Both dermatologists and pathologists sometimes find daunting the evaluation of melanonychia (especially subungual melanocytic lesions) because of the fear of performing nail surgery due to the risk of dystrophy, difficulties processing and interpreting nail biopsy specimens, and a general lack of experience in the field. Nevertheless, mastery of nail biopsy techniques, correct processing and orientation of specimens, and familiarity with the histologic particularities of the nail apparatus can attenuate the undoubted complexity and facilitate the tasks involved. Longitudinal excision is the biopsy technique that ensures the simplest histologic interpretation, and it is associated with a low risk of nail dystrophy when performed correctly. Clinical and epidemiological data are crucial. Subungual melanoma in childhood, for instance, is very rare and even lesions with atypical clinical and/or histologic features are probably benign. The presence of suprabasal melanocytes and other findings that would suggest malignancy at other sites are considered normal in the nail apparatus. Subungual melanoma shows a lentiginous pattern in the early stages of disease, and detection of an inflammatory infiltrate accompanying atypical lentiginous subungual lesions would appear to be one of the first diagnostic findings.
El miedo a realizar intervenciones en la uña por el riesgo de distrofia residual, la dificultad de procesado y de interpretación de las biopsias ungueales, y la falta global de experiencia en este campo, han hecho que las melanoniquias ungueales en general (y las lesiones melanocíticas subungueales en particular), sean un tema poco atractivo tanto para dermatólogos como para patólogos. A pesar de la indudable complejidad de este campo, el manejo de las técnicas de biopsia ungueal, el correcto procesado y orientación de las muestras, y el conocimiento de las particularidades histológicas del aparato ungueal pueden facilitar mucho esta labor. La biopsia longitudinal excisional ofrece la interpretación histológica más sencilla, y tiene bajo riesgo de generar distrofia ungueal si se realiza correctamente. Los datos clínicos y epidemiológicos son fundamentales: el diagnóstico de melanoma subungueal en la infancia es excepcional, e incluso lesiones con características clínicas y/o histológicas atípicas constituyen con toda probabilidad lesiones benignas. La presencia de melanocitos suprabasales y otros hallazgos que serían sospechosos de malignidad en lesiones en otras localizaciones se consideran normales en el aparato ungueal. El melanoma subungueal tiene un patrón lentiginoso en estadios precoces, y parece que la presencia de un infiltrado inflamatorio acompañando a lesiones subungueales lentiginosas atípicas es uno de los primeros hallazgos diagnósticos del melanoma subungueal.
Traditionally, diagnosis and treatment of melanonychia has not been a fashionable topic for either dermatologists or pathologists. This is due in part to the low prevalence of these lesions. The resulting limited experience with this condition means that dermatologists are reticent about performing biopsies because of concerns that these may cause nail dystrophy.1–6 Often, this leads to partial or inadequate biopsies that may be insufficient to obtain a reliable histologic diagnosis.3,6–8 For the same reason, pathologists encounter problems in the interpretation of nail biopsies.8,9 In addition to the low number of such biopsies, pathologists are faced with inappropriate specimens due to the way the biopsy was performed (for example, because the matrix in a melanocytic lesion was not included or because of the transverse instead of longitudinal orientation of the biopsy) as well as incorrect specimen processing.7,10 The histological preparation obtained from a nail biopsy, whose consistency requires special processing techniques, therefore often includes retraction artefacts or the preparation is incorrectly oriented.2,10,11 To make matters worse, the nail is a site with its own particularities and the density and distribution of melanocytes differ from those in the rest of the skin.7–9,11,12
