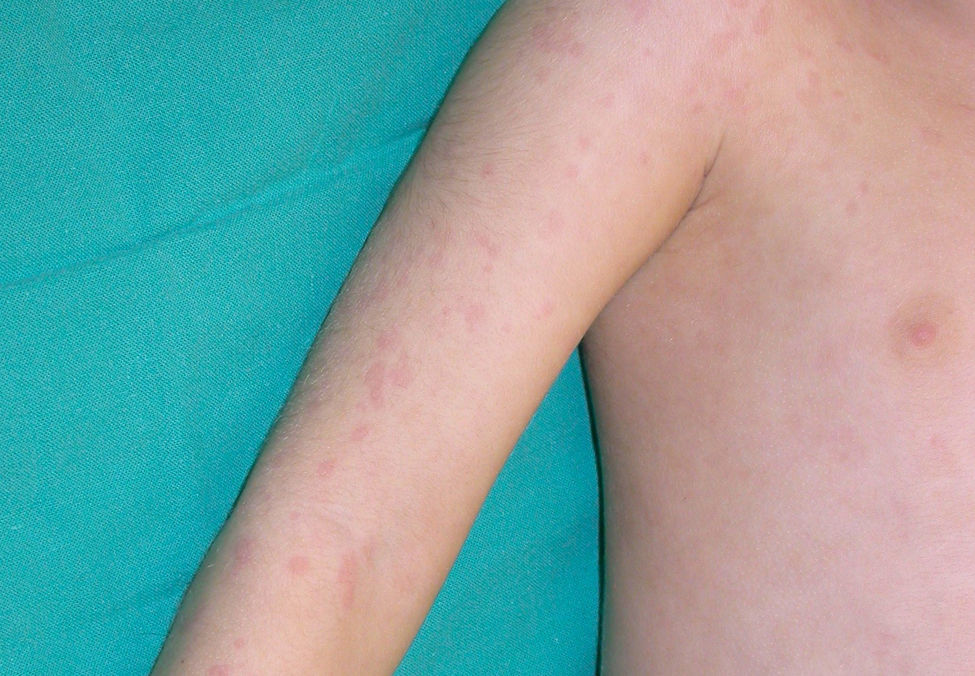
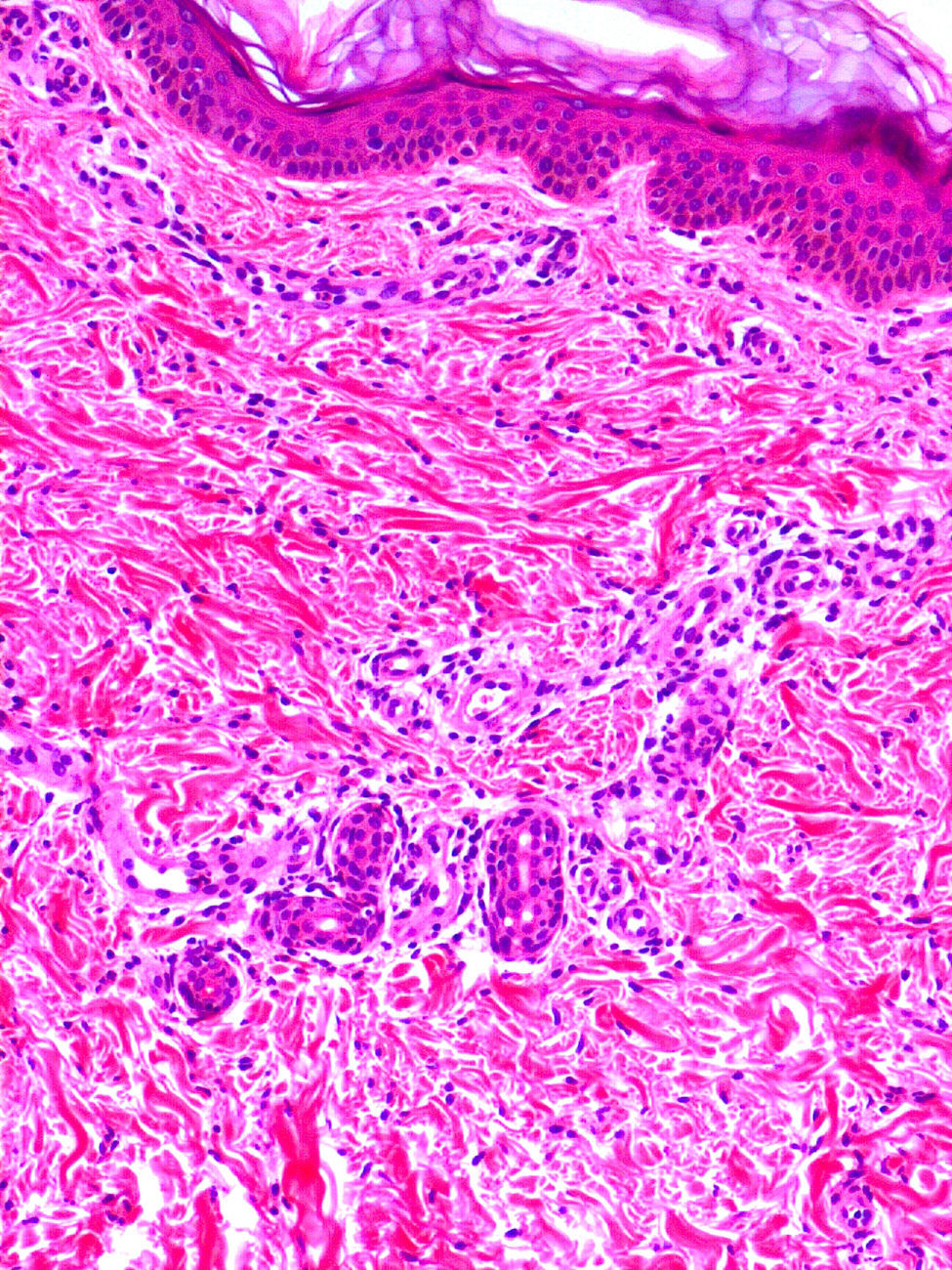
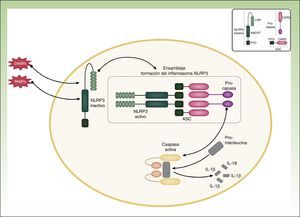
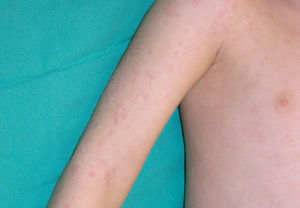
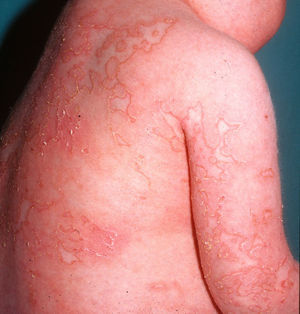
Cutaneous ManifestationsA characteristic skin manifestation of CINCA/NOMID is a migratory nonpruritic urticaria-like eruption that, unlike in other CAPS, persists for lifetime and has no clear triggers such as cold (Fig. 2). Biopsy shows a superficial and deep perivascular mixed inflammatory infiltrate composed of lymphocytes, neutrophils, and eosinophils together with neutrophilic eccrine hidradenitis5 (Fig. 3).
Noncutaneous clinical manifestations are more severe in NOMID and can have serious consequences for neurological development. Common manifestations are short recurrent episodes of fever, enlarged lymph nodes, joint symptoms, hepatosplenomegaly, secondary amyloidosis, neurological involvement with cerebral atrophy, mental retardation, chronic aseptic neutrophilic meningitis, seizures, transient hemiplegia, sensorineural deafness, morning headaches, anterior uveitis, increased intracranial pressure, papilledema, and blindness.6 Characteristic facial features such as flattening of the nasal bridge, macrocephaly, a prominent forehead, and ocular hypertelorism may also be seen. The severity of joint disease is variable. Approximately 50% of children develop severe joint deformities in the first year of life due to cartilage overgrowth and bony overgrowth of epiphyses, which can simulate bone tumors in radiological studies. Typical laboratory study findings include elevated acute phase reactants with leukocytosis, thrombocytosis, eosinophilia, and increased immunoglobulin (Ig) levels in serum.
