Pivotal trials with omalizumab for treatment of chronic spontaneous urticaria (CSU) are generally run over 12 to 24weeks. However, in clinical practice, many patients need longer treatment. In this article, we present an algorithm for treatment with omalizumab.
Material and methodsThe consensus document we present is the result of a series of meetings by the CSU working group of “Xarxa d’Urticària Catalana i Balear” (XUrCB) at which data from the recent literature were presented, discussed, compared, and agreed upon.
ResultsTreatment with omalizumab should be initiated at the authorized dose, and is adjusted at 3-monthly intervals according to the Urticaria Activity Score Over 7days, the Urticaria Control Test, or both.
ConclusionsThe algorithm proposed is designed to provide guidance on how to adjust omalizumab doses, how and when to discontinue the drug, and how to reintroduce it in cases of relapse.
Los ensayos pivotales de omalizumab en urticaria crónica espontánea (UCE) tienen un periodo de tratamiento de entre 12 y 24semanas. Sin embargo, muchos pacientes en práctica clínica requieren periodos de tratamiento más prolongados. Por ello el objetivo es presentar un algoritmo de manejo del fármaco.
Materiales y métodosEl documento de consenso que detallamos nace de la puesta en común, aceptación, revisión y confrontación de la literatura reciente del grupo de trabajo de UCE «Xarxa d’Urticària Catalana i Balear» (XUrCB).
ResultadosSe inicia el tratamiento a dosis autorizada y se ajusta la dosis en intervalos trimestrales en función del Urticaria Activity Score de los últimos 7días (UAS7) y/o el Urticarial Control Test (UCT).
ConclusionesEl algoritmo propuesto pretende servir de guía respecto a cómo ajustar dosis, cómo y cuándo parar el fármaco y el modo de reintroducirlo en casos de recaída.
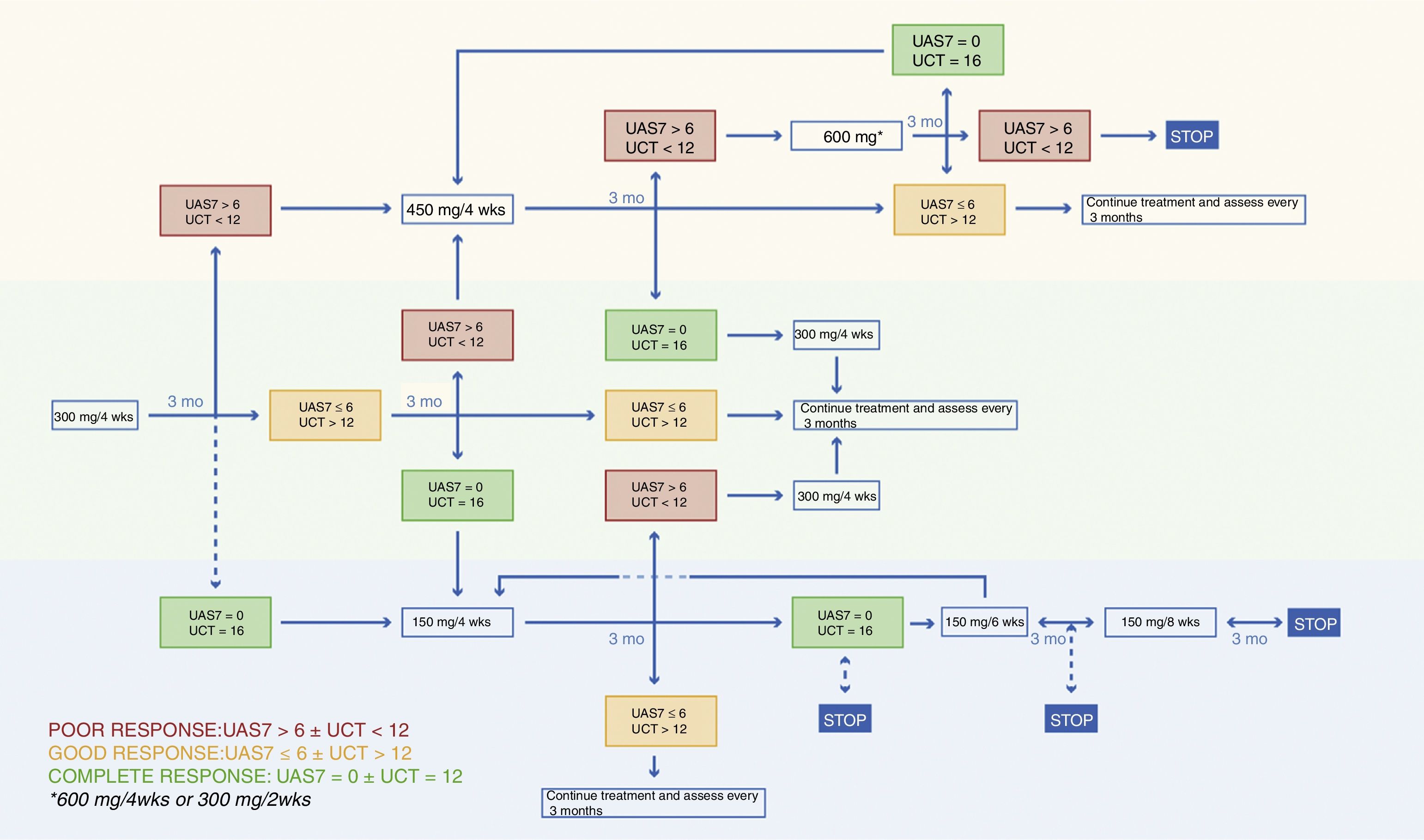