Nevertheless, it is possible to perform suitable biopsies of melanocytic nail lesions causing only very limited permanent damage, using relatively simple techniques.1,4,6,11 On the other hand, although histological interpretation of these lesions is not easy, a suitable biopsy and appropriate specimen processing and orientation, as well as awareness of the specific characteristics of nail melanocytes, can be of help.4,7,9,13 The present article aims to analyze the clinical and histological characteristics of melanocytic lesions of the nail and offer a diagnostic algorithm for pigmented lesions of the nail apparatus. The article also includes a detailed review of the different biopsy techniques.
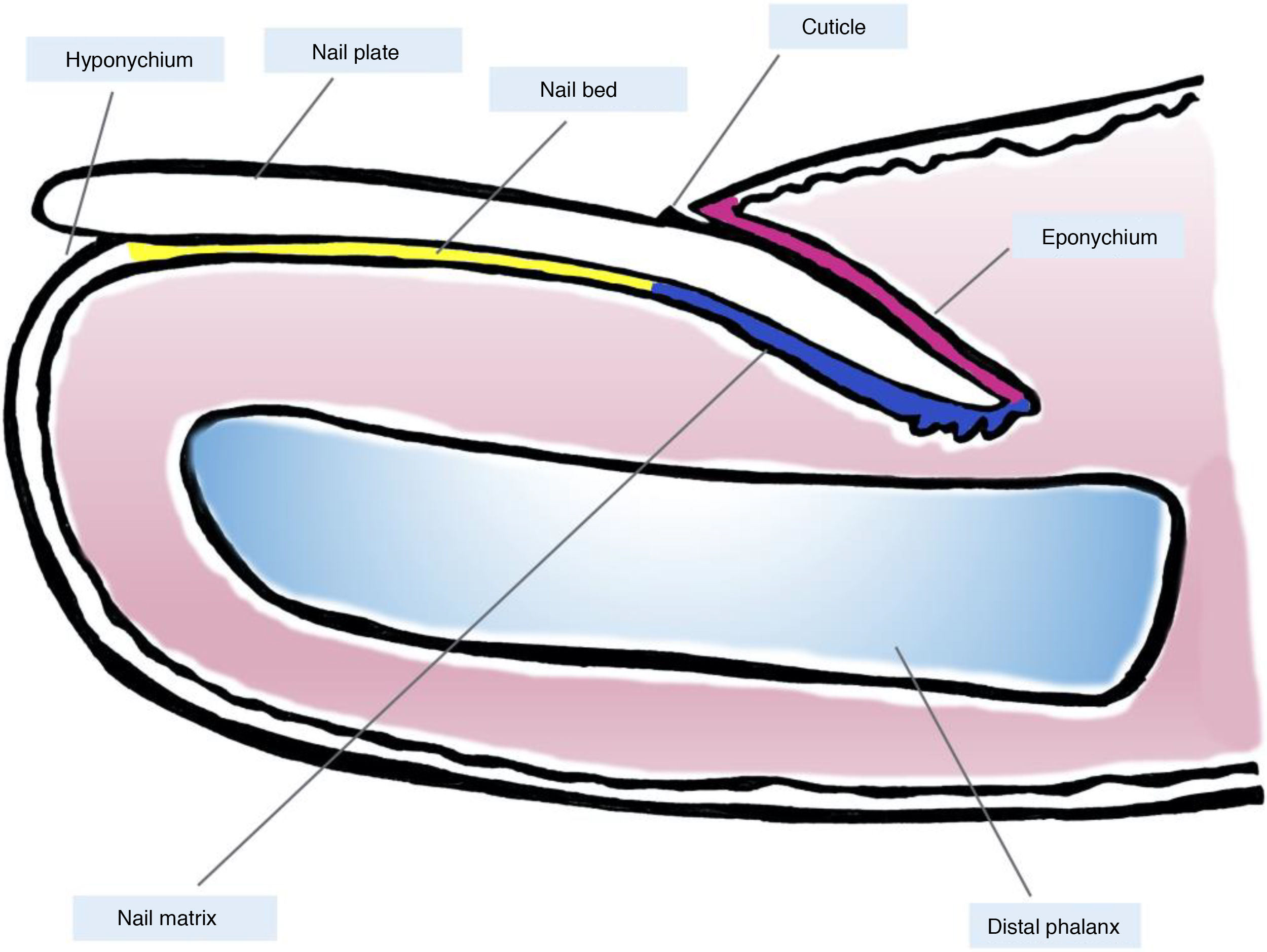
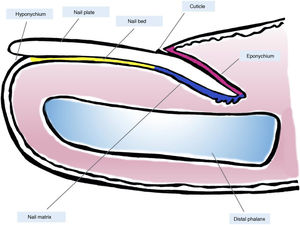
Clinical and Histological Anatomy of the Nail ApparatusKnowledge of the clinical and histological anatomy of the nail is essential for subsequent evaluation of melanocytic lesions.7,11,12,14,15 The nail apparatus consists of the nail matrix, nail bed, nail plate, nail cuticle, and connective tissue that supports these structures (Fig. 1).7,11,14,15 The plate is separated from the rest of the finger by nail folds (1 proximal and 2 lateral ones).13 The matrix, the epithelial part most proximal to the nail apparatus, is responsible for producing the nail plate and continues distally with the bed.7,11,14 Both the matrix and the plate are covered by the proximal nail fold. In some fingers, it is possible to see the lunula, a whitish convex structure with a halfmoon form that corresponds to the most distal part of the matrix, whereas the nail bed is the main structure that is visible through the plate.11,13,14
Macroscopic anatomy of the normal nail: the nail apparatus is covered in its most proximal part by the proximal nail fold. The epithelium of the ventral face of this fold is denoted eponychium (in pink) and produces the true cuticle (in black), which is firmly adhered to the nail plate. The nail matrix (in blue), divided into the proximal and distal matrix, produces the plate and continues distally into the bed. Both the matrix and the bed are covered by the nail plate. Finally, the bed (in yellow) ends distally in the hyponychium.
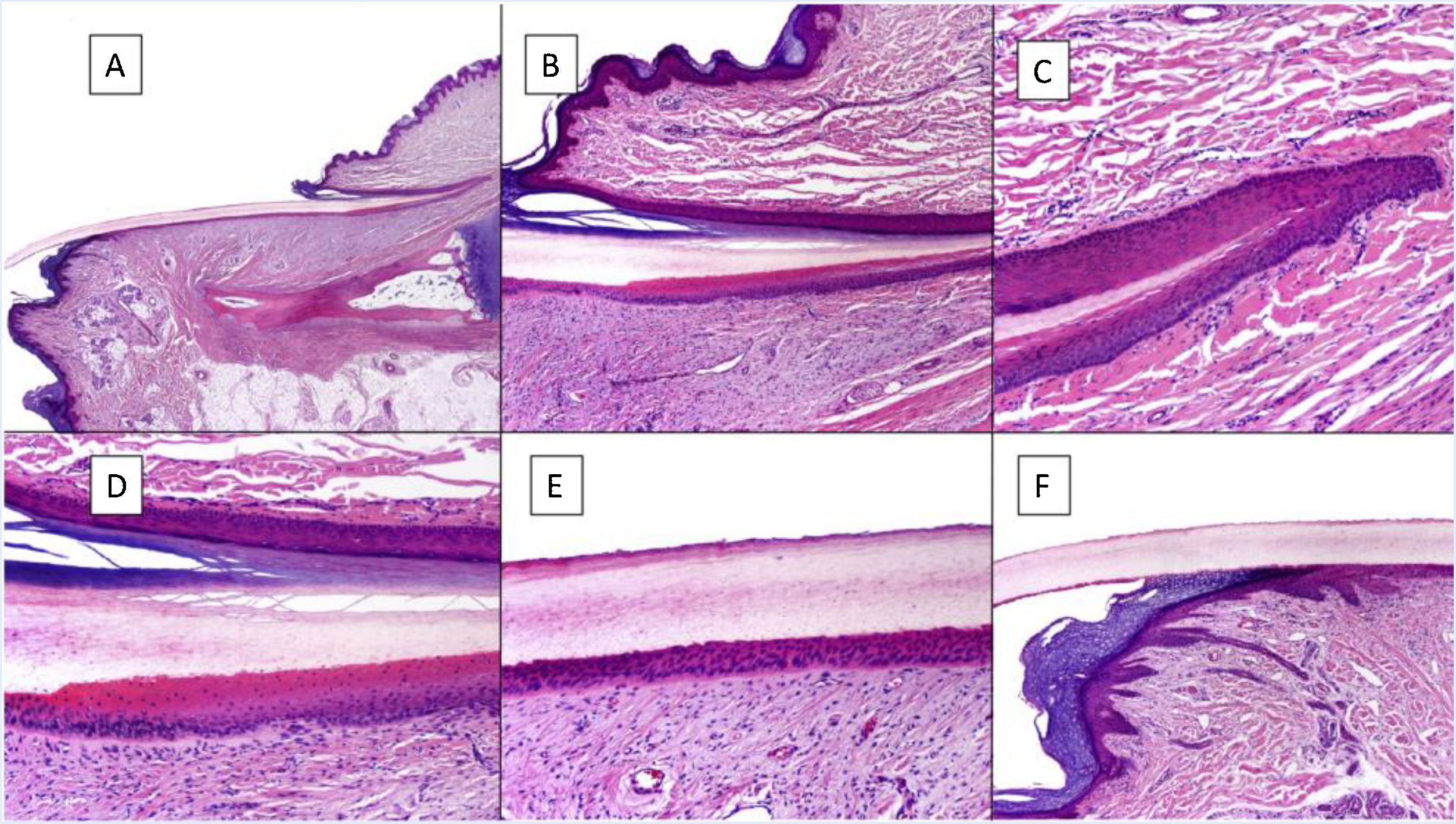
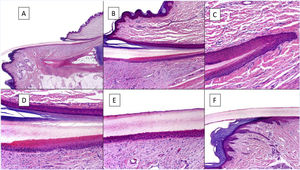
Histologically, the best correlation with macroscopic anatomy of the nail is obtained with longitudinal biopsies (Fig. 2).7,14,16,17 The proximal nail fold consists of acral skin and is covered by a film of keratin that forms the false cuticle. This continues in the ventral face of the fold with the true cuticle, produced by the eponychium.7,11,14,16 The matrix is subdivided into proximal and distal parts. In cuts close to the medial line of the finger, the epithelium of the proximal matrix shows large longitudinal and oblique crests, whereas in lateral areas, it is flatter and thinner.9,14 The proximal matrix is the structure mainly responsible for the production of the nail plate (up to 80%), especially the dorsal and exposed face.3,7,9,11,14,16,18 The ventral face of the lamina originates from the distal matrix. Therefore, proximal damage is more likely to generate permanent nail dystrophy.3,7,11,14,16,18,19
Histological anatomy of a normal nail. A, Microscopic anatomy of a normal nail in a longitudinal section. B, Detail of the nail matrix and eponychium. C, Fold between the proximal matrix and the eponychium. D, In the lower part, typical keratinization can be seen without any granular layer of the matrix to form the plate with a similar aspect to trichilemmal keratinization. In the upper part, the eponychium can be seen with characteristic orthokeratotic keratinization. E, Detail of the nail bed with its typical flattened epidermis without the stratum granulosum covered directly by the nail plate. F, Detail of the hyponychium with an epidermis with orthokeratotic keratinization with similar characteristics to that of the rest of normal acral skin (not subungual).