Hereditary Periodic Fever SyndromesIn this second group of autoinflammatory diseases, we have included syndromes characterized by the presence of a nonspecific skin eruption in the form of erythematous papules or macules or plaques that are frequently associated with abdominal pain and recurrent episodes of fever, separated by variable periods of time. This variable incidence of febrile episodes is one of the main distinguishing features of hereditary periodic fever syndromes, which consist, on the one hand, of familial Mediterranean fever, Marshall syndrome (periodic fever, aphthous stomatitis, pharyngitis, adenitis, and mevalonate kinase deficiency) and hyperimmunoglobulinemia D syndrome (HIDS), in which fever tends to last for less than a week, and on the other hand, of tumor necrosis factor receptor-associated syndrome (TRAPS), in which fever can last for several weeks. We will also review PLAID/APLAID, AISLE, and NAIAD in this section.
Familial Mediterranean FeverFamilial Mediterranean fever (FMF) is an autosomal recessive autoinflammatory syndrome. It results from mutations in the MEFV gene, which is located on chromosome 16p13 and encodes the pyrin (or marenostrin) protein. Over 200 MEFV mutations have been reported to date, and the most severe cases of FMF are associated with homozygous M694V mutations. Diagnosis, however, is essentially clinical as not all patients with typical FMF manifestations have a mutation in MEFV. It is therefore likely that other factors are involved in the development of FMF, including, possibly, mutations in other genes.
Cutaneous ManifestationsPatients typically have a well-delimited, unilateral or bilateral erythematous edematous erysipeloid plaque on the anterior surface of the lower extremities. The plaque is most often located under the knees or on the dorsum of the foot. It generally has a maximum diameter of 15cm and tends to recur at the same site. Purpuric lesions are also observed on the face, trunk, and extremities. Other cutaneous manifestations that are more common in patients with FMF than in the general population are Schönlein-Henoch purpura (5% of children) and polyarthritis nodosum. It is important to note that many patients do not present skin lesions, and when they do, these can be highly variable. Skin biopsy tends to show a predominantly neutrophilic infiltrate with karyorrhexis.
Associated Clinical ManifestationsAssociated clinical manifestations tend to present before the age of 30. They consist of recurrent episodes of high fever (38.5°C-40°C) accompanied by severe asthenia, monoarthritis of large joints (mainly affecting the lower extremities), and acute abdominal pain, possibly with peritonitis. These episodes have a mean duration of 1 to 3 days. Apart from peritonitis, patients may develop other types of serositis, such as pleuritis and pericarditis, which both cause chest pain, as well as scrotal pain due to inflammation of the vaginal tunic.7 Neurological involvement is uncommon, although there have been rare reports of meningitis during acute episodes. Chronic meningitis has not been described. Typical serological alterations during acute episodes include leukocytosis and increased acute phase reactants, ESR, CRP, and fibrinogen. ESR and CRP levels remain elevated during flares. This persistent increase reflects the existence of subclinical inflammation, which, if left untreated, often leads to secondary amyloidosis, which is the most common and most serious outcome of FMF.
TNF Receptor–Associated Periodic SyndromeTNF is an inflammatory cytokine that has a key role in pyrexia, cachexia, production of other cytokines, expression of adhesion molecules, activation of leukocytes, and resistance to cell pathogens. The TNF receptor (TNFR) acts by antagonizing and regulating the actions of circulating TNF. TRAPS is due to a gain-of-function mutation in the TNFR gene that results in dysregulated TNF production. It is an autosomal dominant disorder with mutations in the TNFRSF1A gene, which codes for TNFR1 (also known as p55 or CD120a). The vast majority of TNFRSF1A mutations identified to date are substitution mutations located at exons 2, 3 and 4 of the gene, which codes for the extracellular domains of TNFR1. De novo mutations have also been described in some patients.
Cutaneous ManifestationsCutaneous lesions have been described in 69% to 87% of patients with TRAPS. The most common manifestation (observed in 40% of patients) is a centrifugal migratory erythematous plaque associated with underlying myalgia, explaining why this condition is sometimes referred to as painful erythema. The plaque migrates from a proximal to a distal site in a process that can last minutes or days. The migration often coincides with the spread of the myalgia. On other occasions, lesions present as urticaria-like plaques or a generalized maculopapular erythematous rash that can coalesce into annular or serpiginous plaques, frequently leaving behind significant ecchymosis. Periorbital edema and conjunctivitis can be diagnostic of TRAPS when accompanied by consistent clinical findings. Skin biopsy shows a perivascular, interstitial mononuclear cell infiltrate. There have been rare reports of leukocytoclastic vasculitis and recurrent panniculitis.