An algorithm is needed to guide the treatment of chronic spontaneous urticaria (CSU) with omalizumab because the pivotal trials are generally 3 to 6 months in length and patients with CSU often require more prolonged treatment.1–3 Furthermore, since CSU can go into spontaneous remission, there is also a need to establish guidelines on how and when treatment should be discontinued, another question not answered by the data from clinical trials. Thirdly, our algorithm introduces, for the first time, a dose increase to 450mg or 600 mg/4 wks for patients in whom the standard regimen does not achieve adequate disease control, which is defined as a weekly Urticaria Activity Score (UAS7) of 6 or less.4 The algorithm proposed by this working group is shown in Figure 1.
Materials and MethodsThis consensus document has been developed on the basis of sharing, approval, revision, and comparison of the recent literature. The authors of this document are all members of a working group called the Xarxa d’Urticària Catalana i Balear (XUrCB), whose members are based in Catalunya and the Balearic Islands. The group was set up 2 years ago to share experiences and reach a consensus on the management of patients with CSU.
The working group met every 3 months. An initial algorithm was proposed based on data from pivotal trials, reports in scientific journals on the use of omalizumab in clinical practice, and the experience of the group members themselves who, between them, have treated over 300 patients with omalizumab. The Medline, Embase, and Cochrane databases were searched using the OVID platform. After a series of meetings and following a study, accepted for publication,4 on the effectiveness of increasing the dose of omalizumab to 450 or 600mg in certain cases, the group agreed on the treatment algorithm for omalizumab shown in Figure 1.
Candidates for Treatment With OmalizumabSince CSU is a condition that can persist for months or years, patients require safe and effective long-term treatment. The EAACI/GA2LEN/EDF/WAO guidelines recommend second generation anti-H1 antihistamines at licensed doses as a first-line treatment for CSU, and increasing the dose of the selected antihistamine by up to 4 times as a second-line option.5 However, a substantial proportion of patients continue to experience symptoms even at the higher antihistamine doses.6,7 It is these patients with treatment-refractory CSU who are candidates for treatment with omalizumab.