Keratinization of the nail plate, which is produced in the keratogenic band of the matrix, occurs abruptly without stratum granulosum, in a very similar fashion to trichilemmal keratinization.9,14,16 The epithelium of the nail bed continues distally to the matrix, is thin, and presents longitudinal crests, penetrating the underlying dermis, which can only been observed in transverse cuts.7,11,14 Finally, the distal limit of the nail consists of the hyponychium, separated from the rest of the finger by the distal nail fold.11,14,16 The dermis that supports the nail apparatus has limited cellularity and lacks any cutaneous appendages. The hypodermis is also limited.9,11,14,16
The nail is a special site in terms of melanocyte distribution.7,11,14,16,18 The normal number of melanocytes in the nail apparatus is almost 6 times lower than observed in other sites: 200/mm2 compared with 1150/mm2 in normal skin.7,9,11,14,20 Melanocytes are scarce in the eponychium and nail bed (0 to 50/mm2), are in a quiescent state, and are mainly found in the basal layers of the epithelium.7,11,14,16,18 In the matrix, the melanocyte density is somewhat higher (100 to 200/mm2) and the melanocytes may be found in the suprabasal layers, although this is not considered a pathologic finding, particularly in the proximal matrix.7,15,16,18,20 The melanocytes in the proximal matrix are also in a quiescent state and hardly produce any melanin, whereas those in the distal matrix are more active (approximately 50% produce melanin).7,11,16 For this reason, most pigmented melanocytic lesions of the nail apparatus arise in the matrix, and particularly in the distal part (up to 85%); therefore, they present clinically with longitudinal melanonychia.7,16 However, up to 25% of subungual melanocytes originate in the nail bed and, in view of the limited melanin synthesis by these melanocytes, they are often amelanotic.2,7,11,14,21
Given their low density and quiescent state, it may be difficult to visualize nail melanocytes with hematoxylin-eosin staining, and an immunohistochemical technique is needed.7,18,20,22 Cytoplasmic staining with melan-A and HMB45 is less precise and may even stain keratinocytes in lesions with a very active pigment synthesis.7,20,23,24 Nuclear stains, such as MiTF and SOX10, are cleaner and can more exactly determine melanocyte density and site in the epithelial layers.7,20,23,24 Nail melanocytes do not stain well with S100 and so its use should be limited.7,9,11,13,16,20
Nail Biopsy TechniquesOne of the main reasons, if not the main reason, for performing a biopsy of the nail apparatus is to rule out subungual melanoma.7,16,19,25 There are many different techniques and to select the most appropriate we should bear in mind both the need to obtain a usable specimen for study and the technical difficulty and risk of permanent nail dystrophy.2,11,14,16,25 Taking into account that most melanocytic lesions lie in the nail matrix, this structure should be included in almost all cases. However, given that most lesions appear in the distal matrix, it may not be necessary to include the proximal matrix in the biopsy, thereby reducing the risk of dystrophy.16,17,25 Nail dermoscopy is useful for the study of longitudinal melanonychia and facilitates identification of the clinical signs of alarm.6,26–28 Intraoperative nail dermoscopy (once the nail plate has been removed) can, moreover, more precisely locate the source of pigmentation.5,6,26,29,30
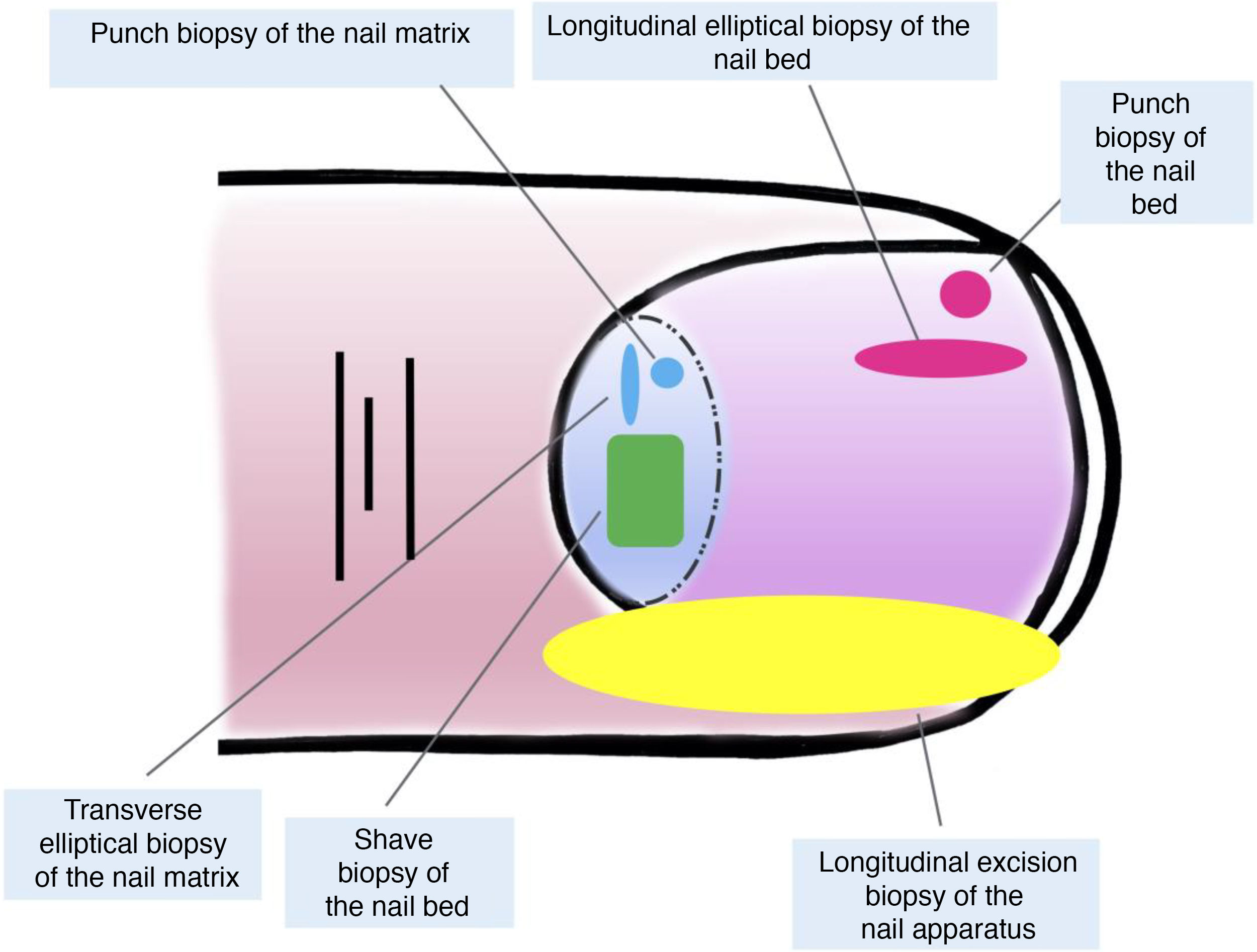
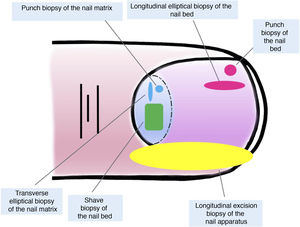
Both longitudinal elliptical and punch biopsies can be used for nail bed biopsies.16,19,21 Given that most pigmentation of melanocytic origin arises in the matrix, an isolated biopsy of the nail bed is insufficient in this context, as the histological material of interest would not be obtained for study.7,9
