Methotrexate (MTX) is a drug with a good safety profile that is frequently used in dermatology. Although serious complications due to acute MTX toxicity typically occur in the context of antineoplastic doses (up to 1-3g/m2 in some tumors), they occasionally occur in patients treated with considerably lower doses, and the early identification of these effects is essential. We present 3 patients who developed acute myelosuppression secondary to treatment with MTX. The initial signs in all the patients were similar forms of skin lesions; clinical observation of these lesions can be the key to the early diagnosis of this important condition.
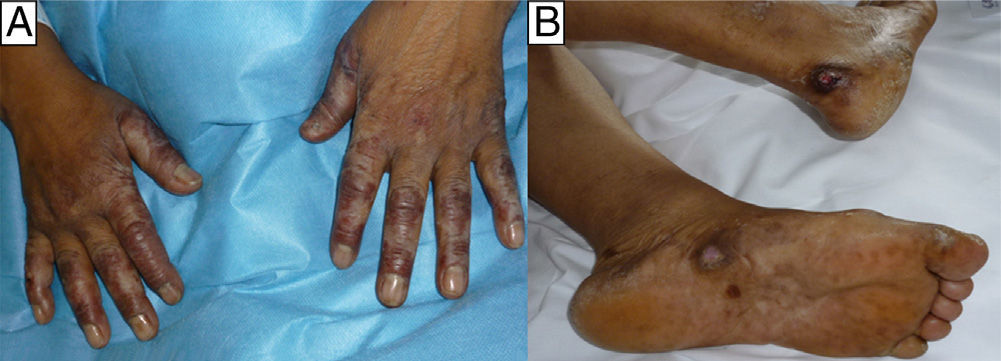
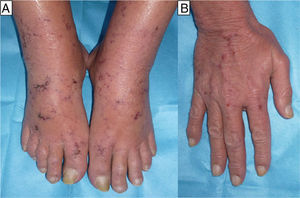
The first patient was a woman of 65 years of age with rheumatoid arthritis. She had been on treatment for 1 month with MTX, 20mg/24h, ibuprofen, 600mg/8h, and prednisone, 10mg/24h. She came to the emergency department with a 3-day history of fever and mucositis associated with painful edema of the hands of sudden onset and ulcers on both feet (Fig. 1). After 48hours of in-hospital observation, she developed severe pancytopenia with a neutrophil count of 400/μL (normal range, 1800-7600/μL), hemoglobin (Hb) of 9g/dL (normal range, 11.4-15.1g/dL), mean cell volume of 100fL, and platelet count of 66 000/μL (normal range, 140 000-450 000/μL). Blood and urine cultures, microbiology study of the mucosas, and serology for human immunodeficiency virus, syphilis, hepatitis B and C viruses, parvovirus B19, cytomegalovirus, Epstein-Barr virus, and toxoplasma were negative.