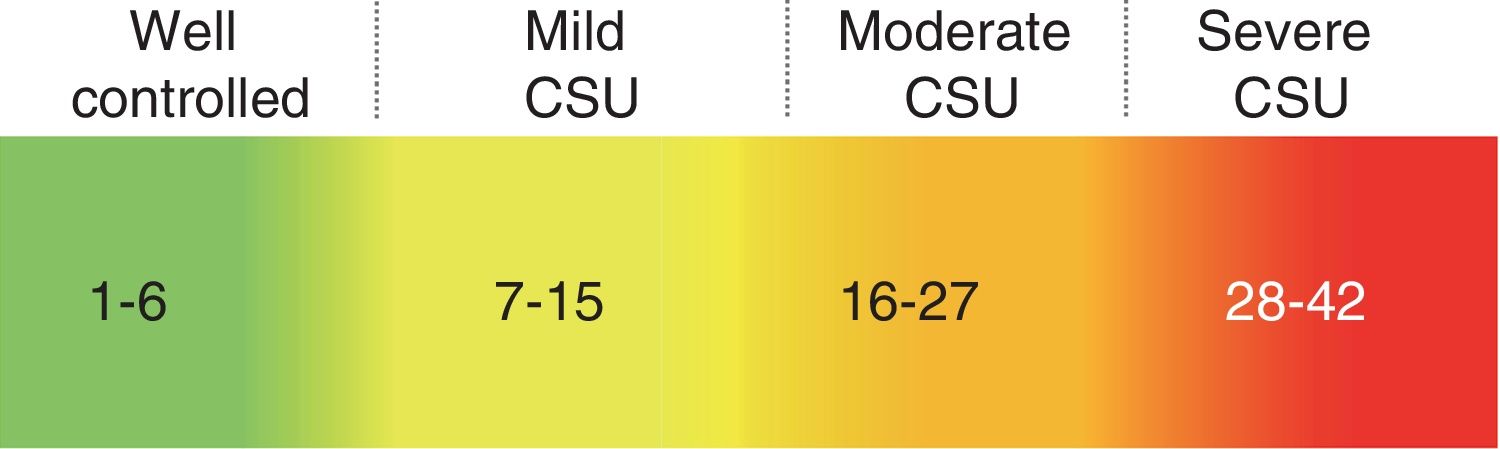
Tools for Assessing Response to TherapyUsing the UAS7, the instrument recommended for assessing disease activity in CSU,5,8 patients record the wheal count and itch intensity every day for the last week. The resulting score (range 0-42) can be used to define 5 disease activity categories.9 These 5 categories are as follows: urticaria free (UAS7 0), well-controlled urticaria (UAS7 1-6), mild-activity urticaria (UAS7 7-15), moderate-activity urticaria (UAS7 16-27), and severe-activity urticaria (UAS7 ≥ 28) (Fig. 2). This scale was validated for the assessment of disease activity in urticaria in 2008.10
Categories of chronic spontaneous urticaria (CSU) disease activity.
Source: Adapted from Stull et al.9
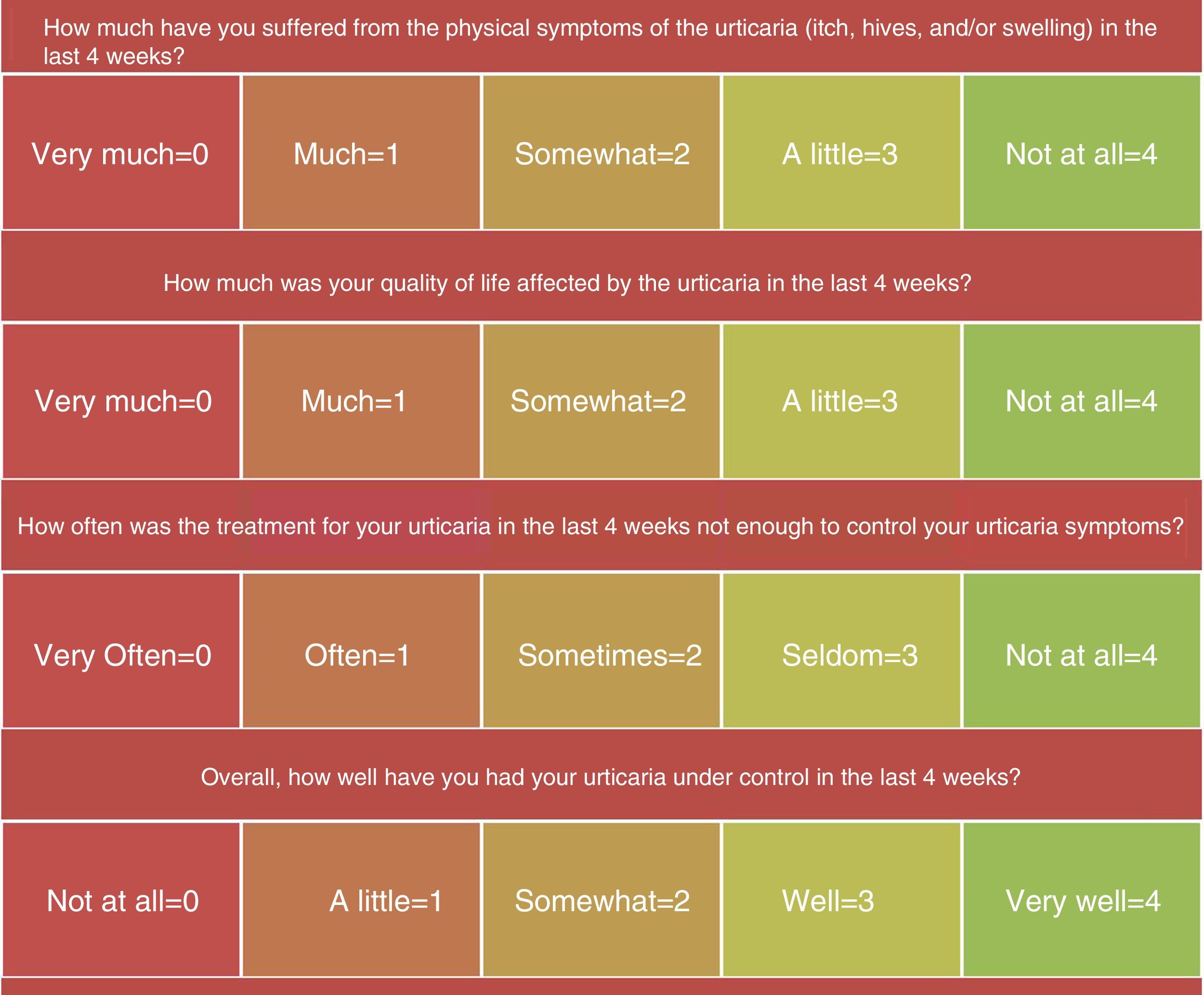
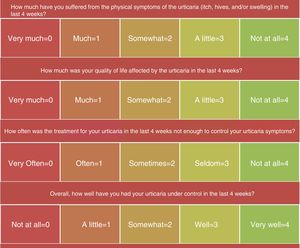
Although the UAS7 is very useful, it has two limitations: first, it does not assess the activity of inducible urticaria, a condition often associated with CSU; and second, it does not consider angioedema, which affects many patients with CSU and has a negative impact on their quality of life. For this reason, we are proposing using the Urticaria Control Test (UCT) as part of the follow-up protocol for these patients, especially when considering dose adjustments. The UCT is a simple questionnaire that can be completed at the time of the consultation and a transcultural adaptation to Spanish is available (Fig. 3). Patients answer questions about the intensity of their symptoms (itch, hives, and swelling), the impact of the disease on their quality of life, and how often the disease was controlled by the treatment. Finally they provide an overall assessment of how well their urticaria was controlled in the preceding 4 weeks.11,12 The UCT score ranges from 0 and 16, with 16 corresponding to complete control of the disease. A UCT score greater than 12 is considered to be indicative of good disease control.