Isolated biopsies of the matrix are mainly of the elliptical type, in this case, transverse (in general, the first should be transversely oriented),21,31 punch biopsies, and longitudinal excision biopsies, which we will discuss in more detail later.2,14,19,21 Elliptical biopsies and punch biopsies can be performed directly or with prior avulsion of the nail plate.2,5,13,14,19 After avulsion, if the pigmentation studied has a small diameter, a punch biopsy can be performed.2,13,14,19,25 For broad pigmentations, longitudinal transverse biopsy of the matrix is the preferred option.14,19,25 In recent years, shave biopsy of the matrix has received particular attention.5,6,13,21,25,32 This technique is performed after withdrawing the proximal nail fold and avulsion of the proximal plate. With the scalpel blade, shaving of the part of interest of the matrix or excision of its entirety is performed. Subsequently, the nail plate is replaced and the incision in the fold is closed by direct approximation.6,21,25,32 This shave biopsy allows complete inclusion of the matrix when broad bands of melanonychia are present and has a low risk of permanent nail dystrophy.4,5,6,7,21,32,33
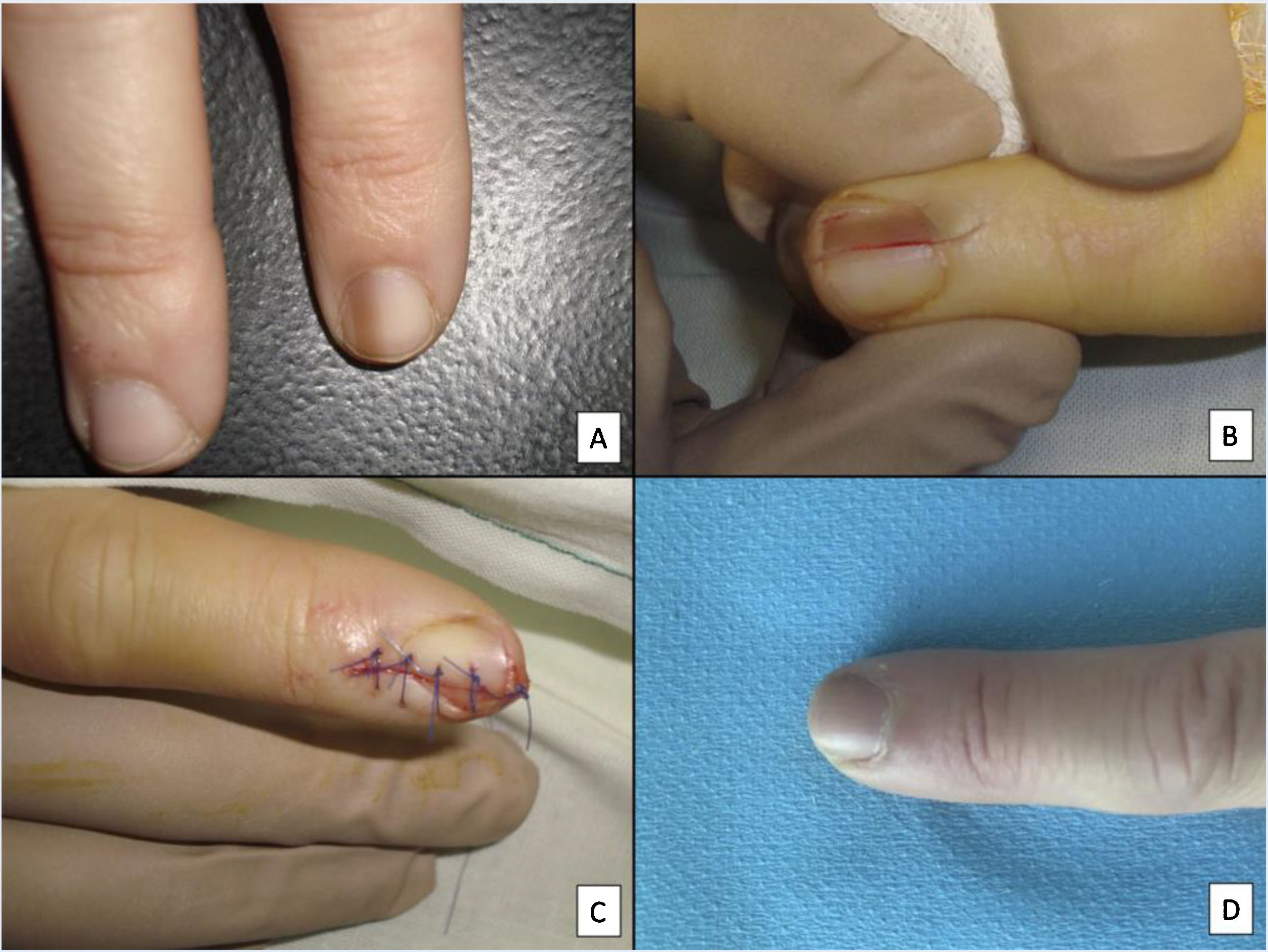
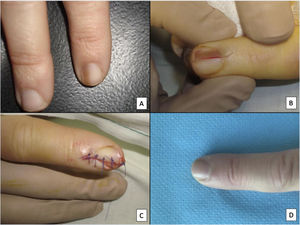
Although all these techniques undeniably have a role to play, most authors consider that longitudinal excision biopsy (or incision biopsy in the case of broad lesions) is the technique of choice for histological study of the nail apparatus.7,16,17,20,21,31 Longitudinal biopsies offer the best anatomical correlation and allow study of the distribution of the lesion within the global architecture of the nail apparatus, thereby facilitating analysis.16,21,31,34 In general, longitudinal excision biopsy is considered the technique of choice for study of fine longitudinal melanonychias and those at a lateral site, with the result of a narrower nail but with a low risk of permanent dystrophy.7,11,14,16,21,31 However, when the melanonychia is located in the central area of the nail plate, there is less consensus and many authors prefer one of the other techniques described above, given that permanent dystrophy in the central area of the nail would generate permanent indentation.4,7,11,14,16,21,31 In our experience, longitudinal biopsy can be used safely, even in centrally located bands of melanonychia (Fig. 3). In this case, the technique consists of removing a cylindrical specimen that includes the entire pigmented band (or the most representative part of it if it is too broad), from the hyponychium to the proximal nail fold, going as deep as the periosteum of the distal phalanx. After excision, a direct approximation of the defect is performed with stitches, from the plate on the surface to the deepest fibrocollagenous supportive tissue. To reduce the risk of dystrophy, the need for stitches in the proximal area of the matrix can be avoided, suturing instead in the proximal nail fold and the corresponding area of the distal matrix and proximal bed. Performed this way, this technique has a low risk of permanent dystrophy and the only potential consequence is that of a narrower nail plate (as is the case when the technique is performed at a lateral site). Therefore, in our opinion, longitudinal excision biopsy should be considered as the technique of choice in most cases given that it provides the best architectural overview of the lesion. Following the method described, with the borders in contact and the matrix correctly aligned, the risk of permanent nail dystrophy is low. Fig. 4 shows the different biopsy techniques for the nail apparatus.
