Immune checkpoint inhibitors (CI), used to boost the immune system, have substantially improved the prognosis of patients with advanced melanoma.1,2 However, they are associated with a unique spectrum of side effects: immune-related adverse events (IRAEs), which are important to be recognized and promptly treated.3 Despite clinical trials data regarding IRAEs, real-life comparison between IRAEs, when CI is used in metastatic disease (MD) or as adjuvant therapy (AT), is still missing. Therefore, we conducted a retrospective, observational, cohort study to assess the frequency, type, and severity of IRAEs in melanoma patients receiving AT compared with those receiving MD regimen (MDR). Additionally, we explored any clinical factor related to IRAEs appearance or treatment-effectiveness correlation. We included all melanoma patients treated with a standard regimen of CI in daily-practice conditions for at least 3 months.
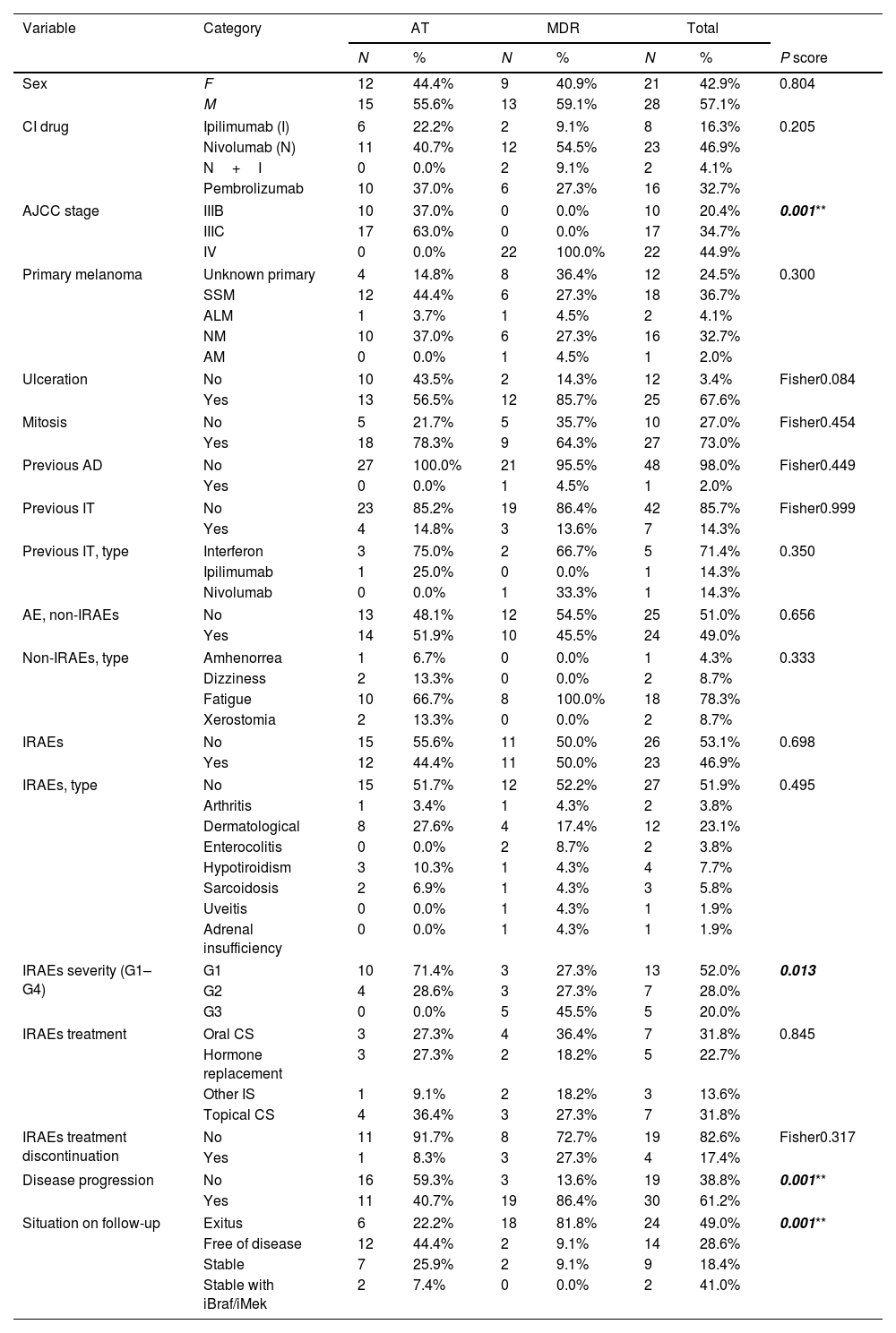
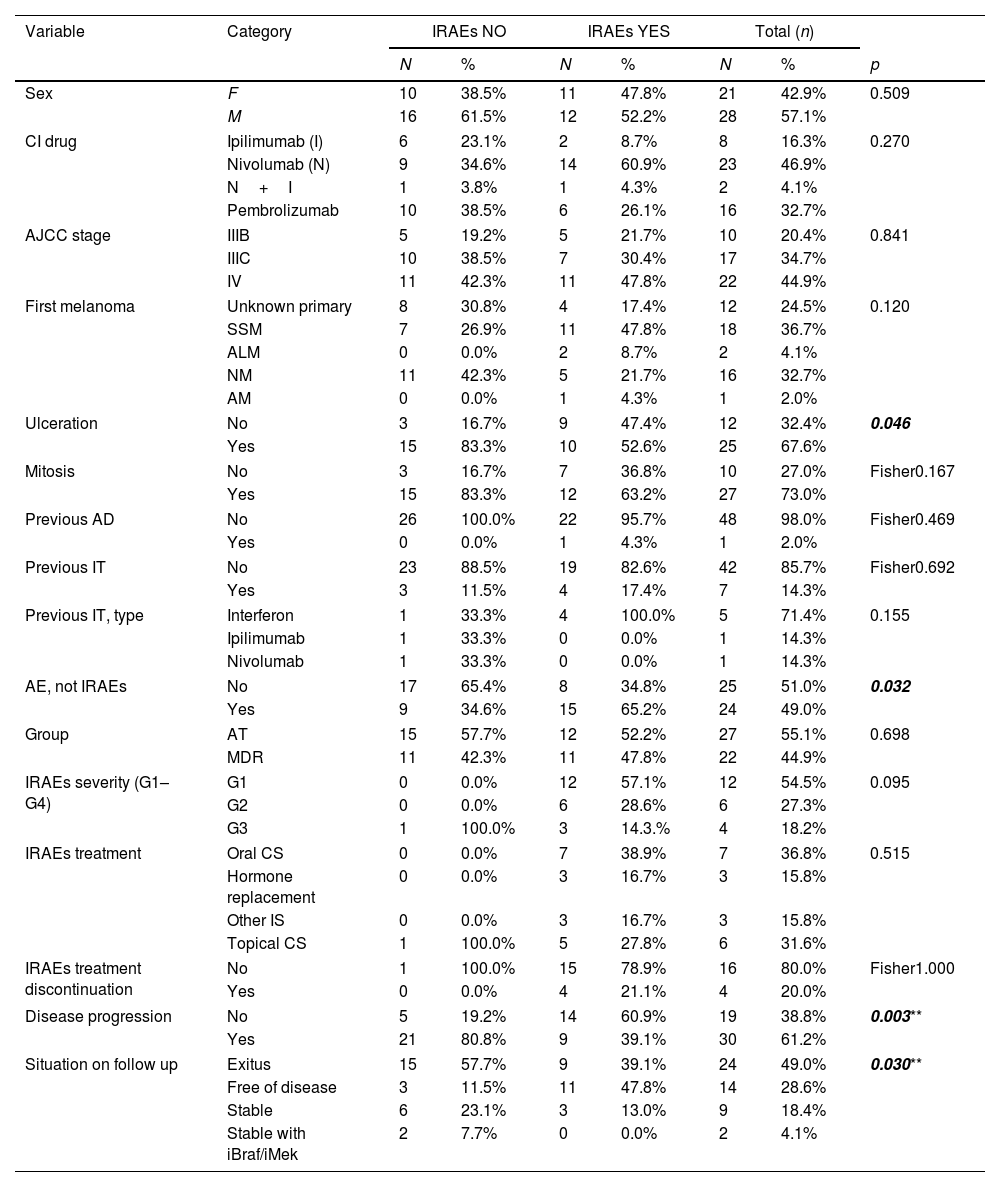
Our cohort included 49 patients with either nivolumab (n=23), pembrolizumab (16), ipilimumab (n=8) or ipilimumab+nivolumab (n=2). Baseline characteristics are shown in Table 1. Out of all of them, 51.9% (n=27) where on AT and 42.3% (n=22) on MDR. We found that 46.9% of the patients experienced an IRAE. They were typically mild to moderate in intensity, being severe (grade 3 of the common terminology criteria for adverse events3) in 18.2% of patients, and mostly resolved with topical/oral corticosteroids (Table 1). When comparing AT versus MDR, IRAEs frequency, type or management was similar in both groups (Table 1). IRAEs severity differed