Starting DoseThe starting dose for omalizumab in patients with CSU is 300mg administered every 4 weeks because this was the dose that achieved the outcomes in Phase III trials that best complied with the efficacy objectives and provided the best control of angioedema. However, in a study of data from real-life clinical practice, a considerable number of patients benefited from treatment with lower doses (150mg/4 wks) and 86% of patients achieved a greater than 90% reduction in UAS7 4 weeks after the first injection.13 A possible strategy in patients who do not have angioedema or inducible urticaria could be to start with a dose of 150mg and assess response at week 4; if the UAS7 is 6 or less, the patient would continue on the lower dose. This strategy would improve cost-effectiveness. However, since the aforementioned study was based on a small sample size and had no control group, the strategy should only be considered in individual patients or in settings where the pressure on health expenditure is greater.13
Dose AdjustmentWe propose that decisions on dose adjustment should be made taking into account the patient's UAS7 and/or UCT scores. A UAS7 score of 6 or less is considered to indicate well-controlled urticaria. Achieving a UAS7 of 6 or less was one of the end points of the ASTERIA and GLACIAL trials, and a recent study has shown a very good correlation between a UAS7 of 6 or less and good scores on the Dermatology Life Quality Index (DLQI), the Chronic Urticaria Quality of Life Questionnaire (CU-Q2Ol), and patient-reported interference with daily activities.9 Moreover, the current EAACI/GA2LEN/EDF/WAO guideline has set complete control of symptoms as a therapeutic objective, which is why we believe that a UAS7 of 6 or less is a suitable threshold for increasing or decreasing the dose of omalizumab in these patients.5
Achieving a UAS7 under 7 is not easy, and owing to the high cost of omalizumab, the dose may be maintained even when this objective is not achieved provided an improvement over the baseline situation is observed and the UCT score is over 12.