Associated Clinical ManifestationsClinical manifestations of TRAPS appear more frequently during childhood or adolescence (mean age of 10 years at diagnosis), but they can appear at any time between the first year of life and the sixth decade. Febrile episodes are common and can last for weeks, although on average they last for approximately 2 weeks. Many patients report sudden, intense abdominal pain during febrile episodes. Similarly to in FMF, this pain can be misdiagnosed as acute abdomen, and approximately a third of patients are sent for abdominal surgery. As mentioned, skin lesions in TRAPS are associated with musculoskeletal involvement with severe myalgia. Deep-tissue biopsy shows monocytic fasciitis, which can also be observed by magnetic resonance imaging. Approximately 50% of patients develop eye problems, such as recurrent conjunctivitis or anterior uveitis. Other potential manifestations include arthralgia or arthritis, pleuritis, pericarditis, scrotal pain, headache, ascetic meningitis, optic neuritis, and behavioral changes. Amyloidosis is the most feared outcome in TRAPS. It has been reported in up to 24% of patients with cysteine residue mutations and 2% of patients with noncysteine mutations who do not receive adequate treatment.7 Blood test alterations during febrile episodes include polyclonal gammopathy, leukocytosis, thrombocytosis, increased ESR, PCR, ferritin, serum amyloid A protein, and fibrinogen, which depending on the severity and chronic nature of the condition may not return to normal levels during afebrile periods.
Mevalonate Kinase Deficiency–Hyperimmunoglobulin D SyndromeHIDS can be divided into 2 entities: classic HIDS, which is associated with the MVK mutation, and variant HIDS, which does not have this mutation or any biochemical evidence of reduced mevalonate kinase activity. Variant HIDS has been linked to low-penetrance mutations in the TNFRSF1A gene and patients often have more latent symptoms. The mutated gene in classic HIDS is the kinase mevalonate gene, located on chromosome 12q24. IgD levels are typically, though not necessarily, elevated. Furthermore, no correlation has been observed between IgD elevation and disease severity or frequency of flares. Considering that IgD levels may be within normal limits in HIDS, they are probably not directly responsible for its clinical manifestations. Their increase is more likely to be a response to an initial autoinflammatory process triggered by trauma, vaccination, stress, or other factors.
Cutaneous ManifestationsSkin lesions occur in 80% of patients with HIDS and can be highly variable. The most common manifestation is a macular or a maculopapular erythematous rash, with varying degrees of confluence, that mainly affects acral sites. Other lesions include urticaria, erythematous papules or nodules, or petechia, and less frequently, lesions similar to those seen in Sweet syndrome, cellulitis type lesions, Henoch-Schönlein purpura, erythema elevatum diutinum, and other forms of vasculitis. Some patients may develop mouth and genital ulcers.
Associated Clinical ManifestationsEpisodes of fever and chills tend to appear within the first 4 years of life (before the age of 12 months in 80% of patients) and generally last between 3 and 7 days. They typically recur every 4 to 6 weeks and are often triggered by vaccination, trauma, surgery, or stress. They are also frequently associated with abdominal pain together with diarrhea and vomiting, serositis, headache, hepatosplenomegaly, polyarthralgia, and nonerosive large-joint arthritis. Many patients with HIDS have soft, swollen bilateral cervical lymph nodes. Blood tests show increased acute phase reactants, leukocytosis, and neutrophilia. IgD serum levels are persistently elevated (≥100U/mL) in over 90% of patients, and 80% of these also have increased IgA (≥260mg/dL). Elevated IgD levels, however, are not specific to HIDS (they are also found in FMF and TRAPS) and as already mentioned, IgD may even be within normal ranges in HIDS, particularly in patients younger than 3 years old.7 A moderate increase in mevalonic acid may be observed in urine during flares.
PLCG2-associated Antibody Deficiency and Immune Dysregulation and Autoinflammation PLCG2-associated Antibody Deficiency and Immune DysregulationPLAID syndrome (PLCG2-associated antibody deficiency and immune dysregulation), also known as familial atypical cold urticaria, is a recently described disorder associated with immunodeficiency, hypogammaglobulinemia, and autoimmune disease caused by deletions in the phospholipase C, gamma-2 (PLCG2) gene. Patients present with urticaria, erythema, burning, and granulomas after exposure to cold and they may also have fever, recurrent sinusitis and lung infections, asthma, and autoimmune disorders. Laboratory studies show increased IgE, decreased circulating IgM, IgG, IgA, and CD19+ B cells, and positive antinuclear antibodies (ANAs).
