Circulating microRNAs (miRNA) are involved in the posttranscriptional regulation of genes associated with lipid metabolism (miRNA-33) and vascular function and angiogenesis (miRNA-126). The objective of this exploratory study was to measure plasma levels of miRNA-33 and miRNA-126 in patients with plaque psoriasis and evaluate their association with clinical parameters.
Material and methodsWe studied 11 patients with plaque psoriasis. The median Psoriasis Area Severity Index (PASI) was 13 (interquartile range [IQR], 9-14) and body surface area involvement was 12 (IQR, 11-15). Eleven healthy controls matched for age and sex were also included. We analyzed cardiovascular risk factors and subclinical carotid atheromatosis. Plasma miRNAs were evaluated using quantitative real-time polymerase chain reaction.
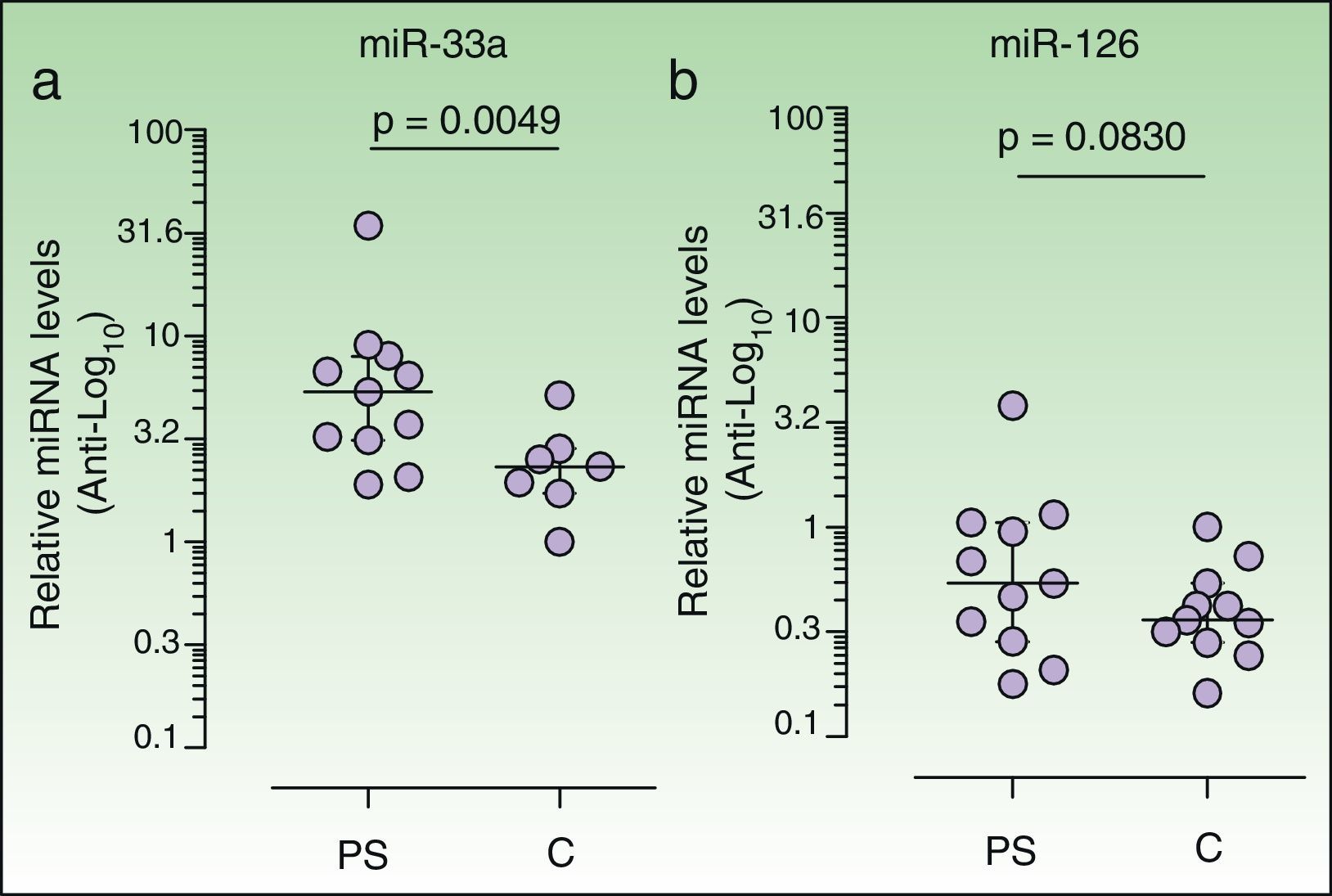
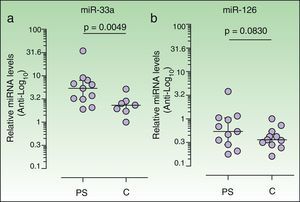
ResultsCarotid intima-media thickness was greater in patients (.57mm; IQR, .54-.61; n=11) than in controls (.50mm; IQR, .48-.54; data available for 9 controls) (P=.0055, Mann-Whitney). Expression of miRNA-33 in patients (5.34; IQR, 3.12-7.96; n=11) was significantly higher than in controls (2.33; IRQ, 1.71-2.84; only detected in 7 of 11 controls) (P=.0049, Wilcoxon signed rank). No differences in miRNA-126 levels were observed between patients and controls. In patients (n=11), we observed a positive correlation between miRNA-33 and insulin levels (r=.7289, P=.0109) and a negative correlation between miRNA-126 and carotid intima-media thickness (r=–.6181, P=.0426).
ConclusionIn psoriasis patients plasma levels of lipid and glucose metabolism-related miRNA-33 are increased and correlated with insulin. The study of circulating miRNA-33 in psoriasis may provide new insights about the associated systemic inflammatory abnormalities.