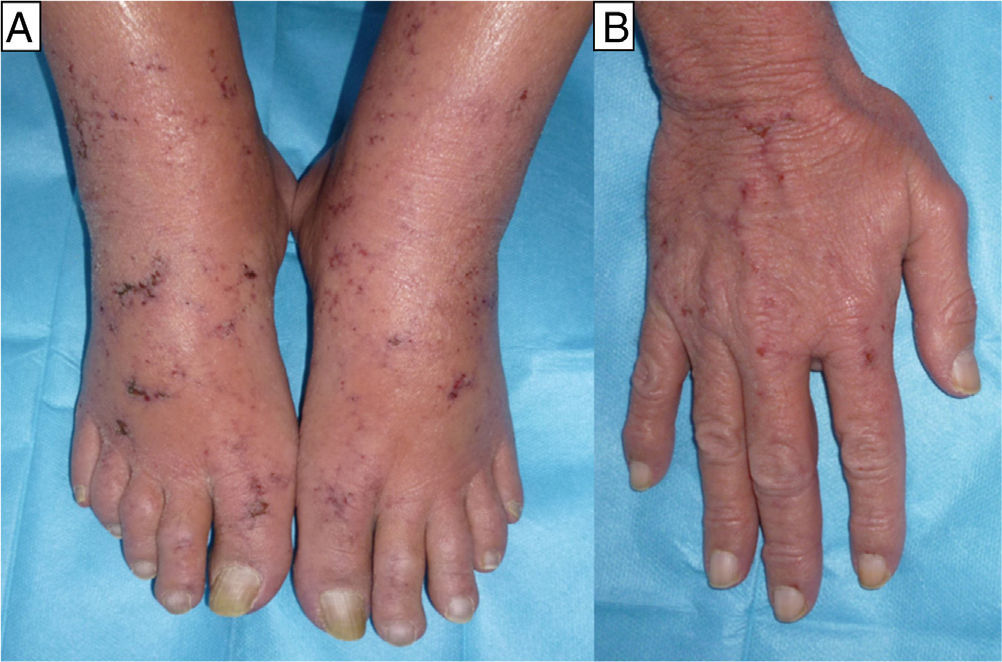
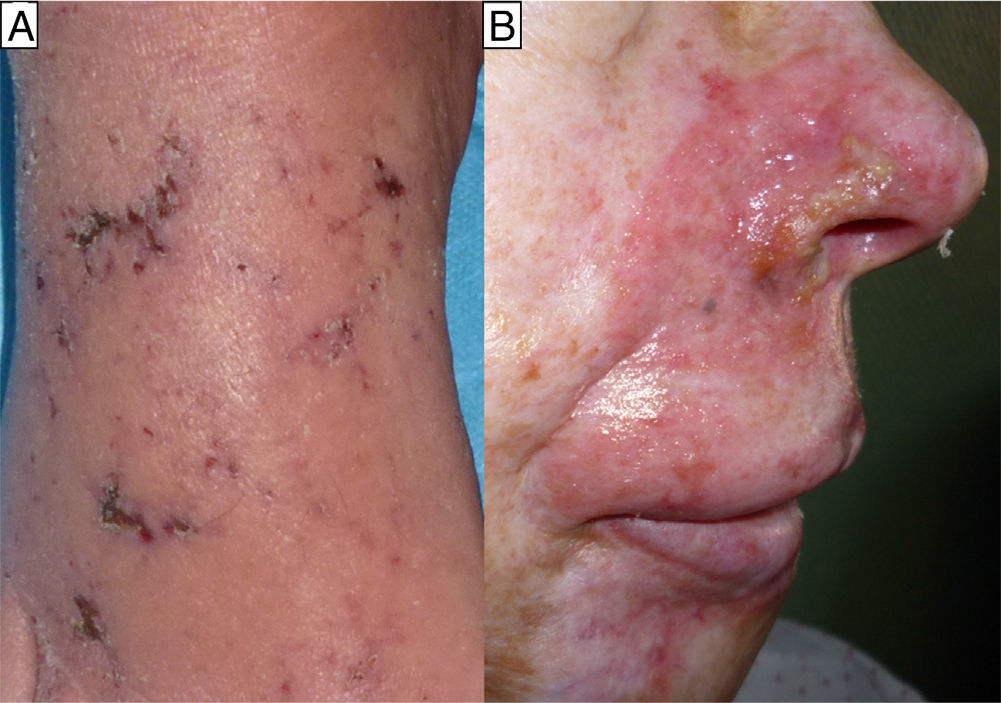
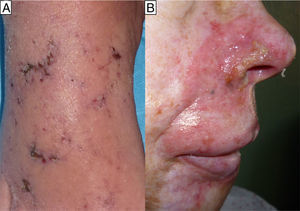
The second case was a 60-year-old man and the third case a 55-year-old woman; both patients had a history of mycosis fungoides for which they had been treated with MTX, the man for 4 months at a dose of 20 mg/wk and the woman for 6 months at a dose of 25mg/wk. For the previous month they had also been treated with sulfamethoxazole/trimethoprim (SMX/TMP) 800/160mg 3 times a week as prophylaxis for sepsis of cutaneous origin. They were seen for very painful acral erosions that had been present for a week (Figs. 2 and 3). Blood tests in the man revealed a marked fall in the Hb to 7.9g/dL from a value of 11.7g/dL 2 weeks earlier, and in the woman revealed moderate bicytopenia that had not previously been detected (Hb of 10g/dl and a white cell count of 2000/μL with 600 neutrophils/μL). Cultures of the skin lesions were negative for viruses and bacteria in both patients.