APLAID syndrome (autoinflammation PLCG2-associated antibody deficiency and immune dysregulation) is similar to PLAID, but is additionally associated with autoinflammatory disorders caused by point mutations in PLCG2. Clinical manifestations include bronchiolitis, recurrent lung infections, cellulitis, arthralgia, enterocolitis, mild immunodeficiency without autoantibodies, and serious eye problems such as corneal erosions, blisters, ulcers, ocular hypertension, and cataracts. Laboratory studies show similar findings to in PLAID, but the ANAs are negative. The PLCC2 mutation in PLAID is a gain-of-function mutation that results in increased signaling. The mutation responsible for APLAID, by contrast, decreases the threshold required for PLCC2 activation. Both syndromes have highly variable cutaneous manifestations, ranging from cold-induced urticaria-like lesions to vesicular-pustular eruptions that worsen with heat and may be seen in both entities. Fixed granulomatous rashes are more common in PLAID, while vesicular-bullous eruptions and recurrent sterile cellulitis plaques are more common in APLAID. In both syndromes, the histopathologic study shows a dense interstitial, perivascular dermal infiltrate composed of neutrophils, lymphocytes, histiocytes, and eosinophils, and leukocytoclastic vasculitis with prominent karyorrhexis. The mutation described for APLAID causes an increase in intracellular calcium, which could activate the NLRP3 inflammasome, generating IL-1 (which would explain why patients partially respond to treatment with anti-IL-1). Depending on the mutational variants present, patients may develop different phenotypic traits, consisting of both cutaneous and systemic manifestations that may or may not be triggered by cold.8,9
Autoinflammatory Syndrome With LymphedemaAutoinflammatory syndrome associated with lymphedema (AISLE) is due to mutations in the MDFIC gene, which contains the MyoD family inhibitor domain.
Cutaneous ManifestationsThe main cutaneous manifestation of AISLE is an extensive urticaria-like eruption.
Associated Clinical ManifestationsOther manifestations include fever accompanied by progressive swelling of the scrotum and lower extremities. Histopathologic examination shows a reduction in the number and size of lymphatic vessels in the area.
NLRP1-associated Autoinflammation With Arthritis and DyskeratosisNLRP1-associated autoinflammation with arthritis and dyskeratosis (NAIAD) was described by Grandemange. Constitutive activation of NLRP1 leads to increased activation of caspase 1 and a subsequent increase in IL-18 production.
Cutaneous ManifestationsNAIAD is characterized by erythematous-brownish hyperkeratotic papules with a prickly appearance on the trunk and extremities. Dyskeratosis is the main finding and the associated cutaneous manifestations are similar to those seen in phrynoderma, a vitamin A deficiency disorder.
Associated Clinical ManifestationsRecurrent fever and arthritis are common in patients with NAIAD.
Pustular SyndromesDeficiency of the Interleukin-1-Receptor AntagonistDeficiency of the interleukin-1-receptor antagonist (DIRA) is caused by mutations in IL1RN (IL-1 receptor antagonist) that give rise to a shorter, truncated protein that lacks IL-1 antagonist activity, resulting in the activation of an inflammatory cascade mediated by IL-1 that causes severe bone and skin inflammation.
Cutaneous ManifestationsPatients typically present generalized erythematous plaques with superficial pustules, simulating pustular psoriasis, at birth or in the first months of life. There may also be diffuse ichthyosiform scaling that often spares the palms and soles. Mouth ulcers and nail changes in the form of pitting or anonychia are common. Skin biopsy shows subcorneal or spongiform pustules with neutrophils in the epidermis and a dermal neutrophilic infiltrate with variable involvement of the hair follicles, blood vessels, and eccrine glands; immunohistochemical studies show pronounced IL-17 overexpression.10
Associated Clinical ManifestationsFlares tend to be accompanied by fever, conjunctivitis, pulmonary infiltrates with respiratory distress, thrombotic episodes, and characteristic bone alterations, such as periostitis, heterotopic ossifications, cervical spinal fusion, and chronic recurrent sterile multifocal osteomyelitis. This last condition, which frequently affects the epiphyses of the long bones, can cause delayed growth. Blood tests show increased acute phase reactants, mild chronic anemia, and neutrophilic leukocytosis without fever. If the autoinflammatory response is severe enough, it can cause multiorgan failure and death.10 Mutations in DIRA have also been linked to preterm delivery as well as intrauterine multiorgan involvement and death.11
Deficiency of Interleukin-36-Receptor AntagonistMutations associated with deficiency of the IL-36 receptor antagonist (DITRA) have been linked to both familial (recessive autosomal inheritance) and sporadic cases of generalized pustular psoriasis (GPP).