Se han identificado microARN (miARN) circulantes implicados en la regulación postranscripcional de genes del metabolismo de lípidos (miARN-33) y de la función vascular y angiogénesis (miARN-126). El objetivo de este estudio exploratorio ha sido evaluar los niveles plasmáticos de miARN-33 y miARN-126 en pacientes con psoriasis en placas y su relación con parámetros clínicos.
Material y métodosSe estudiaron once pacientes con psoriasis en placas (PASI [mediana] [25-75% percentil] 13 [9-14] y BSA 12 [11-15]) y un grupo pareado en edad y sexo de 11 controles sanos. Se analizaron factores de riesgo cardiovascular y la ateromatosis carotídea subclínica. Los miARN plasmáticos se evaluaron mediante la reacción en cadena de la polimerasa cuantitativa a tiempo real (qRT-PCR).
ResultadosLa media del grosor de la íntima carotídea (GIM) estaba aumentada en pacientes (0,57mm [0,54-0,61], n=11) respecto a controles (0,50mm [0,48-0,54], datos disponibles n=9) (test Mann-Whitney, p=0,0055). La expresión de miARN-33 en pacientes (5,34 [3,12–7,96], n=11) estaba significativamente aumentada respecto a controles (2,33 [1,71-2,84], n=7; solo se pudo detectar en 7 de 11) (test de Wilcoxon signed Rank, p=0,0049). No se observaron diferencias en los niveles de miARN-126 entre pacientes y controles. En pacientes se observó una correlación positiva entre miARN-33 e insulina (r=0,7289, p=0,0109, n=11); y una correlación negativa entre miARN-126 y GIM (r=–0,6181, p=0,0426, n=11).
ConclusiónLos pacientes con psoriasis presentaban niveles plasmáticos aumentados de miARN-33 (metabolismo de lípidos y glucosa), que se correlacionaban con los niveles de insulina. La valoración de miARN-33 circulante puede contribuir al conocimiento de las alteraciones inflamatorias sistémicas en psoriasis.