In all 3 patients, biopsy revealed reactive epidermal hyperplasia with areas of ulceration with a fibrin-covered base and dermal eosinophilia. The administration of MTX was interrupted and symptomatic treatment was started, leading to resolution of the skin lesions in less than 1 month in all cases. In the first patient it was necessary to add specific treatment (folinic acid and granulocyte colony stimulating factor) because of the severe pancytopenia, which showed a favorable response.
MTX is an antimetabolite. It is a folic acid analog that competitively and reversibly inhibits the enzyme dihydrofolate reductase (DHFR), a key enzyme for cell DNA synthesis. This mechanism explains its antiproliferative activity and the profile of adverse effects at high doses; acute myelosuppression is the manifestation that carries the greatest threat to life.1 When used as at anti-inflammatory doses, the number of drug molecules bound to the enzyme is insufficient to achieve inhibition, and it is postulated that the effect a low doses may be related to the formation of intracellular polyglutamates.2 However, the marked inter- and intraindividual variability in its metabolism3 and the possible coexistence of factors that increase its cellular availability means that, in practice, we must considered all patients on treatment with MTX to be susceptible to develop serious complications. Our 3 cases presented factors that could favor cytotoxicity: in the first patient, incorrect dosage (daily instead of weekly) leading to overdose, and treatment with ibuprofen, which interferes with the renal excretion of MTX and increases its free fraction in the plasma by displacing MTX from its protein binding sites; in the other patients, prophylaxis with SMX/TMP impeded the transformation of para-aminobenzoic acid into folic acid (SMX effect) and directly inhibited DHFR (TMP effect).
Skin erosions and/or ulcers as the manifestation of acute cytotoxicity due to MTX is very rare. In the medical literature reviewed, we found only 5 cases in patients with no previous dermatosis.4–8 All were men on low-dose treatment for rheumatoid arthritis and only one of them presented general manifestations with fever, mucositis, and acute severe pancytopenia, similar to our first patient.7 Cell turnover is increased in patients with psoriasis and/or mycosis fungoides, and this increases the cutaneous tropism of MTX.9,10 Lawrence et al.9 describe 2 distinct clinical patterns with different prognoses in patients with psoriasis: type I, with erosions and/or ulcers that develop on plaques and heal within a few days after the withdrawal of MTX; and type II, with lesions on healthy skin that take weeks to resolve despite the withdrawal of treatment. The clinical recognition of this condition is important, as it must be differentiated from treatment inefficacy or a flare-up of the dermatosis; this typically occurs at the beginning or end of treatment, which could lead either to an increase in the dose or to the reintroduction of MTX. The common factor in all the cases described, both in individuals with lesions on underlying skin disease and in those with lesions on healthy skin, was the disproportionate pain of the lesions and their characteristic distribution on acral areas, as was observed in our patients.
Based on all we have described, we believe that the appearance of painful acral erosions and/or ulcers in patients receiving treatment with MTX must lead us to consider severe underlying cytotoxicity. Two fundamental factors in the prevention of potentially lethal adverse effects are detailed evaluation of all the concomitant medication and ensuring that patients understand the indicated MTX regimen.
Please cite this article as: Maroñas-Jiménez L, Castellanos-González M, Sanz Bueno J, Vanaclocha Sebastián F. Erosiones y úlceras acrales: manifestación precoz de toxicidad aguda grave por metrotexato. 2014 105:319–321.