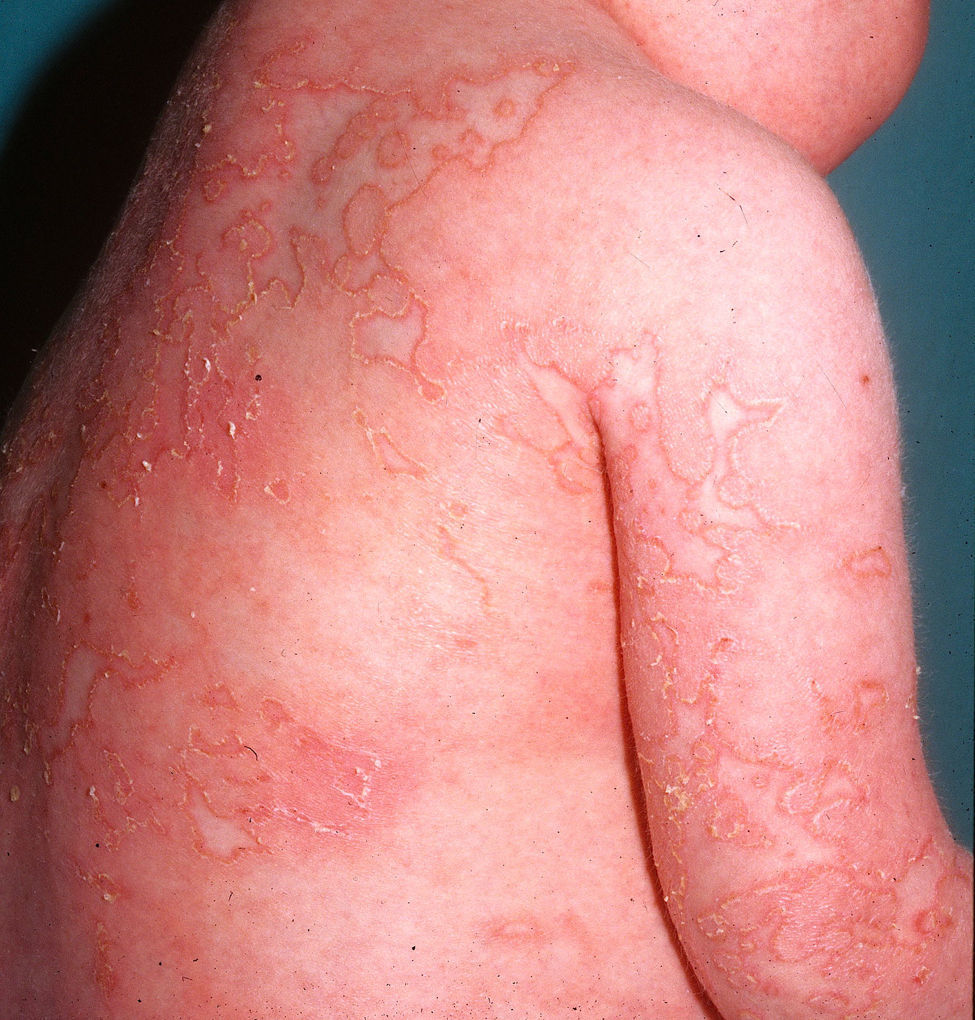
Cutaneous ManifestationsCutaneous lesions in DITRA present as an acute generalized pustular eruption on an erythematous base12 (Fig. 4), similar to that seen in a GPP flare. DITRA lesions are also characterized by recurrent bouts followed by diffuse superficial scaling. In some cases, the lesions can present as psoriasis vulgaris or pustular acral lesions with nail destruction in the form of continuous acrodermatitis. It is possible that many patients who experience GPP flares in the absence of previous manifestations of psoriasis vulgaris could have the DITRA mutation. The frequency of flares varies considerably from one patient to the next, and the lesions may become chronic and acquire the form of erythematous plaques without pustules.12 Histopathologic examination shows the typical features of GPP with parakeratosis, psoriasiform acanthosis, and spongiform pustules, and a predominance of CD8+ T cells, CD3+ T cells, and macrophages.