Longitudinal excision biopsy of the nail apparatus. A, Pale brown longitudinal melanonychia, which histologically corresponds to nail hypermelanosis. B, Under local anesthetic, longitudinal excision is performed in a cylindrical shape from the proximal nail fold to the hyponychium, including the entire nail apparatus. C, Direct closure of the defect by suture from nail plate at the surface to the deep nail connective tissue. D, After 1 year, the result is a normal nail without any sign of permanent dystrophy, just narrower.
Finally, correct processing of the histological specimen is just as demanding as obtaining it in the first place. The nail plate is a very hard tissue that is difficult to cut, and this often leads to tractional artefacts, epithelial detachments, and, ultimately, a poor orientation of the cut, thereby hindering interpretation.3,7,10,11,16 There are different methods for softening the nail piece before cutting,3,9–11,13,31 but these are outside the scope of the present article. Perhaps simplest is immersion of the piece in distilled water for a few hours before inclusion in formalin. Preparations that contain acids should never be used as these can damage the genetic material of the sample and compromise immunohistochemical and molecular biology studies.11,13
Melanocytic Lesions of the Nail ApparatusMost melanocytic lesions of the nail apparatus present as a longitudinal melanonychia. This consists of a pigmented band that extends from the matrix to the free border of a nail and that is caused by the presence of pigment in the nail plate.6,7,9,35 Occasionally, melanonychia occupying the entire width can be seen in the nail plate. Of note is that longitudinal melanonychia is not exclusive to melanocytic lesions and many other entities present with similar clinical manifestations; these include Laugier syndrome, drug-induced exogenous pigmentation, and racial pigmentation.7,16,23,35,36
As with pigmentated lesions at other sites, there is a series of atypical clinical signs considered alarming that require nail melanoma to be ruled out.1,7,16,26,35,37–39 Broad, heterogeneous melanonychia (>5 mm) (composed of different bands of colors), with poorly-defined borders, rapid progression, or accompanied by nail dystrophy are considered highly suspicious.1,7,9,26,39–41 Likewise, melanonychia in triangular form (broader in the proximal part than the distal part) suggests that the lesion that generates it is growing and this also requires melanoma to be ruled out.1,7,14,26
As discussed later, the epidemiological context is very important in the assessment of longitudinal melanonychias.1,7,9,24,26,35,39,42 The appearance of a new melanocytic lesion in an adult, on a single finger, always requires nail melanoma to be ruled out, regardless of the clinical characteristics of the lesion.1,6,7,14,24,43,44 In contrast, given that nail melanoma is exceptional in childhood, even when bands with very atypical clinical characteristics or narrow spacing are present, the lesion is almost always benign.1,6,7,38,43,45–49
For this reason, the pathologist will find it very useful if a clinical photograph is included with the biopsy, along with information on the site of the lesion and age of the patient.7,14,46 However, isolated clinical examination is usually insufficient to determine the nature of a longitudinal melanonychia and a complementary histological study is necessary.1,7,14,17,26,42,50,51
Fig. 5 proposes a diagnostic algorithm for longitudinal melanonychias according to their clinical characteristics.26
Algorithm for management of longitudinal melanonychias (adapted from Piraccini et al.26) In adults, the sudden appearance of a single new band of melanonychia requires subungual melanoma to be ruled out. In children, even in presence of some atypical clinical signs, the lesion is very likely to be benign. In presence of worrying signs or symptoms (bleeding, pain, sudden or noteworthy change in color or thickness), some authors recommend referral to a specialist unit or to consider nail biopsy.
Ungual melanocytic hyperactivation, also called nail hypermelanosis, is the most frequent cause of longitudinal melanonychia in adults (up to 73% of cases).1,7,14,24,52 Clinically, this entity is usually manifest as narrow bands (<5 mm in diameter) with a greyish or light brown coloration.7,14,24 Melanocytic hyperactivation arises due to increased capacity for melanin synthesis in nail melanocytes, located mainly at the suprabasal level.1,7,12,15 Although there may be a moderate increase in the number of melanocytes, this is not usually noteworthy in hematoxylin-eosin staining and the main histological finding is increased pigmentation.1,7,9,12,14 Often, melanonychias have a marked clinical presentation and intensely pigmented bands are usually associated with mainly unremarkable histological findings.7 On the other hand, increased pigmentation in the matrix may hinder clear distinction between melanocytes and keratinocytes not only with hematoxylin-eosin staining but also with cytoplasmatic immunohistochemical staining, given that these products stain melanosomes that can also be present in keratinocytes.7,14,20,24 As described above, nuclear immunohistochemical markers allow a much clearer visualization of nail melanocytes.7,20,24 Finally, it is possible to find isolated melanophages in the superficial dermis of these lesions.7,14