statistically, grading less in the AT group. This data might be of importance when deciding treatment initiation in the different stage III setting that we face in clinical practice. IRAEs lead to treatment discontinuation in 4 patients (n=1, AT; n=3, MDR) and no CI-related deaths were reported. In terms of disease progression, patients with IRAEs were less likely to progress and they had longer disease-free survival. Finally, melanoma ulceration was also related to a higher number of IRAEs (Tables 1 and 2). Ulceration is a biologic entity and over the years, researchers have identified several characteristics linked to the ulcerated melanoma phenotype such as: increased tumor vascularity, a loss of cell-to-cell adhesion as well as increased density of various tumor infiltrating immune cells and neutrophils, dendritic cells, macrophages and an increased number of PD-L1 positive tumor cells. This might explain the stronger response to interpheron alpha treatment in this subset of melanoma and could account to the greater rate of IRAEs4–6 As previously published, this analysis revealed that most IRAEs’ first occurrence took place early during treatment (Table 2).4,5 Similarly, dermatological, endocrine, gastrointestinal, and hepatic effects were the more frequently reported (Table 1).3
Baseline characteristics of the patients. Comparison between groups of patients who are on adjuvant therapy or in metastasis disease regimens.
| Variable | Category | AT | MDR | Total | ||||
|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | P score | ||
| Sex | F | 12 | 44.4% | 9 | 40.9% | 21 | 42.9% | 0.804 |
| M | 15 | 55.6% | 13 | 59.1% | 28 | 57.1% | ||