Associated Clinical ManifestationsCutaneous flares have a variable frequency and are associated with high fever, general malaise, and asthenia. Other organs are not affected. Flares generally start in childhood but adult onset has been described. Multiple triggers have been identified and include bacterial and viral infections, menstruation, pregnancy, and drugs, among others. High fever and a poor general state of health are the main distinctive features of DITRA, but involvement in most cases is limited to the skin, unlike in DIRA. Laboratory studies show elevated acute phase reactants and lactate and low levels of albumin, zinc, and calcium.
Pyogenic Sterile Arthritis, Pyoderma Gangrenosum, AcnePyogenic sterile arthritis, pyoderma gangrenosum, and acne (PAPA), also known as familial recurrent arthritis, is a rare autosomal dominant disease characterized by the triad of pyogenic arthritis, pyoderma gangrenosum, and cystic acne. The genetic mutation is located on chromosome 15 q24-25.1, which encodes the proline-serine-threonine phosphatase-interacting protein (PSTPIP1), which has incomplete penetrance and highly variable expression.13 There have been reports of PAPA syndrome in patients without the above genetic mutation, although new mutations are being discovered.14,15 PSTPIP1 is a cytoskeletal protein that is strongly expressed in hematopoietic cells and modulates the activation of T cells, cytoskeletal organization, and release of IL-1β. The mutation causes IL-1β overproduction.
Cutaneous ManifestationsCutaneous manifestations of PAPA syndrome typically begin in childhood but worsen considerably at puberty. They consist of severe forms of cystic acne, pathergy, and recurrent sterile ulcers with elevated violaceous borders that are very similar to those seen in pyoderma gangrenosum. An association has also been reported with psoriasis and rosacea.15
Associated Clinical ManifestationsPatients with PAPA syndrome may develop fever, although episodes are inconsistent and do not appear to follow any clear pattern. Fever may be accompanied by recurrent episodes of sterile erosive arthritis. This arthritis may be spontaneous or triggered by minor trauma, and although it tends to disappear after puberty, it occasionally persists into adulthood and is severely disabling. Other less common clinical presentations are recurrent otitis, pharyngeal papillomatosis, lymphadenopathy, splenomegaly, thrombocytopenia, hypergammaglobulinemia, hemolytic anemia, and sulfonamide-induced pancytopenia.
Majeed SyndromeMajeed syndrome, like DIRA, is characterized by considerable inflammation of the bones. It responds well to IL-1 antagonists, supporting the hypothesis of an IL-1β-dependent pathogenesis and highlighting the important role of this cytokine in bone inflammation.16 It mainly presents as chronic recurrent multifocal osteomyelitis, neutrophilic dermatosis, and congenital dyserythropoietic anemia with microcytosis. Various homozygous LPIN2 mutations have been identified in patients with Majeed syndrome. The LPIN2 gene encodes a protein that modulates the transcription of coactivators that regulate genes involved in lipid metabolism.
Cutaneous ManifestationsSkin lesions are not a characteristic finding in Majeed syndrome. Pustular involvement is more common and there have also been reports of dermatoses similar to those seen in Sweet syndrome and psoriasis.
Associated Clinical ManifestationsOsteomyelitis flare-ups are invariably accompanied by high fever, pain, and swelling of the large joints. Foci of osteomyelitis are more common in the clavicles, sternum, and the long bones, and less common in the vertebral bodies and jaw. This chronic inflammation causes delayed growth, low stature, and flexion contractures, and radiographic images show osteolytic lesions and areas of sclerosis.
Autoinflammatory Syndrome Associated With Pyrins and Neutrophilic DermatosisAutoinflammatory syndrome associated with pyrins and neutrophilic dermatosis is a new autoinflammatory familial pustular neutrophilic dermatosis described by Masters at the 8th International Congress of Familial Mediterranean Fever and Systemic Auto-Inflammatory Diseases in 2015. It is inherited in an autosomal dominant manner and is caused by monoallelic mutations in the MEFF gene that are different to those involved in FMF, as they affect a highly conserved region of the pyrin protein that constitutes the nexus between this protein and an inhibitory protein known as 14-3-3.
Cutaneous ManifestationsCharacteristic cutaneous manifestations are multiple facial pustules and pyoderma gangrenosum–like lesions.17
Associated Clinical ManifestationsThe skin lesions tend to be accompanied by fever, arthromyalgia, and myositis, and increased acute phase reactants.