Subungual LentigoSubungual lentigo is a lesion composed of an increased number of melanocytes distributed in the basal layer of the epidermis with a lentiginous pattern and without forming nests.1,7,9,14,51 The entity is also known as benign melanocytic hyperplasia, or melanocytic macules, and there are even authors who consider subungual lentigo and melanocytic hyperactivation together under the term melanotic macule.15,24,52 Given that the term melanotic macule is used for lesions at other cutaneous-mucosal sites to designate increased pigmentation without an increased number of melanocytes (which in the nail would be denoted melanocytic hyperactivation), many authors prefer not to use this term.22 The clinical manifestations of subungual lentigo overlap with those of melanocytic hyperactivation: narrow light brown or grey bands are usually present.1,7,12 Histopathologically, a marked increase in the number of matrix melanocytes (often dendritic) is observed as well as pigment synthesis (Fig. 6).1,7,14,51 It is important to remember that isolated suprabasal melanocytes in the epithelium of the nail matrix are not considered a pathological finding.1,3,7
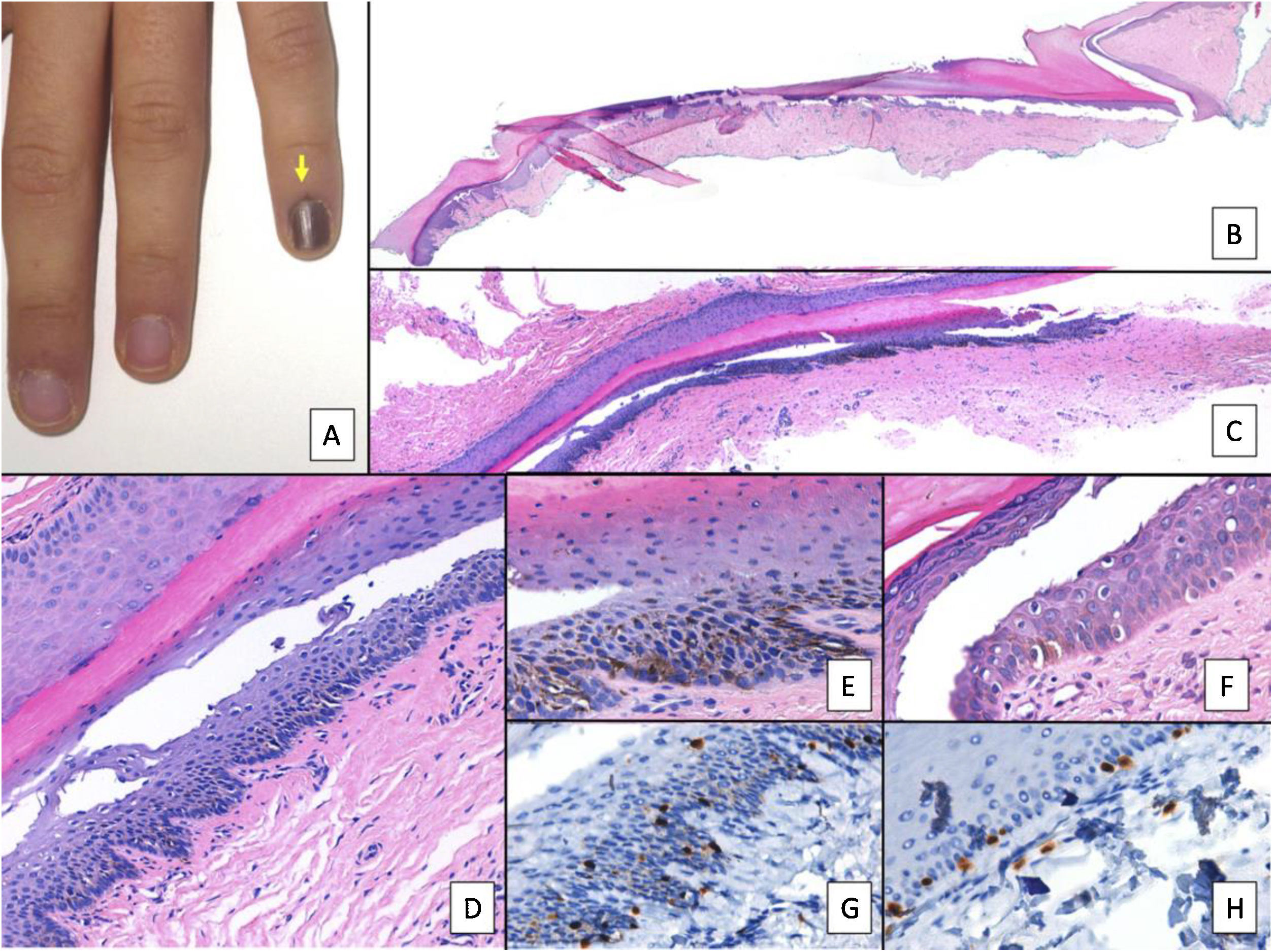
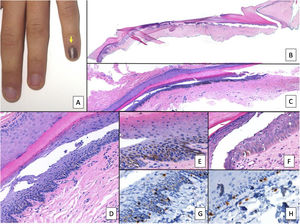
Nail lentigo A, Patient aged 11 years who consulted for melanonychia of the 5th finger of the left hand. The melanonychia band occupied the entire nail, with pigmentation of the proximal nail fold (arrow: Hutchinson sign). B, Longitudinal excision biopsy that shows the anatomy of the nail apparatus in its entirety. C, Oblique longitudinal section in which the following is observed (from top to bottom); eponychium, nail plate with band of onychokeratinization, and the matrix. An increase in pigmentation is observed in the basal layers. D, At greater magnification (x200), lentiginous melanocytic proliferation can be seen in the basal layers of the matrix and abundant melanin pigment. Suprabasal ascent of some melanocytes can be observed, along with several artefacts of the fixing process. E, Detail of the matrix (x400) with a notable substantial increase in pigmentation. F, Detail of the eponychium (x400) in which a slight increase in the number of melanocytes is observed, without any signs of atypia. Once again, keratinocytes are present as artefacts with a vacuolated appearance. Melanocytic hyperplasia at this level is responsible for the clinical Hutchinson sign. G, Detail of the matrix (x400) with SOX10 staining. This confirms a small increase in the number of melanocytes scattered in the basal layer, without forming nests or rows. A suprabasal ascent of some isolated cells can be seen. H, Detail of the eponychium (x400) with SOX10 staining. This highlights a slight increase in the number of melanocytes, separated from each other by normal keratinocytes.
Melanocytic nevus is the most common melanocytic nail lesion in childhood (accounting for up to 75% of lesions).1,7,14,23,45,53 Clinically, it consists of a more or less regular longitudinal melanonychia.1,7,14,43,54 Melanocytic nevi, particularly congenital ones, can lead to pigmentation of the proximal nail fold (Hutchinson sign) without being suggestive of malignancy.1,7,45,54,55 At times, particularly in infancy, melanocytic nevi of the nail matrix may have an atypical clinical presentation, with broad or irregular melanonychia, Hutchinson sign not associated with melanoma, and even complete melanonychia.7,45,49,54–56
Histopathologically, proliferation is observed of melanocytes arranged in nests that occupy the basal layers of the epithelium of the nail matrix.1,7,15,51,54,56 Given the firmness of the overlying nail plate, the nests can be squashed and confluent, as frequently observed in longitudinal biopsies.7 A suprabasal increase of isolated melanocytes can be observed, without any other atypical features present.1,3,7–9,51,56
Nail MelanomaNail melanoma is an uncommon tumor, but diagnosis is often made late, and this partly explains its poor prognosis.1,7,34,41,42,50,51,57 In fact, the extent of invasion at diagnosis is usually significantly greater than that of surface spreading melanoma.7,8,34,41,42,57 The most frequent histopathological variant of subungual melanoma is acral lentiginous melanoma.8,16,27,42,58–60 Superficial spreading melanoma and nodular melanoma occur less frequently in the nail.7,8,12,23,59,60 In absolute terms, nail melanoma has a similar incidence in different ethnic groups, but the low frequency of other types of melanoma in black and oriental patients means the percentage in these groups with respect to all types of melanoma is higher.1,3,8,14,58,61–63