| CI drug | Ipilimumab (I) | 6 | 22.2% | 2 | 9.1% | 8 | 16.3% | 0.205 |
| Nivolumab (N) | 11 | 40.7% | 12 | 54.5% | 23 | 46.9% | ||
| N+I | 0 | 0.0% | 2 | 9.1% | 2 | 4.1% | ||
| Pembrolizumab | 10 | 37.0% | 6 | 27.3% | 16 | 32.7% | ||
| AJCC stage | IIIB | 10 | 37.0% | 0 | 0.0% | 10 | 20.4% | 0.001** |
| IIIC | 17 | 63.0% | 0 | 0.0% | 17 | 34.7% | ||
| IV | 0 | 0.0% | 22 | 100.0% | 22 | 44.9% | ||
| Primary melanoma | Unknown primary | 4 | 14.8% | 8 | 36.4% | 12 | 24.5% | 0.300 |
| SSM | 12 | 44.4% | 6 | 27.3% | 18 | 36.7% | ||
| ALM | 1 | 3.7% | 1 | 4.5% | 2 | 4.1% | ||
| NM | 10 | 37.0% | 6 | 27.3% | 16 | 32.7% | ||
| AM | 0 | 0.0% | 1 | 4.5% | 1 | 2.0% | ||
| Ulceration | No | 10 | 43.5% | 2 | 14.3% | 12 | 3.4% | Fisher0.084 |
| Yes | 13 | 56.5% | 12 | 85.7% | 25 | 67.6% | ||
| Mitosis | No | 5 | 21.7% | 5 | 35.7% | 10 | 27.0% | Fisher0.454 |
| Yes | 18 | 78.3% | 9 | 64.3% | 27 | 73.0% | ||
| Previous AD | No | 27 | 100.0% | 21 | 95.5% | 48 | 98.0% | Fisher0.449 |
| Yes | 0 | 0.0% | 1 | 4.5% | 1 | 2.0% | ||
| Previous IT | No | 23 | 85.2% | 19 | 86.4% | 42 | 85.7% | Fisher0.999 |
| Yes | 4 | 14.8% | 3 | 13.6% | 7 | 14.3% | ||
| Previous IT, type | Interferon | 3 | 75.0% | 2 | 66.7% | 5 | 71.4% | 0.350 |
| Ipilimumab | 1 | 25.0% | 0 | 0.0% | 1 | 14.3% | ||
| Nivolumab | 0 | 0.0% | 1 | 33.3% | 1 | 14.3% | ||
| AE, non-IRAEs | No | 13 | 48.1% | 12 | 54.5% | 25 | 51.0% | 0.656 |
| Yes | 14 | 51.9% | 10 | 45.5% | 24 | 49.0% | ||
| Non-IRAEs, type | Amhenorrea | 1 | 6.7% | 0 | 0.0% | 1 | 4.3% | 0.333 |
| Dizziness | 2 | 13.3% | 0 | 0.0% | 2 | 8.7% | ||
| Fatigue | 10 | 66.7% | 8 | 100.0% | 18 | 78.3% | ||
| Xerostomia | 2 | 13.3% | 0 | 0.0% | 2 | 8.7% | ||