The pathogenesis of psoriasis, a chronic inflammatory disease of the skin and joints, involves genetic, environmental and immunological factors.1,2 Alterations in the expression of over 1000 genes have been described in psoriatic lesions.3,4 Moreover, recent studies have described a key role for small RNAs (20-25 nucleotides long) known as microRNAs (miRNAs or miRs) in controlling the gene expression of inflammatory proteins in skin affected by psoriasis.5 miRNAs have also been implicated in keratinocyte differentiation6 and T-cell function in psoriasis.7
miRNAs are evolutionarily conserved (miRBase; http://www.mirbase.org) and bind to complementary sequences in the 3’ untranslated regions of the target mRNA, leading to inhibition of translation and mRNA degradation.8 miRNA binding to its mRNA target results in a reduction in the amount of protein produced.8miRNAs can have many mRNA targets and act as regulators of gene expression to maintain the homeostasis of many cellular processes. miRNAs are one of the most abundant and important classes of gene regulatory molecules and constitute an additional level of regulation of gene expression.9 Recent studies have demonstrated the presence of miRNAs in circulating blood.10 While the cellular origin of these circulating miRNAs is unclear, it is proposed that they are derived in part from cells of the cardiovascular system.11 The alterations observed in plasma miRNA levels in response to physiological and pathological changes12 have led to increased interest in the potential of miRNAs as biomarkers10 and/or therapeutic targets.13 Altered miRNA expression has also been described in chronic inflammatory autoimmune diseases.14–16A recent study of psoriasis patients described alterations in the levels of circulating miRNAs and found that inhibition of tumor necrosis factor with etanercept decreased the expression levels of miRNAs implicated in inflammation and autoimmunity.17
Recent studies have identified circulating miRNAs that are involved in the posttranscriptional regulation of lipid metabolism genes18; these miRNAs include miR-33, which is implicated in the posttranscriptional regulation of cholesterol metabolism genes.18,19 miR-33 also targets genes involved in sterol transport, the metabolism of high-density lipoproteins (HDL), fatty acid oxidation, and glucose metabolism.18 In vivo silencing of miR-33results in an increase in HDL levels and promotes reverse cholesterol transport.18 There is considerable research interest in the potential of miR-33 as a novel therapeutic target in atherosclerosis and other metabolic diseases.18 Circulating miRNAs identified in cardiovascular diseases20 include miR-126, which participates in the posttranscriptional regulation of genes that regulate vascular function and angiogenesis.21
Research suggests that patients with psoriasis have an increased incidence of cardiovascular risk factors, including metabolic syndrome, obesity, dyslipidemia, diabetes, and hypertension.22,23 Moreover, important similarities between the inflammatory mechanisms of atherosclerosis and psoriasis have been described.24 The aim of our exploratory study was to measure plasma levels of miR-33 and miR-126 in patients with plaque psoriasis. We then investigated the relationship between the expression of these miRNAs and other clinical parameters.
Materials and MethodsPatients and controlsWe studied 11 adult patients with a clinical diagnosis of plaque psoriasis who had attended our hospital's dermatology department. All patients were consecutively enrolled in the study and gave their written informed consent. Seven patients had never received systemic therapy and the other 4 had not received systemic treatment in the preceding 4 to 6 weeks. Samples were collected and tested between February and May 2012. During the same period a control group of 11 healthy volunteers matched for age and sex was selected and underwent the same tests. The exclusion criteria for patients and controls included the presence of cutaneous lymphoma or other cancers and established cardiovascular disease (heart attack or stroke).
Clinical and laboratory parametersThe severity of psoriasis was assessed using the Psoriasis Area Severity Index (PASI) and the involved body surface area (BSA). The weight, height, and waist circumference of patients and controls were measured and used to calculate body mass index (BMI, kg/m2). Patients with a BMI > 25 were considered overweight. Systolic and diastolic blood pressures were measured after 10 minutes on 3 occasions, and the average value was calculated. In addition, serum levels of HDL-cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), triglycerides, glucose, insulin, C-reactive protein (CRP), and fibrinogen were measured and the erythrocyte sedimentation rate (ESR) determined in both patients and controls. The criteria of the National Cholesterol Education Program's Adult Treatment Panel III (ATP-III) report were used to assess the prevalence of metabolic syndrome.25 Participants were considered to have metabolic syndrome if they fulfilled 3 or more of the following criteria: waist circumference>102cm in men and>88cm in women; ESR>10mm/h; hypertriglyceridemia>150mg/dL; HDL-C<40mg/dL in men and<50mg/dL in women; blood pressure>130/85mmHg; fasting glucose>110mg/dL.
