Common acne affects approximately 85% of young people, often persisting to adult age.1 It is a chronic inflammatory disease of the pilosebaceous unit and its pathogenesis involves the production of sebum, bacterial colonization by Cutibacterium acnes, and certain keratinization abnormalities.2 Clinical presentation is characterized by the concomitance of open and closed comedos, inflammatory papules, pustules, nodules, and scars,1 which present on the face, and also on the chest and/or back in up to 50% of patients.3 Its active presence and its scars affect the emotional wellbeing and have a detrimental effect on the quality of life and mental state of the patient, leading to depression, social isolation, and suicidal thoughts in a considerable number of cases.4
The principal treatment that has managed, in spectacular form, to reverse most of the negative effects of acne is the use of retinoids. These are structural and functional analogs of vitamin A that can mediate in intranuclear retinoid receptors by activating genes that respond to retinoic acid.1
Systemic retinoids are mostly used in moderate to severe cases or those that have proven refractory to previous treatments.3 The representative par excellence is isotretinoin, the major adverse effect of which is teratogenicity, making prevention of its use in pregnancy essential, regardless of the dose.5 Topical retinoids are used in single-drug therapy or in combination with other molecules, such as benzoyl peroxide, antibiotics, etc., and as maintenance therapy following systemic therapies.6 Its anti-inflammatory, antiseborrheic, and microcomedo-formation limiting properties support its unquestionable efficacy.1–6
The first generation of retinoids consists of nonaromatic compounds: 12-cis-retinoic acid, all-trans retinoic acid, and 9-cis-retinoic acid, which are derived from vitamin A and present modifications to the terminal polar group and on the polyene chain.7 Second-generation retinoids are characterized by being monoaromatic with modifications to the ring. They are also more lipophilic and have greater bioavailability than those of the previous generation, and include etretinate and acetretin.7 Third-generation retinoids are characterized by their polyaromatic structure (through cyclization of the polyene chain) and their greater rigidity. Examples of this group include adapalene, tazarotene, and bexarotene.7
More than 20 years have passed between the final approval of a new active ingredient against acne and authorization of the sale of trifarotene, a new topical retinoid that inaugurates the 4th generation.8 The US Food and Drug Administration approved this drug in October 2019 (it was later approved by the European Medicines Agency) for use in acne of the face and torso.9,10 Despite the need for a treatment focusing on the chest and/or back, little scientific evidence has been available to date regarding the management of acne in these locations.11–13
Trifarotene is a retinoid aimed specifically at the retinoic acid receptor (RAR)-γ (the most common isoform in the skin), with low affinity for RAR-α and RAR-β.8 Its mechanism of action provides it with anti-inflammatory, comedolytic, and depigmenting effects.8 This last property was shown in preclinical trials in SKH2 mice.14 Depigmenting activity in the tails of mice was compared using topical adapalene 0.1%, trifarotene 0.01%, and all-trans retinoic acid 0.1%, daily for 6 weeks. The last 2 drugs showed significant activity, whereas adapalene (possibly due to its lesser penetration in the skin) did not produce significant depigmentation.14