| IRAEs | No | 15 | 55.6% | 11 | 50.0% | 26 | 53.1% | 0.698 |
| Yes | 12 | 44.4% | 11 | 50.0% | 23 | 46.9% | ||
| IRAEs, type | No | 15 | 51.7% | 12 | 52.2% | 27 | 51.9% | 0.495 |
| Arthritis | 1 | 3.4% | 1 | 4.3% | 2 | 3.8% | ||
| Dermatological | 8 | 27.6% | 4 | 17.4% | 12 | 23.1% | ||
| Enterocolitis | 0 | 0.0% | 2 | 8.7% | 2 | 3.8% | ||
| Hypotiroidism | 3 | 10.3% | 1 | 4.3% | 4 | 7.7% | ||
| Sarcoidosis | 2 | 6.9% | 1 | 4.3% | 3 | 5.8% | ||
| Uveitis | 0 | 0.0% | 1 | 4.3% | 1 | 1.9% | ||
| Adrenal insufficiency | 0 | 0.0% | 1 | 4.3% | 1 | 1.9% | ||
| IRAEs severity (G1–G4) | G1 | 10 | 71.4% | 3 | 27.3% | 13 | 52.0% | 0.013 |
| G2 | 4 | 28.6% | 3 | 27.3% | 7 | 28.0% | ||
| G3 | 0 | 0.0% | 5 | 45.5% | 5 | 20.0% | ||
| IRAEs treatment | Oral CS | 3 | 27.3% | 4 | 36.4% | 7 | 31.8% | 0.845 |
| Hormone replacement | 3 | 27.3% | 2 | 18.2% | 5 | 22.7% | ||
| Other IS | 1 | 9.1% | 2 | 18.2% | 3 | 13.6% | ||
| Topical CS | 4 | 36.4% | 3 | 27.3% | 7 | 31.8% | ||
| IRAEs treatment discontinuation | No | 11 | 91.7% | 8 | 72.7% | 19 | 82.6% | Fisher0.317 |
| Yes | 1 | 8.3% | 3 | 27.3% | 4 | 17.4% | ||
| Disease progression | No | 16 | 59.3% | 3 | 13.6% | 19 | 38.8% | 0.001** |
| Yes | 11 | 40.7% | 19 | 86.4% | 30 | 61.2% | ||
| Situation on follow-up | Exitus | 6 | 22.2% | 18 | 81.8% | 24 | 49.0% | 0.001** |
| Free of disease | 12 | 44.4% | 2 | 9.1% | 14 | 28.6% | ||
| Stable | 7 | 25.9% | 2 | 9.1% | 9 | 18.4% | ||
| Stable with iBraf/iMek | 2 | 7.4% | 0 | 0.0% | 2 | 41.0% | ||
Unless otherwise stated, X2 tests (Chi or Chi square of independence) are performed for qualitative variables and Student's t tests for quantitative ones.