Blood and plasma samplesBlood samples were collected following standard procedures (using K2-EDTA tubes and the BD Vacutainer system; BD Diagnostics) in the hospital's laboratory. Plasma was isolated by Ficoll gradient centrifugation as previously described2 and plasma aliquots were stored at -80°C until analysis.
miRNA extraction and quantitative real-time polymerase chain reactionTotal RNA was isolated from 200μL of plasma from patients (n=11) and controls (n=11) supplemented with 3.5μL of 1.6 ×108 copies/μL of miRNeasy Control Serum/Plasma Spike-In (Qiagen, Catalog No. 219610), using the Qiagen miRNeasy Mini Kit (Catalog No. 21704) in accordance with the manufacturer's instructions. The quality and amount of total RNA was determined using the Experion Automated Electrophoresis System (Bio-Rad) and the NanoDrop system (Thermo Fisher), respectively. cDNA was synthesized from 1μg of total RNA using the miScript II Reverse Transcription Kit (Qiagen) following the manufacturer's recommendations. Quantitative real-time polymerase chain reaction (qRT-PCR) was performed on a CFX96 thermal cycler (Bio-Rad). Every 10μL of PCR reaction mixture contained 1X QuantiTec SYBR Green PCR Master Mix, 1X miScript Universal Primer, 1X miRNA-specific primer, and 10ng cDNA. miRNA-specific primers from Qiagen were used (Homo sapiens miR-33a, Catalog no. MS00003304; and Homo sapiens miR-126-1, Catalog No. MS00003430). The following PCR program was used: 15minutes of incubation at 95¿C (to activate the Hot Start Taq DNA polymerase) followed by 40 cycles of 15seconds at 94¿C, 30seconds at 55¿C, and 30seconds at 70¿C. The fluorescence emitted by SybrGreen was measured at the end of the extension step of each cycle. The specificity of PCR amplification was determined by performing melt curve analysis of the amplicon once the PCR was completed. This involved heating from 55¿C to 90¿C in increments of 0.5¿C every 10seconds. Quantification was performed based on the results of 3 PCR reactions per sample from each participant in the control and patient groups. The threshold cycle (Ct) was calculated using the qRT-PCR software, and was defined as the point at which the amount of amplicon detected began to increase exponentially. The results were normalized internally to the levels of miRNeasy Serum/Plasma Spike-In Control Ce-miR-39_1 (Qiagen primer, Catalog No. MS00019789). Relative transcript levels in each sample were calculated using the 2-ΔΔCT method.26
UltrasonographyPatients (n=11) and controls (n=9) underwent color Doppler ultrasonography (Acuson Antares, Siemens) of the supra-aortic trunks using a 10-5MHz transducer to detect atheromatous plaques, as previously described.2,23 A carotid intima-media thickness (IMT)>1.5cm was considered indicative of the presence of a plaque.
StatisticsResults are expressed as the median and interquartile range (IQR) unless otherwise specified. Plasma expression levels of miRNAs were analyzed using the nonparametric Wilcoxon signed rank test to compare the median of the patient group with that of the control group. Where indicated, the Mann-Whitney test was used to analyze differences between patients and controls. The Fischer test was used for the analysis of qualitative variables and the Spearman test to calculate correlations Differences were considered statistically significant at P<.05 (GraphPad Prism version 5.01, GraphPad Software Inc.).
EthicsThe study protocol was approved by the research and ethics committees of the hospital and research institute.
