Angiolymphoid hyperplasia with eosinophilia (AHE) is a rare, benign vascular proliferation with a chronic clinical course. It typically presents as erythematous or brownish papules and plaques or subcutaneous lesions predominantly affecting the head and neck, especially in the periauricular region.1 Histology reveals endothelial cells of epithelioid appearance within the vascular lumen, associated with an inflammatory infiltrate and abundant eosinophils. This disorder has a broad differential diagnosis with other subcutaneous lesions. Ultrasound is a noninvasive technique that is very useful for the study of these lesions. We present a case of AHE and describe the ultrasound findings.
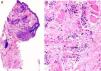
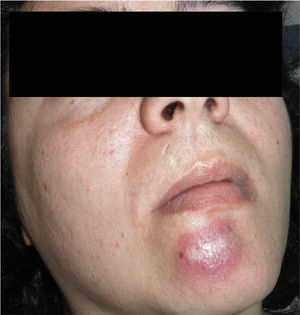
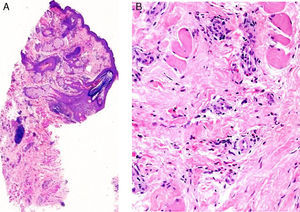
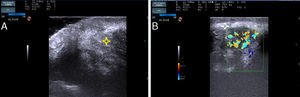
The patient was a 37-year-old woman with no past medical history of interest. She was seen in dermatology outpatients for an asymptomatic lesion that had arisen on her chin several months earlier. On examination there was a brownish nodule of 2cm diameter, with a rubbery consistency (Fig. 1). There were no palpable lymph nodes. The lesion was excised. Histology showed no changes in the epidermis, but vessels lined by endothelial cells of epithelioid appearance were present in the deeper layers, associated with aggregates of lymphocytes and eosinophils (Fig. 2, A andB). Eosinophilia was detected in the blood tests, with no changes in other laboratory parameters. Based on these findings, a diagnosis of AHE was made. Ultrasound monitoring (Esaote My Lab One, using a variable frequency linear probe of 18-22MHz with a lateral resolution of 240microns) was performed throughout follow-up. In the dermis there was a mass with poorly defined borders; this mass was composed of intertwined hyperechoic and hypoechoic bundles, forming an image of a “ball of wool” surrounded by a hyperechoic halo (Fig. 3A). Doppler study (Esaote My Lab One with a linear probe in power Doppler mode; frequency, 18MHz; pulse repetition frequency, 750MHz) showed increased vascularization (Fig. 3B). This image has persisted unchanged despite the treatments performed.
A, At low magnification, the superficial layers show no changes, and lymphocyte aggregates are seen in the deeper layers (hematoxylin-eosin, original magnification ×25). B, Detail showing the presence of numerous eosinophils throughout the stroma (hematoxylin-eosin, original magnification ×200).
Ultrasound image of the lesion. A, A poorly-defined nodule is present in the dermis; hyperechoic and hypoechoic bundles are seen to form a woolly pattern(*). B, Color Doppler study of the lesion showing increased vascularization. Also observe the presence of a hyperechoic halo around the mass(*).
The differential diagnosis of AHE includes a wide range of benign and malignant subcutaneous lesions. Ultrasound is useful as many of these lesions present characteristic sonographic patterns. Of the benign lesions, epidermal cysts appear as well-defined, round anechoic structures in the dermis or subcutaneous tissue, with a tract that connects it to the epidermis, and posterior acoustic enhancement.2 Pilomatrixomas are also easy to exclude as the image they present is of an oval target image with a hypoechoic ring around hyperechoic center formed of calcified material.2 Lipomas appear in the subcutaneous cellular tissue as elongated, isoechoic structures with hyperechoic septa.3 Glomus tumors are seen on ultrasound as well-defined, hypoechoic nodular lesions with prominent vascularization observed on Doppler study.4 Blood vessels can also be observed adjacent to the lesion. Finally, among the benign lesions, we must consider those of vascular origin. Doppler study is a fundamental component of the investigation for the diagnosis and classification of all of these lesions. Hemangiomas present a sonographic pattern that varies according to their phase of development. These lesions are hypoechoic and hypervascular in the proliferative phases and hyperechoic and hypovascular during regression.2 The sonographic image of vascular malformations can be variable. Doppler study allows them to be classified according to blood flow.5 In the differential diagnosis with malignant lesions, we must exclude skin metastases and B-cell lymphoma. The diagnosis of these lesions must be confirmed histologically. Skin metastases, most commonly from melanoma, are seen as oval anechoic structures with dense vascularization.6 B-cell lymphoma presents as a well-defined, hypoechoic nodular lesion with a good vascular supply in the dermis or subcutaneous cellular tissue.7
There is only 1 report of a case of cutaneous AHE that gives a description of its sonographic pattern.8 In that case, a hyperechoic lesion was observed on the forearm and had a peripheral hypoechoic ring and increased blood flow on Doppler study. The absence of other reports is probably because the condition was considered part of the spectrum of Kimura disease until a few years ago. The 2 diseases, though clinically similar, are now considered to be different entities because of the differences in extracutaneous involvement, laboratory tests, and histological findings, leading to a completely different prognosis. A larger number of articles have been published on Kimura disease. The characteristic image is of a heterogeneous hypoechoic mass with poorly defined borders, situated in the dermis and subcutaneous cellular tissue, with intermingled, hyperechoic and hypoechoic curvilinear structures, which has been called a “woolly” pattern.9 The vascularization of the lesions is variable.10 Our case shows the sonographic characteristics of both diseases as the woolly pattern was observed, associated with hypervascularization and a hypoechoic halo.
Skin ultrasound is a useful tool for the diagnosis of subcutaneous lesions. Although the woolly pattern is characteristic, it does not enable us to distinguish between AHE and Kimura disease. These findings testify to the difficulty of differentiating between the 2 diseases and to the need for histological confirmation.
Please cite this article as: Lorente-Luna M, Alfageme-Roldán F, Suárez-Massa D, Jiménez-Blázquez E. Patrón en ovillo de lana como hallazgo ecográfico característico de hiperplasia angiolinfoide con eosinofilia. Actas Dermosifiliogr. 2014;105:718–720.