Some immunosuppressed patients who have drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome have been shown to develop a DRESS-like syndrome that in some international articles has been termed virus reactivation with eosinophilia and systemic symptoms (VRESS). The immunologic basis for the pathogenesis of this entity is an exaggerated response to reactivated herpesviruses. VRESS could be more common than the classically-described DRESS and should be recognized in order to avoid contraindication of drugs necessary for the patient.
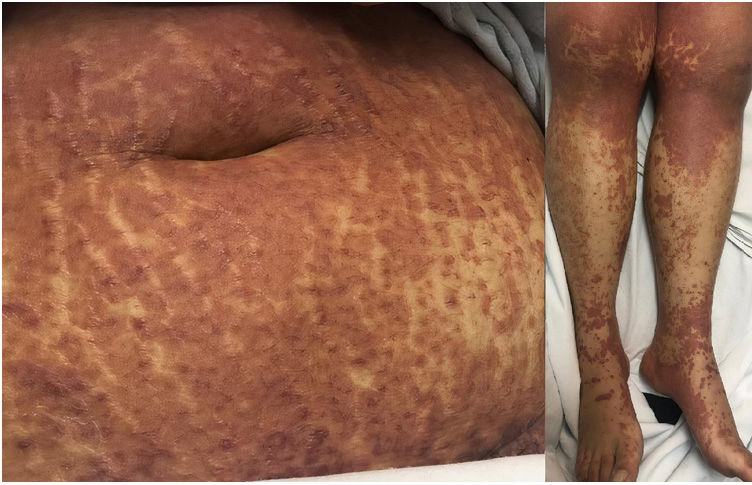
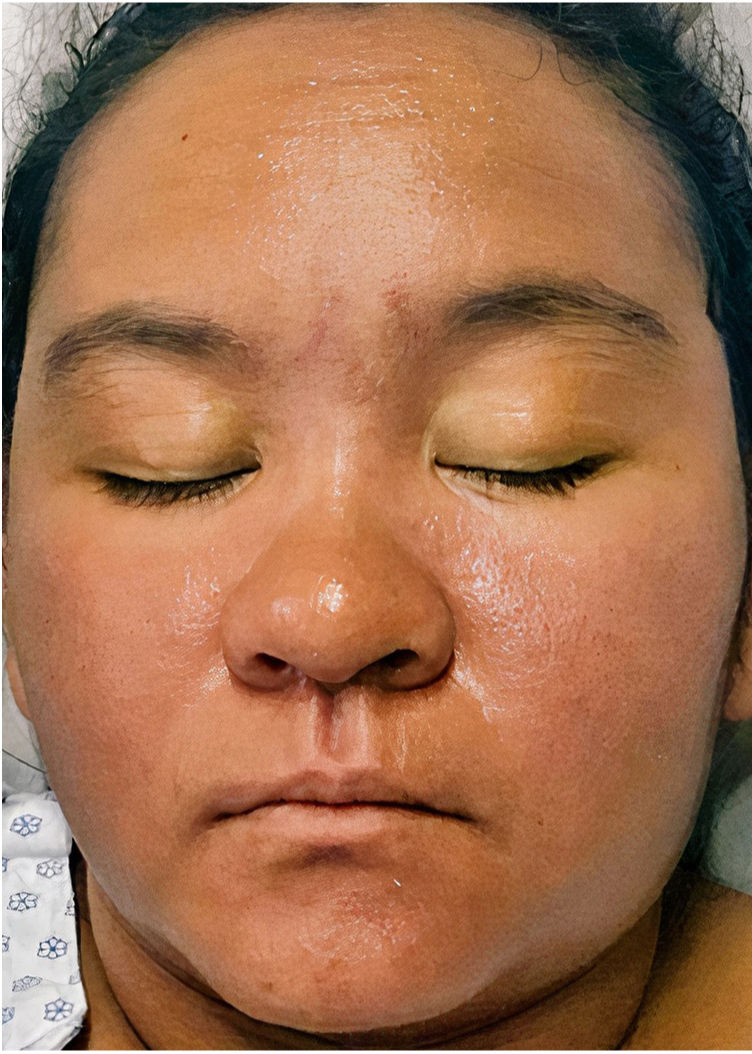
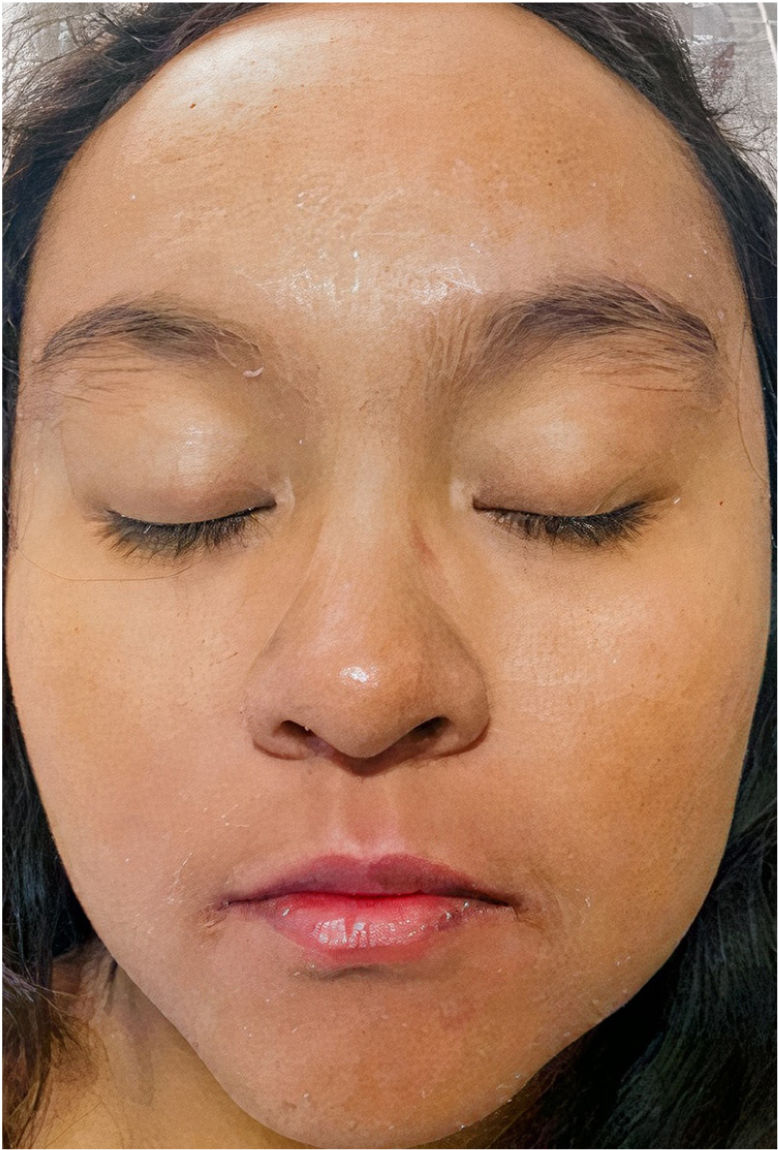
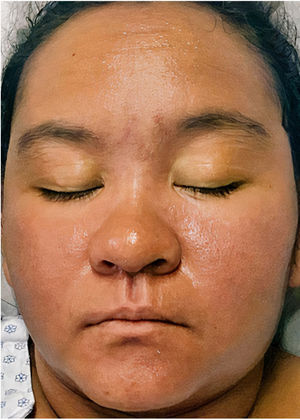
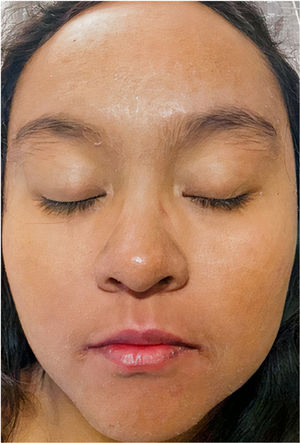
A 22-year-old woman with a history of systemic lupus erythematosus treated with hydroxychloroquine developed a fever of up to 38°C, accompanied by diarrhea, nausea, and vomiting 17 days after initiating treatment with DoTBal (rifampicin, pyrazinamide, ethambutol, and isoniazid) for lymph node tuberculosis. Due to suspicion of infectious gastroenteritis, she was treated with ciprofloxacin and discharged. She discontinued the antituberculosis treatment for 2 days and subsequently presented fever, tachycardia, and tachypnea together with a bilateral, symmetrical, pruritic dermatosis characterized by a morbilliform rash affecting the anterior and posterior trunk as well as the extremities, but sparing the mucosa, palms, and soles. This dermatosis was accompanied by edema and facial erythema (Figs. 1 and 2). Laboratory studies revealed eosinophilia of 9% (549/L), and liver function tests showed a pattern of hepatocellular damage with hyperbilirubinemia (total bilirubin, 4mg/dL; direct bilirubin, 3.1mg/dL; indirect