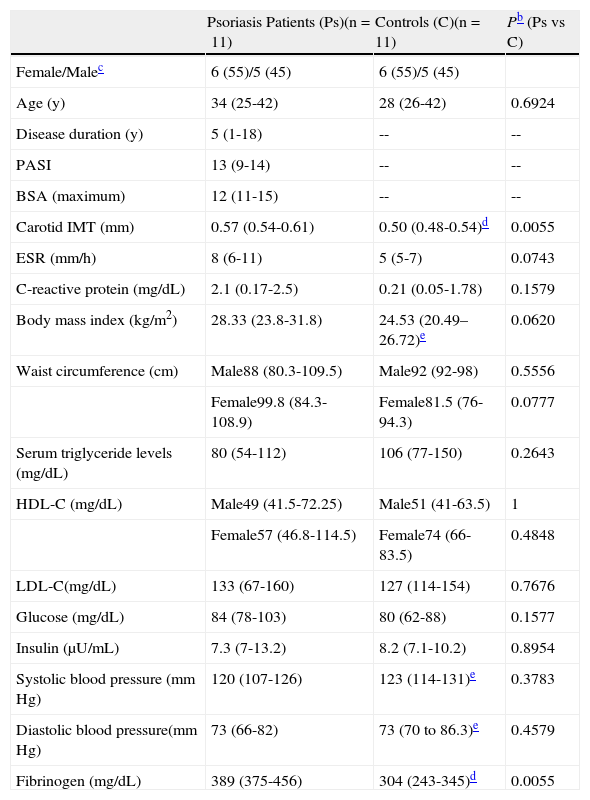
ResultsClinical dataThe patient and control groups both consisted of 5 males and 6 females. While the median age was somewhat higher in the patient group, this difference was not statistically significant (Table 1). All participants were white. In the patient group the median disease activity scores (PASI and BSA) were>10 (Table 1) and the median duration of disease was 5 years.
Clinical Dataa
| Psoriasis Patients (Ps)(n=11) | Controls (C)(n=11) | Pb (Ps vs C) | |
| Female/Malec | 6 (55)/5 (45) | 6 (55)/5 (45) | |
| Age (y) | 34 (25-42) | 28 (26-42) | 0.6924 |
| Disease duration (y) | 5 (1-18) | -- | -- |
| PASI | 13 (9-14) | -- | -- |
| BSA (maximum) | 12 (11-15) | -- | -- |
| Carotid IMT (mm) | 0.57 (0.54-0.61) | 0.50 (0.48-0.54)d | 0.0055 |
| ESR (mm/h) | 8 (6-11) | 5 (5-7) | 0.0743 |
| C-reactive protein (mg/dL) | 2.1 (0.17-2.5) | 0.21 (0.05-1.78) | 0.1579 |
| Body mass index (kg/m2) | 28.33 (23.8-31.8) | 24.53 (20.49–26.72)e | 0.0620 |
| Waist circumference (cm) | Male88 (80.3-109.5) | Male92 (92-98) | 0.5556 |
| Female99.8 (84.3-108.9) | Female81.5 (76-94.3) | 0.0777 | |
| Serum triglyceride levels (mg/dL) | 80 (54-112) | 106 (77-150) | 0.2643 |
| HDL-C (mg/dL) | Male49 (41.5-72.25) | Male51 (41-63.5) | 1 |
| Female57 (46.8-114.5) | Female74 (66-83.5) | 0.4848 | |
| LDL-C(mg/dL) | 133 (67-160) | 127 (114-154) | 0.7676 |
| Glucose (mg/dL) | 84 (78-103) | 80 (62-88) | 0.1577 |
| Insulin (μU/mL) | 7.3 (7-13.2) | 8.2 (7.1-10.2) | 0.8954 |
| Systolic blood pressure (mm Hg) | 120 (107-126) | 123 (114-131)e | 0.3783 |
| Diastolic blood pressure(mm Hg) | 73 (66-82) | 73 (70 to 86.3)e | 0.4579 |
| Fibrinogen (mg/dL) | 389 (375-456) | 304 (243-345)d | 0.0055 |
Abbreviations: ESR, erythrocyte sedimentation rate; HDL-C, high-density lipoprotein cholesterol; IMT, intima-media thickness; LDL-C, low-density lipoprotein cholesterol.