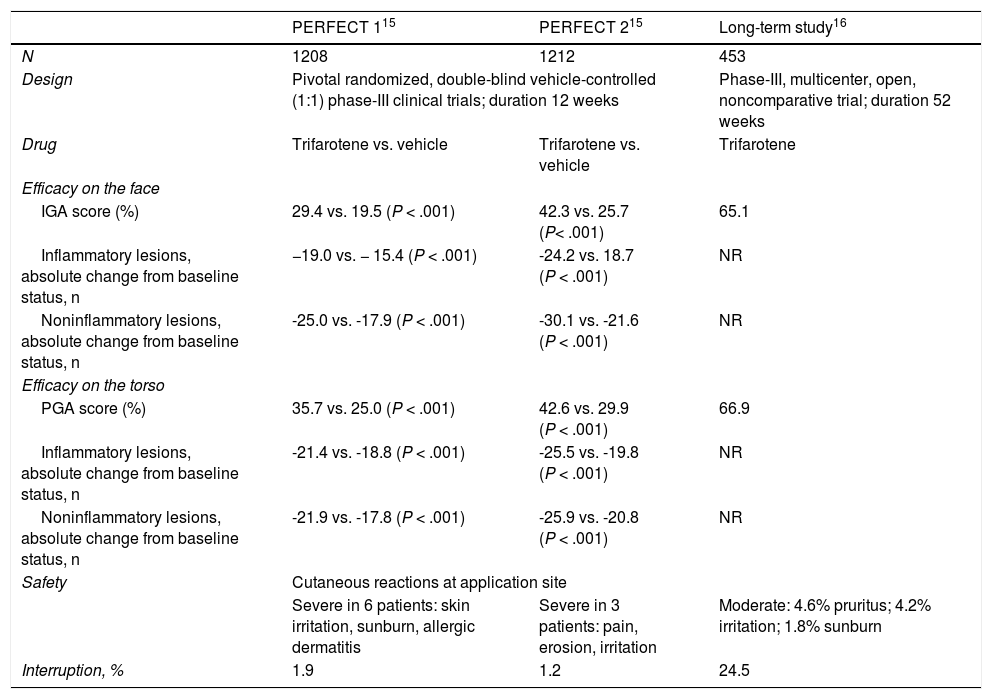
The efficacy and safety of topical trifarotene 50 μg/g was evaluated in 2 pivotal randomized, double-blind vehicle-controlled (1:1) phase-III clinical trials (PERFECT 1, PERFECT 2). Patients over 9 years of age (n = 1208 and 1212, respectively) received the topical retinoid on the face and torso for 12 weeks.15 The primary objectives in both trials were efficacy on the face, measured using the investigator global assessment scale, as a degree of improvement of 2 or more points, and the change from the baseline status to the status at week 12 with regard to inflammatory and noninflammatory lesions. The secondary objectives focused on efficiency on the torso evaluated by means of the physician global assessment, and the change in the number of acne lesions. The primary and secondary objectives were achieved in both studies with statistical significance (P < .001) in favor of trifarotene, as shown in Table 1.15 Onset of the effect of trifarotene was rapid and a reduction in the acne lesions occurred from the 1st week of use in the case of the face and the 2nd week in the case of treatment of the torso.15 With regard to the safety profile, the adverse effects associated with the drug were mainly cutaneous and occurred at the application site. Suspension of treatment was notified in 1.9% and 1.2% of the participants in the PERFECT 1 and PERFECT 2 trials, respectively, due to irritation at the site of application and allergic dermatitis.15
Summary of Publications Regarding Scientific Evidence on Trifarotene.
| PERFECT 115 | PERFECT 215 | Long-term study16 | |
|---|---|---|---|
| N | 1208 | 1212 | 453 |
| Design | Pivotal randomized, double-blind vehicle-controlled (1:1) phase-III clinical trials; duration 12 weeks | Phase-III, multicenter, open, noncomparative trial; duration 52 weeks | |
| Drug | Trifarotene vs. vehicle | Trifarotene vs. vehicle | Trifarotene |
| Efficacy on the face | |||
| IGA score (%) | 29.4 vs. 19.5 (P < .001) | 42.3 vs. 25.7 (P< .001) | 65.1 |
| Inflammatory lesions, absolute change from baseline status, n | −19.0 vs. − 15.4 (P < .001) | -24.2 vs. 18.7 (P < .001) | NR |
| Noninflammatory lesions, absolute change from baseline status, n | -25.0 vs. -17.9 (P < .001) | -30.1 vs. -21.6 (P < .001) | NR |
| Efficacy on the torso | |||
| PGA score (%) | 35.7 vs. 25.0 (P < .001) | 42.6 vs. 29.9 (P < .001) | 66.9 |
| Inflammatory lesions, absolute change from baseline status, n | -21.4 vs. -18.8 (P < .001) | -25.5 vs. -19.8 (P < .001) | NR |
| Noninflammatory lesions, absolute change from baseline status, n | -21.9 vs. -17.8 (P < .001) | -25.9 vs. -20.8 (P < .001) | NR |
| Safety | Cutaneous reactions at application site | ||
| Severe in 6 patients: skin irritation, sunburn, allergic dermatitis | Severe in 3 patients: pain, erosion, irritation | Moderate: 4.6% pruritus; 4.2% irritation; 1.8% sunburn | |
| Interruption, % | 1.9 | 1.2 | 24.5 |
Abbreviations: IGA indicates investigator global assessment scale; NR, not reported; PGA, physician global assessment scale.