Importance of Using the Weekly UAS7 Throughout the Entire MonthThe ideal assessment of UAS7 in patients treated with omalizumab involves recording this data every week after the drug is first administered. This is the only way to obtain a clear picture of the response to therapy throughout the month. Therapeutic response varies from patient to patient and over time during the treatment period. A patient who has a UAS7 score of less than 6 during the first 3 weeks of their treatment may lose this response during the 4th week. This response pattern should not be equated with the response of a patient who presents a UAS7 score greater than 6 during the entire interval between doses and in whom treatment failed to achieve disease control.
Definition of Good Responder, Partial Responder and NonresponderAs mentioned above, a good responder is defined as a patient with a UAS7 score of 6 or less. A partial responder is a patient who achieves a decrease in baseline UAS7 assessed before treatment and whose score ranges from 7 to 15 (mild-activity urticaria). Finally, a nonresponder is a patient in whom treatment does not decrease baseline UAS7 to a value of under 16.
Interval Between AssessmentsWe propose assessing response to treatment with omalizumab every 3 months. This recommendation is based on the results of Phase III clinical trials of omalizumab, in which the percentage of patients who achieved well-controlled urticaria (UAS7 ≤ 6) increased from 41% at week 4 to 57% at week 12. Analysing data from the two pivotal studies, which prolonged treatment until week 24, we observe that this difference declined between weeks 12 and 24 (from 52% to 59%). Moreover, assessing patients every 3 months allows us to compare the results in clinical practice with the primary end points used in clinical trials, since these were also assessed at 12 weeks.
Response TimeThe authors of an article on clinical practice reported that 57% of patients with CSU treated with omalizumab achieved a reduction in UAS7 greater than 90% of the baseline UAS7 during the first week of treatment.13 In the same study, 86% of patients achieved this response at week 4. In our own experience of 286 patients, we observed that, of the patients who achieved a UAS7 of 6 or less with the licensed dose, 83% did so in the first 12 weeks of treatment.4
Dose increase at 3 months may be postponed in patients with mild-activity disease (a UAS7 of 7-15) provided the UCT is under 12 since it has been observed that some patients are late responders and take 12 to 16 weeks to achieve well-controlled disease at a dose of 300mg/4 wks.14
In our study, 17% of patients took over 3 months to respond to a dose of 300 mg/4 wks. For this reason, in a patient who has not achieved a UAS7 of less than 7 by week 12, the dose increase can be postponed for a further 3 months provided the patient's symptoms are not intolerable.
Increased Dose of OmalizumabThe pivotal trials provide no data on the usefulness of increasing the dose of omalizumab, but the strategy has been reported in studies of clinical practice.15–17 In a study of 45 patients, good disease control was observed in all those who received 300 mg/2 wks.15
In our study of 286 patients, the dose was increased to 450 or 600mg/4 wks in 79 patients who failed to respond to the initial dose of 300mg/4 wks (UAS7> 6).4 With the increased dose, 75% of these patients achieved a UAS7 of 6 or less. Thus, we consider that this dose increase strategy is useful in a significant number of patients.
As indicated above, some patients achieve good disease control in the 2 or 3 weeks following the first omalizumab injection, followed by a deterioration in the 4th week. In such cases, it may be reasonable to shorten the interval between injections. To monitor this pattern, it is very useful if the patient not only completes the UAS7 records the week prior to the visit but rather daily over the whole period between visits.