The median IMT in patients was 0.57mm (IQR, 0.54-0.61), as compared with 0.50mm in controls (IQR, 0.48-0.54) (P=.0055). One of the patients presented carotid atherosclerotic plaques.
We observed no significant differences between patients and controls in levels of HDL-C, LDL-C, triglycerides, glucose, or insulin (Table 1). One of the patients had metabolic syndrome according to ATP-III criteria.25 Positive correlations were detected between triglyceride levels and plasma levels of glucose (r=0.7900, P=.0038, n=11), insulin (r=0.8018, P=.0030, n=11), and fibrinogen (r=0.6651, P=.0255, n=11). LDL-C levels were correlated with disease duration (r=0.8219, P=.0019, n=11), and HDL-C levels were inversely correlated with PASI score (r=-0.6391, P=.0343, n=11), BMI (r=-0.6273, P=.0388, n=11), and systolic blood pressure (r=-0.6651, P=.0255, n=11). Analysis of obesity parameters revealed no differences in BMI, ESR, or waist circumference between patients and controls (Table 1). In the patient group we observed a correlation between BMI values and waist circumference (r=0.9091, P=.0001), glucose levels, (r=0.6895, P=.0189), and insulin levels (r=0.7973, P=.0033). Levels of the inflammatory marker and coagulation protein fibrinogen were higher in patients vs controls (Table 1).
Increased plasma levels of miR-33 in psoriasis patientsWe observed a significant increase in the relative plasma expression levels of miR-33 in psoriasis patients (5.34; IQR, 3.12-7.96) with respect to controls (2.33; IQR, 1.71-2.84) (P=.0049, Wilcoxon signed rank test). Plasma levels of miR-33 in 4 of the controls were too low to be correctly quantified, and were thus quantified in only 7 of the 11 controls (Fig. 1A) A positive correlation between plasma levels of miR-33 and insulin was observed in the patient group (r=0.7289, P=.0109) but not in the control group (r = 0.1071, P = .8397, n = 7). We detected no correlation in either group between plasma miR-33 expression and clinical parameters such as weight or BMI. Insulin resistance (IR) was calculated by homeostasis model assessment (HOMA) using the following equation: HOMA-IR = [insulin×glucose]/22.5, with insulin expressed in μU/mL and glucose in mmol/L. No differences in HOMA-IR were observed between patients (1.5; IQR, 1.3-3.4) and controls (1.5; IQR, 1.2-2.02) (P = .4307, Mann-Whitney test). A positive correlation between plasma miRNA levels and HOMA-IR was observed in patients (r=0.6545, P=.0289) but not in controls (r=-0.2143, P=.6445).
Plasma levels of miR-33a and miR-126 in each sample were calculated using the 2-ΔΔCT method26 (see Materials and Methods). Results are presented as the median and interquartile range in logarithmic scale (base 10). Anti-logarithmic values are indicated in the vertical axes. The Wilcoxon signed rank test was used to compare the median of the patient group (n=11) with that of the control group (n=11). Plasma levels of miR-33 expression were lower in controls than in psoriasis patients (a). Plasma miR-33 levels were quantified in 7 of the 11 controls studied (see Results). Analyses were performed using Graph-Pad Prism software (version 5.01).
We observed no significant increase in the relative expression levels of miR-126 in the plasma of patients (0.5384; IQR, 0.2839-1.052) vs controls (0.361; IQR, 0.2806-0.5371]) (P=.0830).
A negative correlation between levels of circulating miR-126 and IMT values was observed in patients (r =-0.6181, P=.0426) but not in controls (r=-0.3333, P=.3807).
Finally, in the group of psoriasis patients we observed no significant correlations between plasma expression levels of miR-33 or miR-126 and the other clinical parameters analyzed.
DiscussionThe main finding of this study is that plasma levels of miR-33, which is implicated in the posttranscriptional regulation of genes involved in cholesterol and glucose metabolism,18,19 are increased in patients with plaque psoriasis as compared with healthy controls and are positively correlated with plasma insulin levels and the HOMA-IR index value.