bilirubin, 0.90mg/dL; alanine aminotransferase, 403U/L; aspartate aminotransferase, 255U/L). Suspecting a drug rash, we consulted the dermatology department, where a punch biopsy was performed. The preliminary histopathological report was compatible with such a rash. A quantitative polymerase chain reaction (PCR) test for human herpesvirus 6 (HHV-6), performed as part of the diagnostic approach, showed a viral load of 705.6copies/mL. The patient was admitted with the definitive diagnosis of DRESS based on the regiSCAR diagnostic criteria (6 points), and treatment with prednisone 0.5mg/kg/day was begun. Six days after admission, despite the initiation of systemic corticosteroid therapy, the dermatosis worsened. A new HHV-6 PCR test showed an increase to 1293copies/mL. On the recommendation of the departments of Infectious Diseases, Dermatology, and Internal Medicine, treatment with valganciclovir 900mg twice daily was initiated. A further HHV-6 CPR performed 4 days after initiation of antiviral therapy showed 22copies/mL, and a final determination the following day yielded a negative result. The patient was discharged 5 days later due to clinical improvement (Fig. 3).
This case demonstrates the paradoxical clinical worsening following discontinuation of the presumed causative drug, the usefulness of valganciclovir treatment, and the complex interaction between herpesviruses, drug hypersensitivity, and the patient's immune system.
DRESS and VRESS have the same immunologic profile as immune reconstitution inflammatory syndrome (IRIS) in HIV patients, with regulatory T (Treg) cells predominating. Shiohara et al. demonstrated that the acute phase of DRESS is associated with an expansion of Treg cells (CD4+ CD25+ Foxp3+), the same phenomenon found in patients with IRIS.1,2