IRAEs, immune-related adverse events; n, number of patients; F, female; M, male; AD, autoimmune disease; CI, check-point inhibitors; IT, immunotherapy; AE, adverse events; AT, adjuvant therapy; MDR, metastasis disease regimens; G, grade; CS, corticosteroids; IS, immunosuppression; SSM, superficial spreading melanoma; ALM, acral lentiginous melanoma; NM, nodular melanoma; AM, amelanotic melanoma.
Comparison between groups of patients who had/not had immune-related adverse events.
| Variable | Category | IRAEs NO | IRAEs YES | Total (n) | ||||
|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | p | ||
| Sex | F | 10 | 38.5% | 11 | 47.8% | 21 | 42.9% | 0.509 |
| M | 16 | 61.5% | 12 | 52.2% | 28 | 57.1% | ||
| CI drug | Ipilimumab (I) | 6 | 23.1% | 2 | 8.7% | 8 | 16.3% | 0.270 |
| Nivolumab (N) | 9 | 34.6% | 14 | 60.9% | 23 | 46.9% | ||
| N+I | 1 | 3.8% | 1 | 4.3% | 2 | 4.1% | ||
| Pembrolizumab | 10 | 38.5% | 6 | 26.1% | 16 | 32.7% | ||
| AJCC stage | IIIB | 5 | 19.2% | 5 | 21.7% | 10 | 20.4% | 0.841 |
| IIIC | 10 | 38.5% | 7 | 30.4% | 17 | 34.7% | ||
| IV | 11 | 42.3% | 11 | 47.8% | 22 | 44.9% | ||
| First melanoma | Unknown primary | 8 | 30.8% | 4 | 17.4% | 12 | 24.5% | 0.120 |
| SSM | 7 | 26.9% | 11 | 47.8% | 18 | 36.7% | ||
| ALM | 0 | 0.0% | 2 | 8.7% | 2 | 4.1% | ||
| NM | 11 | 42.3% | 5 | 21.7% | 16 | 32.7% | ||
| AM | 0 | 0.0% | 1 | 4.3% | 1 | 2.0% | ||
| Ulceration | No | 3 | 16.7% | 9 | 47.4% | 12 | 32.4% | 0.046 |
| Yes | 15 | 83.3% | 10 | 52.6% | 25 | 67.6% | ||
| Mitosis | No | 3 | 16.7% | 7 | 36.8% | 10 | 27.0% | Fisher0.167 |
| Yes | 15 | 83.3% | 12 | 63.2% | 27 | 73.0% | ||
| Previous AD | No | 26 | 100.0% | 22 | 95.7% | 48 | 98.0% | Fisher0.469 |
| Yes | 0 | 0.0% | 1 | 4.3% | 1 | 2.0% | ||
| Previous IT | No | 23 | 88.5% | 19 | 82.6% | 42 | 85.7% | Fisher0.692 |
| Yes | 3 | 11.5% | 4 | 17.4% | 7 | 14.3% | ||
| Previous IT, type | Interferon | 1 | 33.3% | 4 | 100.0% | 5 | 71.4% | 0.155 |
| Ipilimumab | 1 | 33.3% | 0 | 0.0% | 1 | 14.3% | ||
| Nivolumab | 1 | 33.3% | 0 | 0.0% | 1 | 14.3% | ||
| AE, not IRAEs | No | 17 | 65.4% | 8 | 34.8% | 25 | 51.0% | 0.032 |
| Yes | 9 | 34.6% | 15 | 65.2% | 24 | 49.0% | ||
| Group | AT | 15 | 57.7% | 12 | 52.2% | 27 | 55.1% | 0.698 |
| MDR | 11 | 42.3% | 11 | 47.8% | 22 | 44.9% | ||
| IRAEs severity (G1–G4) | G1 | 0 | 0.0% | 12 | 57.1% | 12 | 54.5% | 0.095 |
| G2 | 0 | 0.0% | 6 | 28.6% | 6 | 27.3% | ||
| G3 | 1 | 100.0% | 3 | 14.3.% | 4 | 18.2% | ||
| IRAEs treatment | Oral CS | 0 | 0.0% | 7 | 38.9% | 7 | 36.8% | 0.515 |
| Hormone replacement | 0 | 0.0% | 3 | 16.7% | 3 | 15.8% | ||
| Other IS | 0 | 0.0% | 3 | 16.7% | 3 | 15.8% | ||
| Topical CS | 1 | 100.0% | 5 | 27.8% | 6 | 31.6% | ||
| IRAEs treatment discontinuation | No | 1 | 100.0% | 15 | 78.9% | 16 | 80.0% | Fisher1.000 |
| Yes | 0 | 0.0% | 4 | 21.1% | 4 | 20.0% | ||
| Disease progression | No | 5 | 19.2% | 14 | 60.9% | 19 | 38.8% | 0.003** |
| Yes | 21 | 80.8% | 9 | 39.1% | 30 | 61.2% | ||
| Situation on follow up | Exitus | 15 | 57.7% | 9 | 39.1% | 24 | 49.0% | 0.030** |