Withdrawal of Treatment Due to Complete ResponseIn patients with a UAS7 of 0 we propose a dose reduction to 150 mg/4 wks and a further assessment in 3 months. At that time, if the complete response is maintained, we recommend increasing the injection interval, initially to 6 weeks and then 8 weeks. If the patient's UAS7 is still 0 after 2 doses of 150 mg administered at 8 week intervals, we propose withdrawal of treatment with follow-up for at least 6 months to monitor possible relapse. This interval and the dose used before cessation of treatment coincide with the recommendations of a Danish algorithm in which in the case of relapse, treatment with omalizumab was restarted at 150 mg/8 wks.18
Retreatment in the Case of RelapseIn the case of relapse, the strategy most used in the literature is restarting treatment at the last effective dose and dosing interval. Analysis of data from 25 patients with CSU treated with omalizumab who experienced relapse of disease after withdrawal of treatment following a complete response showed that 100% of the patients responded rapidly to retreatment (after just 1 dose).17 Retreatment in that series was not associated with a higher rate of adverse events and the authors of the study concluded that retreatment with omalizumab is safe and effective in patients who experience relapse. In most cases, renewed symptoms occurred 4 to 8 weeks after the last omalizumab injection, but in 2 patients they did not occur until later (4 and 7 months).17,18
Data from a Spanish study of 110 patients with CSU treated with omalizumab provides evidence on the percentage of relapse following withdrawal of treatment.19 Treatment was withdrawn in 41 of the 110 patients following a complete response. Of these, 47% required retreatment due to relapse.
An analysis of data from Phase III trials of omalizumab undertaken to identify predictors of relapse after withdrawal of treatment showed, using the LASSO statistical method, that of the multiple variables analyzed, risk of relapse was related to baseline UAS7 score and the speed of response in the first 4 weeks of treatment.20 A rapid response and a moderate UAS7 were the factors that predicted no relapse or a late relapse. By contrast, in cases characterized by a slow response to omalizumab and a high UAS7, relapse was prompt.20
ConclusionsWe consider that the proposed algorithm is useful because the follow-up period was short in the clinical trials that studied the treatment of CSU with omalizumab. We propose quarterly assessment of patients to consider dose adjustment, with decisions based principally on the UAS7 score but also taking into consideration UCT and weekly UAS7 for at least a month. We feel that this approach provides a more complete assessment of the effectiveness of the treatment. In the case of relapse, we propose retreatment at the last effective dose and dosing interval. Finally, it is important to emphasize that a considerable number of patients who do not respond to the licensed dose of omalizumab may benefit from a higher dose (450 or 600 mg/4 wks).
Conflicts of InterestNovartis provided support for the working group meetings and technical support, but none of their employees participated in the compilation of evidence, the group discussions, or the task of drafting the document.
Isabel Bielsa-Marsol, Montserrat Bonfill-Ortí, Sara Gómez, Carola Baliu-Piqué, Nuria Lamas-Domenech, Alba Álvarez, María Villar-Buil, Marta Vilavella, Jose Ignasio Torné-Gutiérrez, Gloria Aparicio, and Xavier García-Navarro report no conflicts of interest.
Jorge Spertino, Vicente Expósito-Serrano, Ignasi Figueras-Nart, José Manuel Mascaró, Gemma Mele i Ninot, Eduardo Rozas-Muñoz, Laia Curto-Barredo, Joan Garcías-Ladaria, Esther Serra-Baldrich, and Antonio Guilabert have received honoraria from Novartis for training activities relating to chronic urticaria.
José Manuel Mascaró has received speaker fees from the following pharmaceutical companies: Ferrer, MSD, ISDIN, Novartis, and Leo Pharma.
Ana Giménez Arnau has participated in research projects and has received fees for consultancy and training activities from Uriach Pharma, Genentech, Novartis, FAES, GSK, Menarini, Leo-Pharma, GSK, MSD, and Almirall.
We would like to thank Novartis for providing the space to accommodate the meetings of the working group.
Please cite this article as: Spertino J, Curto Barredo L, Rozas Muñoz E, Figueras Nart I, Serra Baldrich E, Bonfill-Ortí M, et al. Algoritmo de tratamiento con omalizumab en urticaria crónica espontánea. Actas Dermosifiliogr. 2018;109:771–776.