Nail melanoma is very infrequent in children (up to 16 years), with a median age of presentation of 7 years (range, 6 months to 16 years). In addition, in many of the 21 cases reported to date in the literature, diagnosis was debatable. In several cases, the histological images shown are of poor quality (or even nonexistent) and do not allow appropriate assessment of the lesion.1,29,36,38,40,45,64–69 Of the 21 cases, only 4 were invasive and 2 had lymph node involvement. None of the previously described cases had visceral metastases or led to death. Some authors suggest that most of the pediatric cases diagnosed as in situ melanoma lack the same biological malignant potential as similar lesions in adult patients.45,46,56 Therefore, even when atypical clinical manifestations are present, we should remember that malignant melanocytic lesions in childhood are exceptional.1,7,26,40,45,48,51,53
Some of the signs of alarm of atypical longitudinal melanonychias have already been described. Nail melanoma occurs mainly on the first finger of the hand and on the big toe,1,7,8,41 and so a new-onset longitudinal melanonychia in an adult, at either of these sites, is considered suspicious.1,9,14,26,51 The Hutchinson sign, which consists of pigmentation in the proximal nail fold and cuticle, is a classic sign of subungual melanoma.1,9,13,14 However, many other entities can produce nonpathologic pigmentation in the cuticle; these include congenital melanocytic nevi, Laugier syndrome, and drug-induced exogenous pigmentations.1,7,14,55 In these cases, nonmelanoma-associated Hutchinson sign is the preferred terminology.1 Pseudo-Hutchinson sign refers to the apparent pigmentation of the cuticle arising from transparency of the underlying nail plate.1,14 Nevertheless, the presence of the Hutchinson sign in melanonychias in adults with other atypical features is considered highly suspicious.1,70,71 In parallel, pigmentation of the hyponychium (which appears in more advanced phases) linked with longitudinal melanonychia corresponds to spread of the lesion to acral skin and its presence is also suggestive of subungual melanoma.1,7,9 Finally, it is important to remember that up to 25% of nail melanomas can be amelanotic, presenting as nail dystrophy or an amelanotic module in very advanced phases.1,7,34
The most frequent histopathologic variant of subungual melanoma is acral lentiginous melanoma and diagnosis in early phases can be very difficult.3,7,8,44,51,72 Initially, the only indication is an increased number of melanocytes, which are large, with pyknotic nuclei and atypical features.7,8,33,72 They are distributed irregularly, with a preference for the basal layer of the nail matrix or, less frequently, the nail bed.7,8,72 As with other acral melanomas, formation of melanocytic nests occurs late in the course of the tumor.8,9 Neoplastic melanocytes are large, with hyperchromatic nuclei and elongated dendrites.1,7,8,14,51,72 The ascent of melanocytes to the upper layers of the matrix, considered physiological in this anatomical site, is much more evident, with disordered pagetoid spread that may be accompanied by the presence of melanocytes in the nail plate (Fig. 7).7,8,23,51,72 This is a finding that can support diagnosis of melanoma and that can only be assessed in biopsies that include the nail plate.1,7,11,23 It appears that the presence of a lymphocyte infiltrate in subungual intraepidermal melanocytic lesions is an important criterion for malignancy, as such an infiltrate is almost never found in subungual melanocytic nevi (Fig. 8).3,8,9,51 Other criteria suggestive of melanoma are the presence of multinucleation and a significant increase in melanocytic density (in contrast to subungual lentigo, where the increase is only moderate).33,51 Lentiginous spread of melanocytes towards the eponychium or hyponychium may constitute, when present, another useful key to diagnosis of subungual melanoma, as it is an indication of the capacity for invasion of surrounding structures.3,72
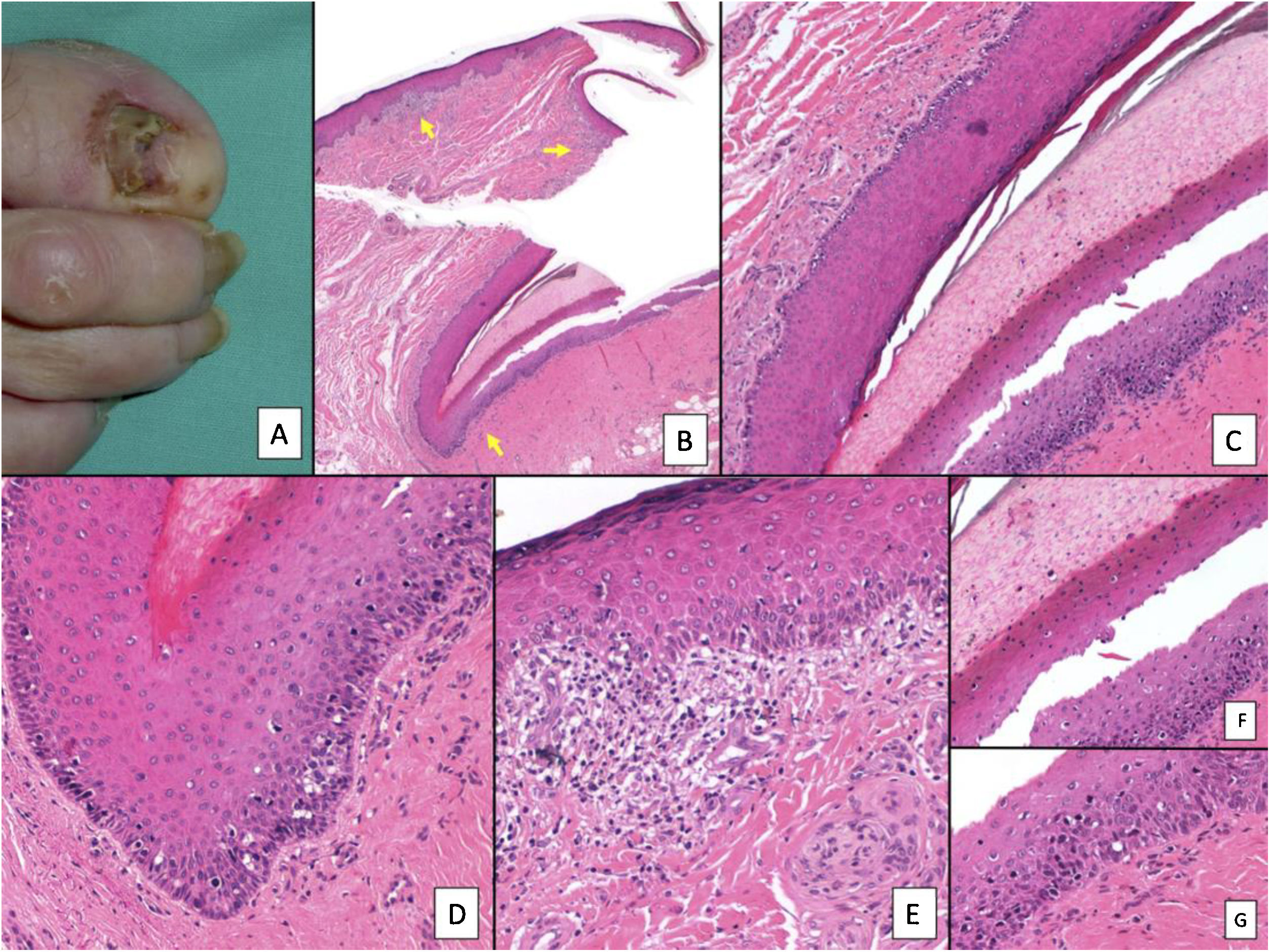
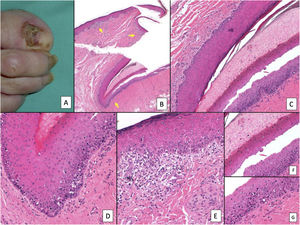
Subungual melanoma. A, Pigmented subungual lesion that generates substantial nail dystrophy, with partial destruction of the nail plate. An evident pigmentation of the exposed bed can be seen, in the hyponychium and the proximal nail fold (Hutchinson sign). B, Low-magnification view of longitudinal nail biopsy (x4), in which an increase in the pigment of the matrix can be seen, as well as sparse foci of accompanying inflammatory infiltrate (arrows). C, Greater detail (x100) of the nail matrix shows a lentiginous proliferation of vacuolated cells (melanocytes), with marked suprabasal ascent; cells thus even become incorporated into the nail plate. This lentiginous proliferation also involves the eponychium. D, At higher magnification (x200), a proliferation of atypical melanocytes can be observed, with suprabasal ascent with invasion of the entire matrix and spread towards the eponychium. E, Detail (x200) of the proximal nail fold. Melanocytic proliferation has now spread to the skin proximal to the eponychium. Once again, a lentiginous proliferation of atypical melanocytes is observed, with presence of atypical cells already in the papillary dermis and accompanying inflammatory infiltrate. Therefore, this melanoma is initially invasive. F, Detail (x200) of the suprabasal ascent of atypical cells, some of which are present in the nail plate. G, Detail (x200) of the nail matrix; ascent to suprabasal layers of atypical melanocytes.