There is currently growing interest in the study of circulating miRNAs given their potential as biomarkers.10 Moreover, circulating miRNAs may play a key role in intercellular communication,27 although this remains to be clarified. miR-33a and miR-33b are encoded in the introns of the genes that encode sterol-regulatory element-binding protein 2 (SREBP-2), a transcription factor that binds to the sterol regulatory element.18,19 It has been reported that miR-33 plays a role in the posttranscriptional suppression of the cholesterol transporter ATP-binding cassette transporter A1 (ABCA1),18 leading to abnormal regulation of cholesterol efflux from macrophages. Moreover, manipulation of miR-33 levels may result in alterations in circulating HDL-C levels.19 Similar results have been reported in mice lacking miR-33, suggesting that blockade of this miRNA may help to prevent atherosclerosis.28 miRNAs are gene regulatory molecules that target multiple mRNAs and regulate numerous cellular processes. In hyperinsulinemia, increases in the expression of SREBP transcription factors and miR-33 may contribute to increases and decreases in plasma levels of triglycerides and HDL-C, respectively, alterations that are included in the criteria for metabolic syndrome.18 Other described targets of miR-33 include genes involved in fatty acid oxidation and glucose metabolism18; these include genes encoding insulin receptor substrate 2 and sirtuin 6 (SIRT6)—a nicotinamide adenine dinucleotide-dependent histone deacetylase implicated in molecular pathways involved in aging, inflammation, and cancer.18 miR-33 also targets genes involved in the cell cycle, cell proliferation, and inflammation.18 Moreover, expression levels of miR-33 are inversely correlated with disease stage in bladder cancer patients, in whom miR-33 targets transforming growth factor β (TGF-β) signaling pathways.29
AngiomiRs, including miR-126, are miRNAs found in plasma, serum, and circulating microvescicles that play key regulatory roles in vascular development and angiogenesis.30 Patients with coronary artery disease or diabetes show reduced levels of circulating miR-126 as compared with healthy controls.20 Moreover, treatment with etanercept decreases miR-126 expression in the serum of patients with psoriasis.17 However, in our study we found no significant differences in miR-126 levels between psoriasis patients and healthy controls, although we did observe an inverse correlation between miR-126 expression and carotid IMT. Only 1 of the psoriasis patients had atheromatous plaques and fulfilled the criteria for metabolic syndrome.
We have presented significant findings that suggest an important biological role of miR-33 in psoriasis. However, the main limitation of our study is the small size of the sample.
In conclusion, analysis of the expression of circulating miRNAs such as miR-33 in the plasma of psoriasis patients can contribute to a better understanding of the mechanisms underlying the systemic inflammatory alterations experienced by these patients.
Ethical DisclosuresProtection of persons and animalsThe authors declare that no experiments were performed on humans or animals during the course of this study.
Data confidentialityThe authors declare that they have followed the protocols of their place of work concerning the publication of patient data and that all patients included in this study were appropriately informed and gave their written informed consent.
Right to privacy and informed consentThe authors declare that no private patient data appear in this article.
FundingFunding was received from the European Social Fund (European Commission-European Regional Development Fund [ERDF/FEDER]); CSIC-PI 200820I216 (to M.Z.); Junta de Andalucía (to J.A.); the Department of Innovation, Science, and Enterprise and the Department of Science and Education (CVI 226 and PC08-CTS-04 046 to J.S. and M.Z.); ME-MICINN (SAF-2008-03685) (to J.S. and M.Z.); SAF-2011-27261 (to J.S.); JAE-Doc CSIC-FEDER (postdoctoral contract to S.G.R.); and the Ministry for Equality, Health and Social Policy of the Junta de Andalusia.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
The two authors contributed equally.
Please cite this article as: García-Rodríguez S, Arias-Santiago S, Orgaz-Molina J, Magro-Checa C, Valenzuela I, Navarro P, et al. Alteraciones en los niveles de expresión del microARN-33 en plasma de pacientes con psoriasis 2014;105:497–503.