Syndromes With Mucocutaneous UlcersPeriodic Fever Syndrome With Aphthous Stomatitis, Pharyngitis, and AdenitisPeriodic fever syndrome with aphthous stomatitis, pharyngitis, and adenitis, also known as Marshall syndrome, is the most common of all the periodic fever syndromes. It is mostly sporadic and tends to spontaneously resolve within the first decade of life. The episodes occur approximately once a month and generally last between 3 to 6 days. No seasonal patterns have been observed.
No genetic defects have been identified to date, although there have been reports of cases with a certain degree of familial aggregation.
Cutaneous ManifestationsPatients typically develop small numbers of small aphthous lip or mouth ulcers that heal without scarring, in addition to nonspecific generalized urticaria-like erythema of varying intensity in a small proportion of patients.
Associated Clinical ManifestationsAcute recurrent pharyngitis and tonsillitis with negative cultures and enlarged cervical lymph nodes are common. There may also be accompanying constitutional symptoms, such as abdominal pain, headache, arthralgia, cough, nausea, and diarrhea. As with other periodic fever syndromes, blood tests may show elevated acute phase reactants during flares.
Periodic Fever Syndrome, Immunodeficiency, and ThrombocytopeniaPeriodic fever, immunodeficiency, and thrombocytopenia (PFIT) is a new autoinflammatory syndrome described by Brogan et al.
Cutaneous ManifestationsSevere oral ulcers that result in deforming scarring and microstomia are the main cutaneous manifestation in PFIT.
Associated Clinical ManifestationsAdditional clinical features include mouth ulcers, failure to thrive, recurrent infections, and thrombocytopenia.
Behçet-like Autoinflammatory Syndrome Associated With A20 HaploinsufficiencyZhou et al.18 published a report on 6 unrelated families with a previously undescribed loss-of-function mutation in the TNFAIP3 gene. This mutation results in haploinsufficiency, which, in turn, leads to an early-onset systemic inflammation syndrome. The mutation causes increased degradation of IkBα, which leads to translocation of NFkB p65 and increased expression of proinflammatory cytokines mediated by this transcription factor.
Cutaneous ManifestationsThe clinical manifestations are very similar to those observed in childhood-onset Behçet disease, with oral and genital ulcers.
Associated Clinical ManifestationsPatients also frequently develop fever, general malaise, eye inflammation, and early-onset arthralgia and arthritis.
Behçet SyndromeBehçet syndrome is a systemic vasculitis that can affect any of the vessels in the body. It is endemic in Eastern Mediterranean countries (Silk Road) and Eastern and Central Asian countries and is associated with HLA-B51.
Cutaneous ManifestationsThe clinical manifestations are heterogeneous and consist of erythema nodosum lesions, pustules, necrotizing ulcers, superficial thrombophlebitis and vasculitis, and lesions similar to those seen in pyoderma gangrenosum or Sweet syndrome, among others. It is associated with positive pathergy reactions. Recurrent mouth ulcers are the first manifestation in a high percentage of patients. They tend to be multiple, painful, and heal without leaving a scar, unlike genital ulcers, which leave characteristic flat scars. Neutrophilic vasculitis and/or thrombosis are characteristic histopathologic findings.
Associated Clinical ManifestationsEye involvement is the main cause of morbidity in Behçet syndrome. The most common condition is posterior uveitis, but anterior uveitis and uveitis with hypopyon are also observed. Patients may also develop arthritis and a large spectrum of manifestations secondary to systemic vasculitis, such as arterial and venous thrombosis, aneurysms, and digestive and neurological symptoms.
Ethical DisclosuresProtection of humans and animalsThe authors declare that no tests were carried out in humans or animals for the purpose of this study.
Confidentiality of dataThe authors declare that they have followed their hospital's protocol on the publication of data concerning patients.
Right to privacy and informed consentThe authors declare that no private patient data appear in this article.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Hernández-Ostiz S, Prieto-Torres L, Xirotagaros G, Noguera-Morel L, Hernández-Martín Á, Torrelo A. Enfermedades autoinflamatorias en dermatología pediátrica. Parte 1: síndromes urticariformes, síndromes pustulosos y síndromes con ulceraciones cutáneo-mucosas. Actas Dermosifiliogr. 2017;108:609–619.