Blume-Peytavi et al16 carried out a long-term open trial over 52 weeks. The study was completed by 75.5% of the initially enrolled participants (n = 342/453, Table 1). Adverse effects associated with the drug were notified in 12.6% of patients and none of the effects was severe. The efficacy rates, measured using the investigator global assessment scale and the physician global assessment, increased from week 12 to week 52, reaching values of 65.1% and 66.9%, respectively. In week 52, 53.8% of patients stated that the acne was not affecting their quality of life.16
Case series in real clinical practice have also been published in the literature. Johnson et al17 published an intermediate analysis at 12 weeks (total duration of 24 weeks), in which 3 patients with acne of the face and torso, with more than 20 lesions in each area, were treated.17 The patients stated their satisfaction with efficacy in both areas, ease of use, and time to onset of improvement. Moreover, tolerability of the product was favorable. All the above key points of treatment with trifarotene are summarized in Table 2.
Key points of treatment with trifarotene.
| First therapeutic novelty approved in more than 20 years8 |
| Indication for acne on the face and torso (chest/back)10 |
| Rapid onset of action, from the 1st week in use on the face and 2nd week in application on the torso15 |
| Adherence greater than 75% after 52 weeks of use16 and interruption below 2% after 12 weeks15 |
| Ease of use reported by patients17 |
| Scientific evidence verified at 12 and 52 weeks, on the face and on the torso, by means of the IGA/IPA scales and lesion count15,16 |
| Associated cutaneous adverse effects, mostly at the application site15 |
Abbreviations: IGA indicates investigator global assessment scale; PGA, physician global assessment scale.
Scientific evidence continues to be generated on the use of trifarotene.8 Clinical trials are focusing on determining the results notified by patients (clinical trial identifier: NCT03915860) and on studying the profile of the drug in combination with oral antibiotics, in cases of severe acne (clinical trial identifier: NCT04451330).
Trifarotene is the first therapeutic novelty in the area of acne in decades. Its arrival has been well received by physicians and by patients, who in many cases needed an alternative to the available treatments. One of the key points is its specific indication for acne on the torso. With regard to this aspect, many patients state that, in general, application of topical treatments on the chest and/or back is complicated and they are sometimes reticent about asking for help. The vehicle used in the formulation of trifarotene, however, makes its use on large areas easy and only a small amount of product is required to cover the entire area.
All the safety and efficacy data on treatment published in the literature are in agreement with what we have been able to observe in our clinic.
In our clinical experience, patients also value the rapid onset of action of this drug. This is very important because it increases adherence to treatment, which tends to be low in this group of patients.18 It should be mentioned, however, that starting treatment with trifarotene should be undertaken with care. Some patients complain of having suffered from flaking, dryness, or erythema. Nevertheless, these adverse effects can be managed effectively with the use of an appropriate moisturizer or by spacing the initial applications until the frequency of continued application is achieved. Trifarotene also metabolized well, avoiding the problem of bioaccumulation in the body. Altogether, this topical retinoid has been shown to be effective and safe in patients refractory to currently available topical treatments and achieves a good response with considerable reduction in acne lesions.
Trifarotene is the first therapeutic novelty in the Spanish market in 20 years. Based on the available scientific evidence and on our clinical experience, trifarotene is effective and safe for the treatment of acne on the face and torso. This drug provides added value in several aspects: ease of application, speed of action, and use on large areas, with the resulting improvement in the quality of life of the patients, and their adherence to and satisfaction with the treatment.
The authors would like to thank Sonia Romero (scientific advisor, Meisys) for her support in writing this article.
Please cite this article as: Guerra-Tapia A, González-Guerra E. Trifaroteno: un nuevo protagonista en el panorama retinoide. Actas Dermosifiliogr. 2021;112:869–872.