The clinical course of VRESS includes 3 phases. The first is the progressive reactivation of the herpesvirus. Some drugs have been shown to epigenetically regulate an increase in the replication of HHV-6 and the Epstein-Barr virus in vitro.3 This finding underscores the need to initiate drugs known to cause DRESS (allopurinol, sulfasalazine, or anticonvulsants) in a progressive manner in order to avoid adverse effects and viral reactivation. The second phase is the powerful immune reaction against the virus. This reaction may be a function of the patient's genetics or a simple imitation of the events connected with IRIS. The third phase is a possible worsening of the disease caused by the rapid reduction of drugs with immunosuppressive or antiviral effects. In our case, the suspension of the antituberculosis treatment, which has immunosuppressive properties, may have increased the patient's exaggerated immune response.4
Drugs that may induce DRESS are thought to have intrinsic immunosuppressive properties in common.5 Subclinical reactivation of the herpesvirus may occur before the administration of these drugs, and this reactivation can only be unmasked by the rapid restoration of antiviral immune responses through their discontinuation. The exaggerated immune reaction against the reactivated herpesvirus is the main difference between VRESS and classic DRESS.
Therapy with valganciclovir may prevent the aggravation of the disease caused by HHV-6 in patients with VRESS, including the syndrome they develop that is very similar to IRIS. Treatment should be accompanied by viral load monitoring to gradually titrate the drugs the patients use.
Conflicts of InterestThe authors declare that they have no conflicts of interest.