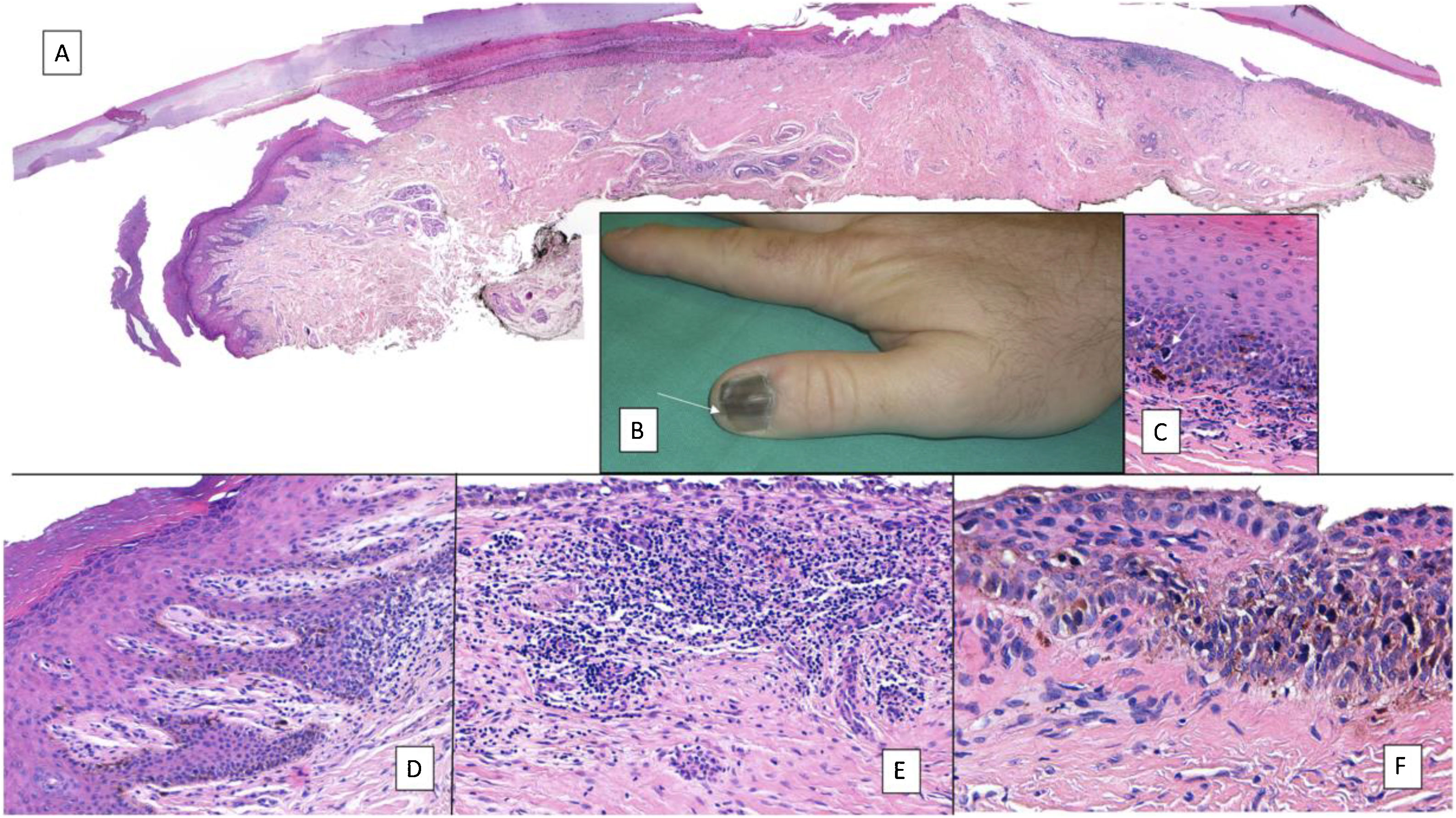
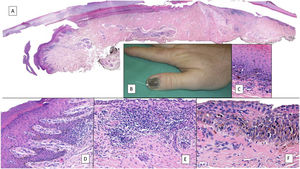
Subungual melanoma. A, Low-magnification longitudinal view of the entire nail apparatus. The patches of inflammatory infiltrate of irregular distribution are already apparent at low magnification of the distal matrix, nail bed, and also in the hyponychium. B, In the clinical image, an irregular and complete melanonychia is shown of the nail of the right thumb. Of note is the punctual pigmentation of the hyponychium (arrow). C, Detail of the matrix with melanocytes with evident atypia (arrow). D, Detail of the hyponychium with proliferation of atypical melanocytes in the basal layer and lymphocyte infiltrate at the base of the epidermal ridge. E, Detail of a nail bed with patchy lymphocyte infiltrate beneath a basal epidermal layer with increased number of melanocytes. F, Detail of the nail matrix with proliferation of atypical melanocytes that are present throughout the full thickness of the nail epithelium.
When nail melanoma becomes invasive, it shows characteristics similar to invasive melanoma at other sites.1,7,9,14
ConclusionsStudy of melanocytic lesions of the nail is complex and may represent a challenge both for dermatologists and pathologists. Clinical and epidemiological data can be as useful if not more so than histopathological findings in diagnosis. Thus, in children, an atypical subungual melanocytic lesion is highly likely to be a melanocytic nevus. The presence of the Hutchinson sign is not always indicative of melanoma, as it has also been reported in many benign entities, but its presence in a melanonychia in an adult along with other atypical features should be considered highly suspicious.
With respect to the histopathology of nail melanocytic lesions, of note is that suprabasal melanocytes are a physiological finding in the matrix. Subungual melanoma, like other acral melanomas, usually shows a lentiginous pattern and only forms nests in advanced phases. Finally, the simplest interpretation of subungual melanocytic lesions is achieved with a longitudinal biopsy that includes the entire nail apparatus.
FundingThis study did not receive any funding.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Ríos-Viñuela E, Nájera-Botello L, Requena L, Nagore E, Requena C. Lesiones melanocíticas subungueales: claves clínico-patológicas y técnicas de biopsia. Actas Dermosifiliogr. 2021;112:573–585.