| Free of disease | 3 | 11.5% | 11 | 47.8% | 14 | 28.6% | ||
| Stable | 6 | 23.1% | 3 | 13.0% | 9 | 18.4% | ||
| Stable with iBraf/iMek | 2 | 7.7% | 0 | 0.0% | 2 | 4.1% | ||
| Quantitative variables | N | Mean (SD) | N | Mean (SD) | N | Mean (SD) | |
|---|---|---|---|---|---|---|---|
| Age (y) | 26 | 60.7 (15.2) | 23 | 61.7 (10.2) | 49 | 61.1 (13.0) | 0.778 |
| Breslow | 18 | 6.5 (6.7) | 19 | 3.1 (2.0) | 37 | 4.7 (5.1) | 0.051 |
| Time to onset of immune-mediated effect (w) | 1 | 12 | 21 | 12.1 (5.3) | 22 | 12.0 (5.2) | 0.972 |
| Time to resolution of the immune-mediated effect (w) | 0 | NA | 5 | 3.0 (1.7) | 5 | 3.0 (1.7) | NA |
| Melanoma progression after CI initiation (w) | 25 | 27.6 (29.6) | 17 | 30.4 (24.0) | 42 | 28.7 (27.2) | 0.752 |
| CI treatment duration (w) | 26 | 21.6 (25.2) | 23 | 19.0 (14.4) | 49 | 20.4 (20.7) | 0.653 |
| Follow-up time after last dose of CI (w) | 26 | 17.7 (32.4) | 23 | 10.3 (12.5) | 49 | 61.1 (13) | 0.289 |
Unless otherwise stated, X2 tests (Chi or Chi square of independence) are performed for qualitative variables and Student's t tests for quantitative ones.
IRAEs, immune-related adverse events; n, number of patients; F, female; M, male; AD, autoimmune disease; CI, check-point inhibitors; IT, immunotherapy; AE, adverse events; AT, adjuvant therapy; MDR, metastasis disease regimens; G, grade; CS, corticosteroids; IS, immunosuppression; SSM, superficial spreading melanoma; ALM, acral lentiginous melanoma; NM, nodular melanoma; AM, amelanotic melanoma; SD, standard deviation; y, years; w, weeks; NA, not applicable.
With several therapeutic options now available in the melanoma treatment setting, an understanding of the benefit–risk ratio in clinical practice is needed to perform treatment decisions. Our safety analysis with nivolumab, pembrolizumab, or/and ipilimumab was consistent with the established safety profiles published before for both, AT and MDR.2,4,7–10 However, fewer discontinuation rates in the pool data analysis were observed in our cohort (8.30%) compared with AT-ipilimumab (35–53%), which is no longer considered a prime candidate for AT),2 or with AT-pembrolizumab (13–14.8%),10 similar when comparing with AT-nivolumab (7.7%).7,8 Moreover, our results confirmed that select IRAEs in AT/MDT settings are manageable using established safety guidelines.3 As seen with AT-pembrolizumab,4 not yet with other drugs in AT,5 IRAEs were associated with a better prognosis.
Limitations include the study's retrospective nature and the small sample size, forcing us to analyze the pooled data. However, to our knowledge, this report is the largest analysis to date comparing the safety profile of CI in both, AT and MDR, in daily practice.
Overall, these findings add to the understanding of the benefit–risk profile of immunotherapy in patients with resected high-risk or advanced melanoma and may ultimately help in guiding clinicians.
Conflict of interestsThe authors declare they have no conflict of interest.